Abstract
Interleukin-17 (IL-17, also known as IL-17A) is a key cytokine that links T cell activation to neutrophil mobilization and activation. As such, IL-17 can mediate protective innate immunity to pathogens or contribute to the pathogenesis of inflammatory diseases, such as psoriasis and rheumatoid arthritis. This review summarizes the basic biology of IL-17 and discusses its emerging role in periodontal disease. The current burden of evidence from human and animal model studies suggests that the net effect of IL-17 signaling promotes disease development. In addition to promoting neutrophilic inflammation, IL-17 has potent pro-osteoclastogenic effects that are likely to contribute to the pathogenesis of periodontitis, rheumatoid arthritis, and other diseases involving bone immunopathology. Systemic treatments with anti-IL-17 biologics have shown promising results in clinical trials for psoriasis and rheumatoid arthritis, although their impact on the highly prevalent periodontal disease has not been investigated or reported. Future clinical trials, preferably using locally administered IL-17 blockers, are required to conclusively implicate IL-17 in periodontitis and, more importantly, to establish an effective adjunctive treatment for this oral inflammatory disease.
Interleukin-17 (IL-17) and IL-17–producing lymphocytes and innate immune cells are emerging as potentially important players in the pathogenesis of periodontitis (63). Nearly all of the IL-17-related research in the field of periodontal disease involves the IL-17A isoform (also simply known as IL-17) and, therefore, will be the focus of this review. To understand the role of IL-17 in periodontitis, it is instructive to first discuss the basic biology of this cytokine and the cells that produce it.
IL-17 is one of the best-studied cytokines in immunology, at least in part owing to its involvement in inflammatory pathology (4, 47, 107). The gene encoding IL-17 was cloned in 1993 from a mouse cytotoxic T lymphocyte hybridoma cDNA library (131) and the role of human IL-17 in inflammation was recognized soon after (45, 160). However, IL-17 entered the spotlight of immunological attention after the discovery of a developmentally distinct CD4+ T helper subset that expresses IL-17 (the so-called Th17 lineage) and mediates tissue inflammation (69, 122). Interleukin-17 or IL-17A is the founding member of a family of cytokines that also includes IL-17B through to IL-17F (58, 81). The cellular source of IL-17C is quite unique among the other isoforms as IL-17C is produced primarily by epithelial cells rather than hematopoietic cells (128). The best-characterized cytokines in the family are IL-17 and IL-17F, which can signal as homodimers or as IL-17A/F heterodimers through the same heterodimeric receptor that comprises IL-17RA and IL-17RC subunits (Fig. 1). IL-17RA is expressed ubiquitously on various cell types as is IL-17RC, although their tissue expression profile displays interesting differences. IL-17RC is predominantly expressed in the epithelial cells of the prostate, kidney and joints, whereas IL-17RA is abundantly expressed in hematopoietic cell compartments (75). If the binding repertoire of IL-17RA and IL-17RC includes distinct ligands, this would explain, at least in part, their different tissue distribution. In this regard, IL-17RA oligomerizes also with IL-17RB and the IL-17RA/RB complex binds IL-17E, also known as IL-25 (tissues that are responsive to IL-25 might therefore express higher levels of IL-17RA than IL-17RC). IL-17RA additionally pairs with IL-17RD, although the cognate ligand (if it exists) for the IL-17RA/RD complex has not been identified (130). The different tissue distribution of IL-17RA and IL-17RC may also serve to allow tissue-specific signaling by IL-17A, IL-17F, and IL-17A/F, since these ligands have differential binding affinities for each of the IL-17RC and IL-17RA subunits, although overall IL-17A binds to the IL-17RA/RC complex with higher affinity than IL-17F does (75). Interestingly, IL-17B and IL-17C can signal through monomeric receptors, IL-17RB and IL-17RE, respectively, whereas the receptor for IL-17D is unknown (81).
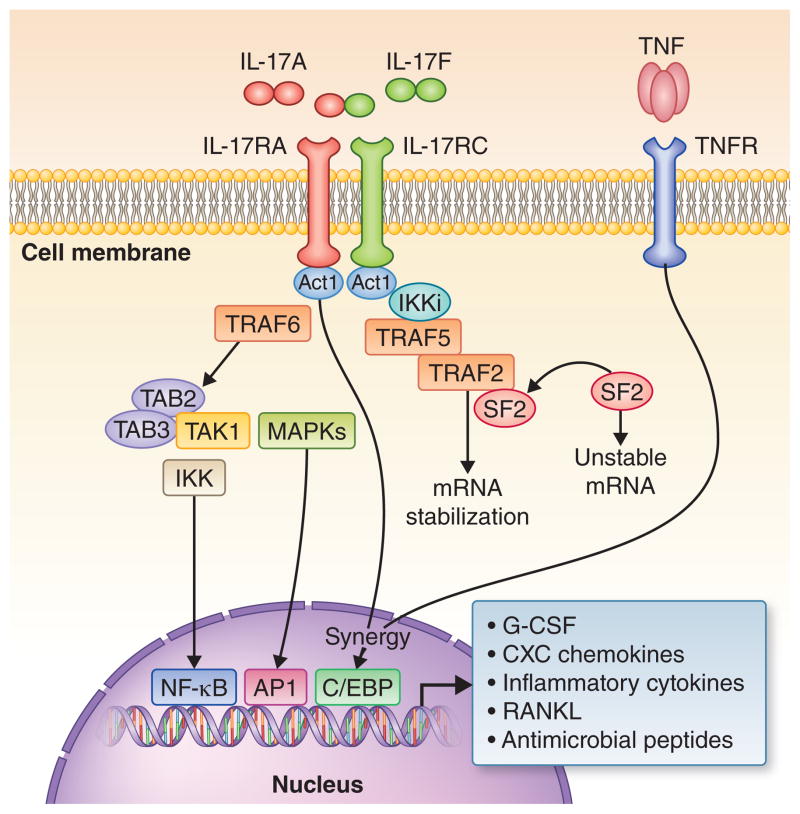
Fig. 1. Interleukin-17 receptor signaling.
The IL-17 receptor complex (IL-17RA/IL-17RC) is ubiquitously expressed and its ligands include IL-17A and IL-17F homodimers, as well as IL-17A/F heterodimers. Ligand-induced IL-17 receptor signaling initiates the recruitment of the essential adaptor molecule Act1, which in turn recruits tumor necrosis factor-R-associated factor 6 (TRAF6), a critical upstream activator of the NF-κB pathway. Specifically, TRAF6 recruits the kinase TAK1 and its binding partners TAB2 and TAB3 leading to activation of the inhibitor of NF-κB kinase (IKK) complex and thereby to induction of NF-κB translocation. It is uncertain whether TRAF6 is also required for the activation of mitogen-activated protein kinases (MAPKs) that link IL-17 receptor signaling to activation of the transcription factor activator protein-1. Interleukin-17 receptor activation also induces the recruitment of the IKK-related kinase IKKi to the IL-17 receptor–Act1 complex. IKKi phosphorylates Act1 generating a docking site for recruitment of TRAF2 and TRAF5. The resulting Act1-TRAF2-TRAF6 complex sequesters splicing factor SF2 resulting in mRNA stabilization of target genes (e.g., chemokines). Another important transcription factor activated downstream of Act1 is CCAAT/enhancer-binding protein (C/EBP). The activation of NF-κB, transcription factor activator protein-1, and C/EBP (C/EBPβ and C/EBPδ) leads to the induction of genes encoding for inflammatory mediators and neutrophil-specific CXC chemokines (e.g., CXCL8), antimicrobial peptides, and the osteoclastogenic factor RANKL. Tumor necrosis factor signaling can synergize with IL-17 signaling through C/EBP to amplify inflammatory mediators such as IL-6 and CXCL8. Drawn on the basis of information from several sources (18, 47, 132, 142).
Although a signature cytokine of Th17 cells, IL-17 is now known to be expressed also by other adaptive and immune cell types, including CD8+ T cells, γδ T cells, natural killer T (NKT) cells, and innate lymphoid cells (29, 144) (Fig. 2). The γδ T cells constitute a relatively minor lymphoid cell subset in lymphoid tissues and blood but they are a major subset at mucosal sites, where they can be triggered to produce IL-17 by innate signals, such as IL-1 and IL-23, without T-cell receptor engagement (144). IL-17 was also shown to be expressed by mouse neutrophils (42, 98) and, more recently, a population of human neutrophils was identified that expresses the transcription factor RORγt and both produces and responds to IL-17 (146, 147). Consistent with a certain degree of inherent plasticity, naïve T cells, memory T cells, and CD4+ Foxp3+ regulatory T cells (Tregs) have all been shown to have the ability to differentiate into an IL-17-producing phenotype (91, 151, 158). The resulting IL-17-producing T cell can express varying concentrations of different effectors such as IL-17 and IL-10, potentially exhibiting either a pathogenic or regulatory phenotype (104).
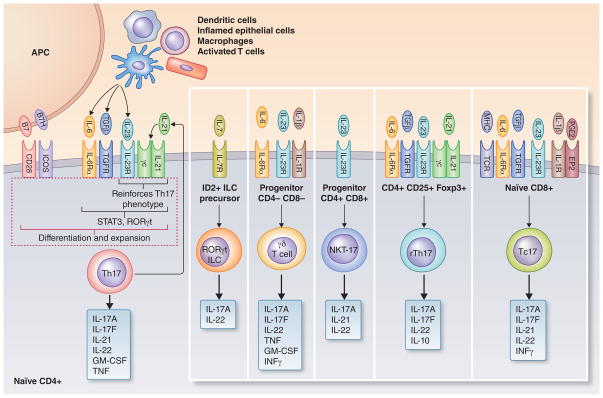
Fig. 2. Differentiation of Th17 and other IL-17–producing cell subsets.
Of the lymphoid cells that are capable of producing IL-17, the differentiation process of CD4+ IL-17–producing T cells (Th17) is the best defined and depends on a specific cytokine milieu provided by innate and adaptive immune cell sources. Moreover, the induction of the Th17 lineage from a naïve T cell requires engagement of CD28 and ICOS by the costimulatory molecules B7 and B7H, respectively, on antigen-presenting cells. Tumor growth factor-β combined with specific immunostimulatory cytokines, such as IL-6, can induce Th17 differentiation in mice. Although in humans the requirement for Th17 development is met with IL-6 and IL-1β, when the starting population is rigorously sorted for naive T cells and hidden sources of tumor growth factor-β in the culture conditions are accounted for, similar factors appear to govern Th17 differentiation in mice and humans. In both species, IL-21 feeds back on developing Th17 cells and reinforces the differentiation process, while IL-23 promotes Th17 cell expansion and survival. The cytokines that induce Th17 differentiation and function act via the transcription factors Stat3 and RORγt, which represents the lineage-specific transcription factor of Th17 cells. The differentiation requirements for other IL-17–producing cell subsets are less well defined; shown are factors that are thought to contribute to the differentiation process. Innate cellular sources for IL-17 production include RORγt+ innate lymphoid cells (ILC), natural killer T (NKT) cells, and γδ T cells which do not require T-cell receptor stimulation for IL-17 production. In addition to the typical Th17 cells, IL-17 can be produced also (albeit at lower levels) by CD4+ regulatory Th17 cells (rTh17), which express high levels of the suppressive cytokine IL-10. Another adaptive immune cell source of IL-17 are CD8+ cytotoxic T cells (Tc17). The up-regulation of the IL-23 receptor (IL-23R) is essential for most subsets to initiate the IL-17 production. Also shown are other important cytokines produced by each cell subset. Drawn on the basis of information from several sources (2, 13, 43, 84, 90, 91, 96, 158, 164).
Interleukin-17 is of particular interest in the pathogenesis of periodontitis because of its involvement in both inflammation and protective antimicrobial immunity (88) (Fig. 3). In the latter regard, IL-17 was shown to mediate protection against extracellular pathogens (73, 88) and together with IL-22 (a cytokine also produced by Th17 and other IL-17–expressing cells; Fig. 2) can induce the production of antimicrobial peptides (101), which are thought to be protective in periodontitis (36, 53). In principle, therefore, IL-17 is a paradigmatic double-edged sword for a disease, such as periodontitis, that is initiated by bacteria although tissue damage is inflicted by the host response (63). Therefore, the biological properties of IL-17 make it difficult to predict its role in inflammatory diseases with a polymicrobial etiology. It is possible that IL-17 exerts both protective and destructive effects, as suggested in distinct mouse models (42, 161), although chronic IL-17 receptor signaling can turn a potentially protective acute inflammatory response into chronic immunopathology (103). Interleukin-17 and IL-17–producing cells that display inflammatory, antimicrobial, and regulatory functions are therefore of keen interest in the development and progression of periodontal disease and their nuances are discussed in this review.
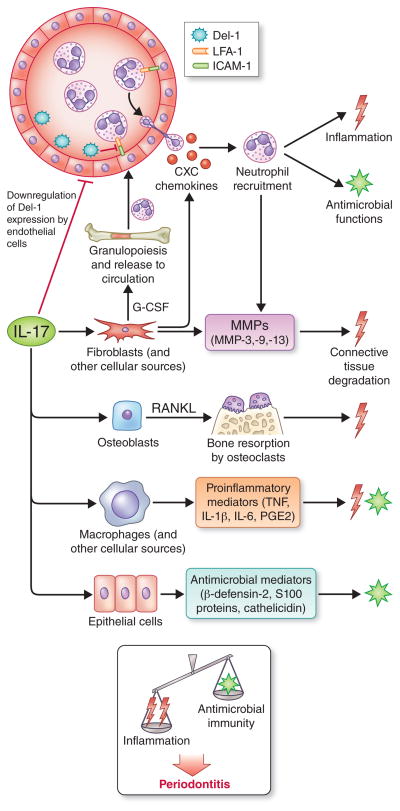
Fig. 3. Biological functions of IL-17 and their role in periodontitis.
IL-17 acts predominantly on innate immune and stromal cells to promote inflammation and antimicrobial functions. By acting through fibroblast upregulation of granulocyte colony-stimulating factor and CXC chemokines, IL-17 can orchestrate bone marrow production and release of neutrophils and their chemotactic recruitment to the periodontium. Additionally, IL-17 inhibits endothelial cell production of Del-1, a glycoprotein that restrains neutrophil extravasation by blocking the interaction of the LFA-1 integrin on neutrophils with the molecule intercellular adhesion molecule-1 (ICAM-1) on endothelial cells. Neutrophils can exert both protective and destructive effects in periodontitis and thus their homeostatic regulation is key to periodontal health. IL-17 can promote the destruction of both connective tissue and the underlying bone by stimulating the production of matrix metalloproteinases (MMPs) and RANKL from the indicated stromal cell types. Although IL-17 can also induce the production of epithelial cell-derived antimicrobial molecules, the current burden of evidence from human and animal model studies suggests that the net effect of IL-17 signaling promotes disease development. Drawn on the basis of information from several sources (5, 40, 42, 48, 81, 101, 107, 153).
Differentiation and function of IL-17–producing T cells
The local cytokine environment contributes to the differentiation of specific T cell subsets with distinct transcription patterns resulting in unique effector functions. In the classical Th1/Th2 paradigm (111), the differentiation of Th1 and Th2 subsets are driven by IL-12 and IL-4, respectively, and the key transcription factors driving their differentiation are T-bet (Th1) and GATA3 (Th2). Th1 cells secrete interferon-γ and are primarily responsible for cell-mediated immunity to intracellular pathogens (bacteria, protozoans, viruses). On the other hand, Th2 cells secrete IL-4, IL-5, and IL-13 and are responsible for humoral immunity, including production of IgE, and activation of mast cells that mediate immune responses to helminths. Similar to the Th1/Th2 paradigm, both Tregs and Th17 differentiate in a specific cytokine milieu and both require tumor growth factor-β. Tumor growth factor-β is sufficient for Treg differentiation but needs to be combined with specific immunostimulatory cytokines, such as IL-6 and IL-21, to induce Th17 differentiation (Fig. 2). In mice, IL-6 together with tumor growth factor-β is sufficient to drive Th17 development. In humans, the requirement for Th17 development is met with IL-6 and IL-1β (2). However, it is thought that when the starting population is rigorously sorted for naive T cells and hidden sources of tumor growth factor-β in the culture conditions are revealed, it then appears that similar factors govern the differentiation of Th17 cells in mice and humans (91). In both species, IL-21 feeds back on developing Th17 cells and amplifies the differentiation process (Fig. 2), whereas innate immune cell-derived IL-23 is required for Th17 cell expansion and survival (91). Acting alone, tumor growth factor-β is suppressive for Th17 development and instead initiates differentiation into Tregs by upregulating the forkhead box P3 (FoxP3) transcription factor (164). Conversely, retinoid-related orphan receptor-gamma t (RORγt), a transcription factor upregulated during differentiation toward Th17, inhibits FoxP3 and thereby suppresses Treg development (164). Additional suppression of FoxP3 can be directly mediated by IL-6 and IL-21 (90, 158).
While the differentiation of Th17 and Tregs appears mutually exclusive, the presence of IL-6 coupled with the production of tumor growth factor-β by Tregs may allow the conversion of Tregs to Th17 suggesting a degree of plasticity (13). The differentiation of Treg toward a Th17 phenotype can start prior to full inhibition of FoxP3, thereby creating a double-positive (IL-17+/FoxP3+) cell type (154). There is also plasticity in the Th17 cell in that it can acquire functional characteristics of Th1 cells, manifested as interferon-γ production (114). Although there is a paucity of literature regarding mechanisms of T-cell differentiation in periodontal tissues, the implications of this T-cell plasticity might contribute to the transition from active inflammation in sites of periodontal disease to a resolution phase.
Although the typical IL-17–producing T cell can be involved in potent inflammatory responses, recently a regulatory Th17 (rTh17) cell subset that expresses the anti-inflammatory IL-10 cytokine has been identified (Fig. 2). The rTh17 cells can be found in vivo in certain autoimmune diseases and were shown to mitigate pathology in a mouse model of colitis (43, 84). It should also be noted that rTh17 cells produce less IL-17 than the typical Th17 cells. Intriguingly, naïve CD4+ T cells can differentiate into either a pathogenic or non-pathogenic Th17 phenotype depending on the subtype of tumor growth factor-β used to induce Th17 differentiation (96). Th17 generated with tumor growth factor-β1 and IL-6 produce IL-17 but cannot drive autoimmune pathology in the absence of IL-23, whereas Th17 generated with tumor growth factor-β3 and IL-6 define a pathogenic effector subset that can induce autoimmunity, as shown in a mouse model of experimental autoimmune encephalitis (96). These studies illustrate that the complexity of the cytokine milieu is key in directing the specific functional characteristics of Th17 effector cells, which can thereby play pathogenic or regulatory roles in inflammatory diseases.
Interleukin-17 and inflammatory interactions with other cytokines
Interleukin-17 is known foremost for its ability to initiate a potent inflammatory response that includes the induction of granulopoiesis factors (granulocyte colony-stimulating factor) and neutrophil-specific chemokines (CXCL1, CXCL2, CXCL5, CXCL8), mediators of the acute phase response (IL-6), proinflammatory/bone resorptive cytokines (tumor necrosis factor, IL-1β, and RANKL), and matrix metalloproteinases (48, 100, 110, 150) (Fig. 3). The targets of IL-17 include primarily epithelial, endothelial and other stromal cells such as fibroblasts, osteoblasts, chondrocytes, and synovial cells (21, 77, 78, 103, 137). Interestingly, IL-17 appears insufficient to mount a robust inflammatory response by itself; however, in cooperation or synergism with other inflammatory mediators, such as tumor necrosis factor, IL-17 can induce a potent inflammatory cascade by upregulating the expression of a plethora of target genes (38, 57, 120, 121). For instance, IL-17 together with tumor necrosis factor induces a sustained neutrophil recruitment during inflammation, in part by synergistically upregulating endothelial cell expression of CXCL1, CXCL2, and CXCL5 (57). IL-17 can additionally stabilize CXCL1 mRNA and enhance IL-1β-mediated cellular release of CXCL8 (39, 71). The production of IL-17 is dependent on the action of certain other cytokines, such as IL-1β and IL-23 (143). In fact, IL-1β has been shown to synergize with IL-23 to induce IL-17 production (37, 106).
Interleukin-1β is a versatile cytokine with a broad range of functions that can shape the lymphocyte response and is commonly found in gingival crevice fluid and tissues clinically diagnosed with periodontal disease (9, 54, 139). Interleukin-1β combined with IL-17 can synergistically increase the production of chemokine C–C motif ligand 20 (CCL20) in human gingival fibroblasts, thereby stimulating the recruitment of Th17 cells (74). Interestingly, in human gingival fibroblasts, IL-1β can also induce hypoxia-inducible factor-1 (148), which is known to control the Th17-Treg balance in favor of Th17 development (31). Collectively, these data suggest that IL-1β and IL-17 cooperatively promote a Th17 environment, which may have pathological implications in the oral gingival tissues. IL-1β has also been shown to synergize with tumor necrosis factor to produce IL-6, which is important for Th17 differentiation (132).
As mentioned earlier, IL-6 and tumor growth factor-β together promote Th17 differentiation, whereas tumor growth factor-β alone initiates Treg development. In this context, tumor growth factor-β and IL-1β have an antagonistic relationship since tumor growth factor-β can cause inhibition of IL-1β production as well as of IL-1R expression, thereby suppressing lymphocyte proliferation (72, 149, 155). Interleukin-1β has also been shown to induce the expression of complement component C3 in intestinal epithelial cells (109), while tumor growth factor-β inhibits complement signaling by reducing the expression of complement factors C3a and C5a (141). These activities affect Th17 development since inhibition of either C5a receptor (C5aR; CD88) or C3a receptor (C3aR) signaling on CD4+ T cells is thought to lead to Treg development at the expense of Th17 (93, 141). In summary, tumor growth factor-β inhibits the induction of IL-17 and other Th17-related cytokines (even though it is required for Th17 differentiation), whereas IL-1β, IL-23, IL-6, tumor necrosis factor, and perhaps also complement appear to collectively work together to promote an IL-17 environment.
Complement and IL-17
The junctional epithelium lies at the base of the gingival crevice and provides a porous border between the underlying connective tissue and the microbial biofilm that accumulates on subgingival tooth surfaces (32). The permeability of the junctional epithelium is due to the fact that the cells are interconnected by only a few desmosomes and occasional gap junctions, with only a few or no tight junctions (16). In this environment, local host- and microbe-derived proinflammatory factors, such as complement, cytokines including IL-17, host or microbial proteases, and microbial Toll-like receptor ligands such as lipopolysaccharide, can be found at high concentrations (56, 59, 61, 95, 136, 152). In the environment of the gingival crevice, neutrophils constitute the overwhelming majority (>95%) of total infiltrating leukocytes (35).
Complement and IL-17 are both involved in the regulation of neutrophil recruitment, a process considered important for periodontal tissue homeostasis, although both excessive and diminished recruitment can precipitate periodontitis (32, 42, 60). Interleukin-17 can initiate neutrophil mobilization and recruitment by inducing the production of granulocyte colony-stimulating factor (a primary regulator of both granulopoiesis and neutrophil release from the bone marrow) and CXC-chemokines (CXCL1, 2, 5 and 8), which function as ligands of CXC-chemokine receptor 2 (CXCR2) (153). CXCR2 is required for neutrophil extravasation into gingival tissues (162). Whereas transmigrating neutrophils initially utilize CXCR2 to follow the chemokine gradient deposited by the endothelium, they subsequently have to move towards a gradient existing in the infected or inflamed tissue. Such gradients could involve chemoattractants derived either from bacteria (e.g., N-formyl-methionyl-leucyl-phenylalanine) or complement C5a fragments generated from local complement activation (89). In this regard, C5aR is abundantly expressed on neutrophils (127) and was shown to facilitate their recruitment to peripheral tissues (133). Interestingly, C5a-induced activation of C5aR also contributes to the induction of granulocyte colony-stimulating factor, at least in acute models of inflammation (14), although it is uncertain whether this function involves cooperation with IL-17.
Although normally tightly regulated (129), the complement system may become deregulated in a local niche, such as the gingival crevice due to a constant influx of microbial inflammatory molecules and the presence of periodontal bacteria that can subvert complement function (61, 65, 156). For instance, Porphyromonas gingivalis, a gram-negative bacterium strongly associated with human periodontitis (66), is very adept at subverting the complement system and has several mechanisms by which it can disrupt or hijack complement components leading to immune evasion and destructive inflammation (61, 67, 126). Not only are complement activation fragments found in abundance in the gingival crevice fluid of periodontitis patients but their levels correlate with clinical parameters of the disease (28, 61, 134). Single nucleotide polymorphisms in the complement component C5 and IL-17 are suspected to predispose to periodontal disease, suggesting possible involvement of both molecules in its pathogenesis (22, 27, 85).
Although complement generally has complex effects on IL-17 expression that include both positive and negative regulation (1, 15, 94, 102, 108, 159), complement was shown to augment IL-17 production in the murine periodontal tissue in cooperation with Toll-like receptors (1). Specifically, C5a-induced activation of C5aR has been shown to synergize with Toll-like receptor-2 in a mouse model of periodontal disease to yield abundant increases in IL-17, IL-1β, IL-6, and tumor necrosis factor that result in significant bone loss (1). Conversely, mice deficient in either C5aR or Toll-like receptor-2 are protected from experimental periodontitis (1, 67, 99).
Interleukin-17 and neutrophil homeostasis
As alluded to above, IL-17 is important for neutrophil homeostasis, and consequently for periodontal health since any deviation from normal neutrophil activity (in terms of numbers or activation status) can potentially cause periodontitis (32, 60). In fact, IL-17 is a key component of a neutrophil rheostat (‘neutrostat’) feedback mechanism that maintains steady-state neutrophil counts (140) (Fig. 4). Specifically, the neutrostat mechanism maintains a fine balance among granulopoiesis, release of mature neutrophils from the bone marrow into the circulation, extravasation of circulating neutrophils, and clearance of apoptotic neutrophils (44, 140, 153). During infection or inflammation, innate immune cell-secreted IL-23 induces IL-17, which promotes granulopoiesis and mobilization of mature neutrophils from the bone marrow by acting through upregulation of granulocyte colony-stimulating factor. Neutrophils released from the bone marrow circulate in the blood and can extravasate into infected or inflamed tissues. Upon senescence, transmigrated neutrophils become apoptotic and are phagocytosed by tissue phagocytes leading to suppression of IL-23 production, in turn, downregulating the IL-17– granulocyte colony-stimulating factor axis for maintaining steady-state neutrophil counts (44, 140, 153) (Fig. 4). In a similar context, the phagocytosis of apoptotic neutrophils associated with resolution of inflammation will signal the down-regulation of neutrophil production, since neutrophils are no longer needed in great numbers.
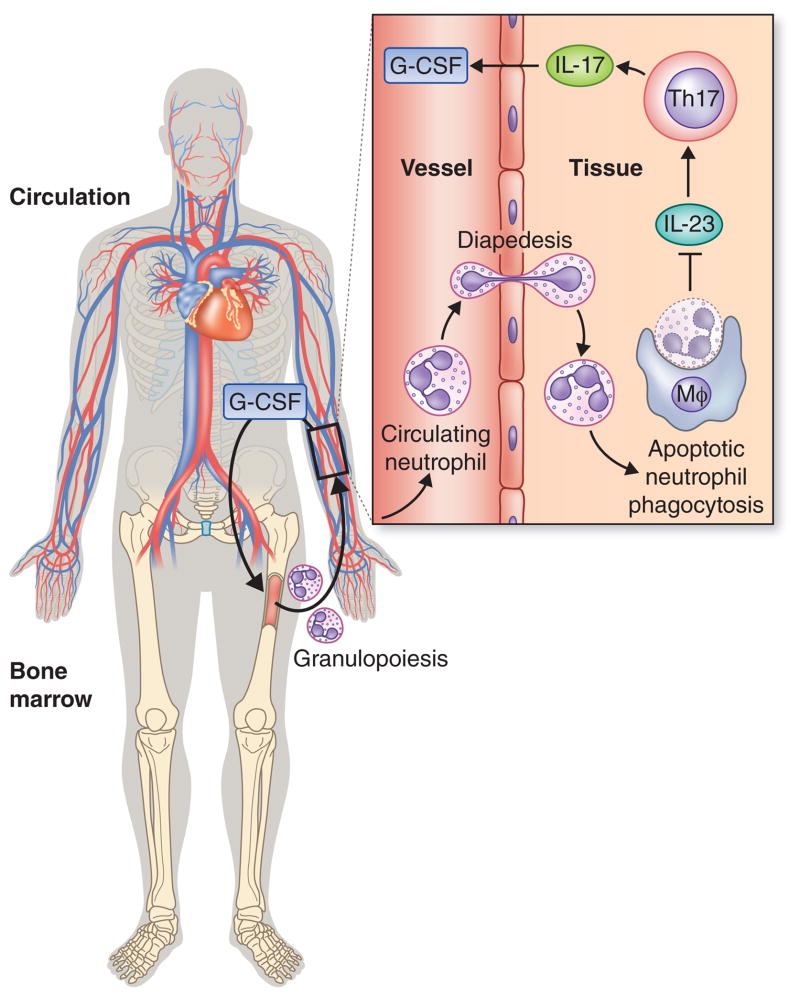
Fig. 4. The neutrostat regulatory feedback loop.
IL-17 promotes granulopoiesis and mobilization of mature neutrophils from the bone marrow by acting through upregulation of granulocyte colony-stimulating factor. Upon release from the bone marrow, circulating neutrophils can normally extravasate into infected or inflamed tissues. Upon senescence, transmigrated neutrophils become apoptotic and are phagocytosed by tissue phagocytes leading to suppression of their IL-23 production, in turn, downregulating the IL-17– granulocyte colony-stimulating factor axis for maintaining steady-state neutrophil counts (140, 153). Disruption of this regulatory circuit in conditions that prevent the extravasation of neutrophils (e.g., leukocyte adhesion deficiency) leads to local overproduction of IL-17, which causes severe inflammation and periodontal bone loss (112).
Therefore, while granulocyte colony-stimulating factor is important for granulopoiesis, neutrophil recruitment into local tissues is equally important to maintain the neutrostat regulatory circuit and hence neutrophil homeostasis. This is best illustrated in disorders that disrupt the normal recruitment of neutrophils to peripheral tissues and thereby abrogate the regulatory signals for IL-23 and IL-17 expression, which are normally associated with apoptotic neutrophil phagocytosis. For instance, adhesion molecule-deficient mice (e.g., lacking β2 [CD18] integrins) display blood neutrophilia and fail to recruit neutrophils in peripheral tissues (such as lungs and gut) where there is unrestrained expression of IL-17 (140). This homeostatic breakdown has recently been linked to IL-17–driven pathology in mouse models and humans. Specifically, impaired neutrophil recruitment to the periodontal tissue of mice lacking either the LFA-1 (CD11a/CD18) integrin or CXCR2 leads to severe periodontal bone loss associated with overexpression of IL-17, whereas local anti-IL-17 treatment reverses the disease phenotype (112). Interestingly, the anti-IL-17 treatment also reduced the periodontal bacterial load (112), apparently because periodontal bacteria rely on inflammation to secure critical nutrients, such as tissue breakdown products (peptides) and heme-containing compounds (66). Importantly, overexpression of IL-17 was also observed in the gingival tissue of patients with leukocyte adhesion deficiency Type I (LAD-I) (112). LAD-I is a disease syndrome caused by mutations on the CD18 subunit of β2 integrins and is associated with frequent microbial infections and with aggressive periodontitis early in life (6, 34, 60, 68, 135). LAD-I periodontitis has been historically attributed to lack of neutrophil surveillance of the periodontal infection. However, the recent demonstration that IL-17 is abnormally upregulated in this condition, together with the demonstration that neutralization of IL-17 in relevant mouse models reverses both destructive inflammation and bacterial dysbiosis, suggests that LAD-I–associated periodontitis is fundamentally caused by a dysregulated host response featuring IL-17 overproduction.
It should be noted that the importance of neutrophil homeostasis for periodontal health is also emphatically evident in additional conditions associated with defects in mechanisms that regulate the production and life cycle of neutrophils (e.g., congenital neutropenia, Chediak-Higashi syndrome, and Papillon-Lefèvre syndrome) (30, 34, 60, 70, 115). Although the underlying molecular mechanisms driving severe periodontitis in these patients are poorly understood, we hypothesize that an IL-17–driven mechanism might be relevant to any conditions associated with poor or no accumulation of neutrophils in extravascular sites, such as defective chemotaxis as seen in the Chediak-Higashi syndrome (34, 60).
Periodontal bacteria and IL-17
In the context of the neutrostat mechanism discussed above, CXCR2 was shown to regulate the IL-17– granulocyte colony-stimulating factor axis in the intestine in a bacteria-dependent manner (105). Although CXCL5 was shown to be the CXCR2 ligand that regulates the IL-17– granulocyte colony-stimulating factor axis in the intestine, CXCL5 has not been explored in gingival tissues. However, commensal bacteria have been shown to induce CXCL2 and to contribute to neutrophil recruitment to gingival tissues (162). Whether CXCL2 plays a similar role in the periodontium, as CXCL5 does in the intestine, is not known at present.
Little is known on the mechanisms by which periodontal bacteria regulate IL-17 or IL-17–producing cells and such investigation could provide additional insight into mechanisms of neutrophil recruitment and activation. Interestingly, Th17 cells can contribute to neutrophil recruitment not only through IL-17 production but also through their capacity to express CXCL8 (124). Conversely, recruited neutrophils can amplify the recruitment of Th17 cells though the production of CCL2 and CCL20 chemokines, which are ligands respectively for chemokine CC-receptor -2 (CCR2) and -6 (CCR6) that are characteristically expressed by Th17 cells (124). This apparent reciprocal relationship between neutrophils and Th17 may have important implications in periodontal health or disease, by either reinforcing a protective immune response to control the periodontal bacteria or by amplifying a destructive inflammatory response.
As stated earlier, IL-17 is a key molecule in protection against extracellular bacteria and fungal pathogens (26, 116). The protective mechanisms involved include the ability of IL-17 to not only orchestrate neutrophil recruitment but also stimulate the production of antimicrobial peptides from epithelial and other cell types, including β-defensin-2, S100 proteins, and cathelicidin (101, 116). In this context, IL-17 receptor signaling was associated with protection in a mouse model of periodontitis induced by implantation of a human periodontal pathogen (P. gingivalis) (161). In contrast, IL-17 receptor signaling was associated with protection against naturally occurring chronic bone loss in mice (42). In the latter model, genetic or aging-associated deficiency of Del-1, an endothelial cell-secreted glycoprotein that antagonizes the LFA-1 integrin (25, 64), leads to unrestrained neutrophil infiltration and IL-17-dependent bone loss (42). This apparent discrepancy might involve the different nature of the two models (chronic versus a relatively acute periodontitis model). Although such explanation is uncertain, chronic IL-17 receptor signaling can potentially turn an acute inflammatory response into chronic immunopathology, as in rheumatoid arthritis (103).
Although it is uncertain how periodontal bacteria may regulate IL-17 production, there is evidence suggesting that P. gingivalis promotes an IL-17 environment, ostensibly to exploit the resulting inflammatory response to obtain nutrients in the form of tissue breakdown products and heme-containing molecules (64, 113, 117, 123). In this regard, stimulation of peripheral blood mononuclear cells from healthy volunteers by P. gingivalis resulted in increased IL-17 production in CD3+ T cells and increased IL-23 production in macrophages (113). Moreover, lipopolysaccharide isolated from P. gingivalis was shown to induce IL-17 and IL-23 production from human periodontal ligament cells (123) while its outer membrane proteins could stimulate IL-17 mRNA expression in peripheral blood mononuclear cells isolated from patients with gingivitis or periodontal disease (117). Remarkably, P. gingivalis appears to skew a Th1 response toward Th17, ostensibly to escape Th1 cell-mediated immunity to which the organism appears to be susceptible (46, 49, 113). In part, the suppression of Th1 cell-mediated immunity by P. gingivalis could be attributed to its ability to inhibit gingival epithelial cell production of Th1-recruiting chemokines (82) as well as T cell production of interferon-γ (46). In general, P. gingivalis has an arsenal of virulence factors by which it can manipulate innate and adaptive immune cells to initiate a nutrient-rich inflammatory response orchestrated by IL-17. Importantly, the presence of P. gingivalis in the subgingival biofilm was associated with increased gingival crevice fluid levels of IL-17 in human periodontitis (136).
Interleukin-17 and inflammatory bone loss
A persisting inflammatory environment can ultimately disrupt bone homeostasis which depends on a triad of proteins within the tumor necrosis factor/tumor necrosis factor-receptor family consisting of receptor activator of nuclear factor-κB ligand (RANKL), its functional receptor RANK, and its decoy receptor osteoprotegerin (17). These proteins are key factors for osteoclast differentiation and function: Osteoclastogenesis is promoted by the binding of RANKL (expressed by osteoblasts as well as activated T cells and B cells) to RANK on osteoclast precursors, whereas osteoprotegerin restrains osteoclastogenesis by inhibiting the interaction of RANKL with RANK. However, the bone-protective effect of osteoprotegerin is diminished in periodontitis since the osteoprotegerin/RANKL ratio decreases with increasing periodontal inflammation (12).
IL-17 has potent osteoclastogenic properties, in part due to its capacity to stimulate RANKL expression by osteoblasts and other stromal cells (92) (Fig. 3) and is, therefore, a focal point of interest in bone-related diseases such as rheumatoid arthritis, osteoporosis, and periodontal disease. IL-17 can additionally induce the expression of matrix metalloproteinases in fibroblasts, endothelial cells, and epithelial cells, thereby potentially mediating destruction of both connective tissue and the underlying bone (107). By expressing both IL-17 and RANKL, Th17 cells can function as a dedicated osteoclastogenic subset that links T-cell activation to inflammatory bone destruction (107). Most of the knowledge regarding Th17 and IL-17 in bone loss regulation comes from studies in rheumatoid arthritis. Periodontal disease has certain similarities with rheumatoid arthritis in that they both feature chronic inflammatory bone loss (33).
Interleukin-17 was also shown to enhance the survival and proliferation of human B cells and their differentiation into antibody-secreting plasma cells (38). In the bone resorptive lesions of chronic periodontitis, B cells/plasma cells are a major source of RANKL (86). This raises the possibility that the influence of IL-17 on B cells and plasma cells may include bone destructive effects, thereby contributing to the progression of periodontal disease. It could be argued that such deleterious effects could be offset by IL-17-mediated enhancement of the antibody response. However, the role of the antibody response in periodontitis remains unclear, although it is generally thought that naturally induced antibodies to periodontal bacteria are of low affinity and poor functionality (50).
The incidence of chronic inflammatory diseases appears to increase during the aging process (20, 52, 62). Mice also show a propensity for age-related periodontal disease, which correlates with increased production of IL-17 and elevated numbers of periodontal neutrophils (42). Intriguingly, neutrophils can induce osteoclastic bone resorption through the expression of membrane-bound RANKL (23), although whether this occurs in the periodontal tissue is uncertain. The increased production of IL-17 is inversely correlated with a decline of Del-1 expression in the periodontal tissue of old mice (42). The inverse relationship between IL-17 and Del-1 also characterizes human gingiva, with IL-17 and Del-1 dominating in inflamed and healthy gingiva, respectively (42). In this regard, IL-17 inhibits the expression of Del-1 in human endothelial cells (138)(Fig. 3); consistent with this, the neutralization of IL-17 in the murine periodontal tissue leads to increased Del-1 expression, reduced neutrophil infiltration, and diminished periodontal bone loss (42). These findings suggest that IL-17 biologics could, at least in principle, find application for the treatment of human periodontitis.
Interleukin-17 in periodontal disease: clinical studies
Numerous studies have shown that human periodontitis is associated with increased levels of locally produced IL-17 as compared with healthy periodontal tissue (3, 5, 7, 10, 11, 19, 40, 41, 76, 80, 83, 97, 113, 118, 119, 136, 145, 152, 163) (Table 1). Moreover, a single nucleotide polymorphism associated with increased expression of IL-17 was found to be more prevalent in patients with chronic periodontitis than in control subjects (27). Carriers of the IL-17 G197A allele showed increased expression of IL-17 and CXCL8, correlating with worse clinical periodontal parameters but increased myeloperoxidase activity compared to individuals with the GG genotype (27). While very important, these studies by themselves do not formally establish a causal role for IL-17 in periodontitis. However, taken together with the pro-inflammatory and osteoclastogenic properties of IL-17 and intervention studies in mouse models discussed above, it is reasonable to suspect that IL-17 is an important player in periodontal immunopathology.
Table 1.
IL-17-related clinical periodontal studies
| Clinical groups analyzed | Experimental procedure | Results | Reference |
|---|---|---|---|
|
Solubilized gingival biopsies measured for IL-17 | IL-17 highest in 4–5mm | (83) |
| Healthy and PD | Examination of T-cell clones established from gingival tissues and peripheral blood | Gingival T cells had increases in IL-17 mRNA | (80) |
| Healthy and PD | Periodontal biopsies measured for IL-17 | IL-17 present and correlated to periodontal disease | (145) |
| Healthy and PD | GCF taken from healthy and periodontal patients measured for IL-17 | IL-17 is increased in PD | (152) |
| Healthy and PD | IHC on gingival biopsies | IL-17 is increased in PD | (11) |
PD
|
Interdental gingival papilla biopsy | IL-17 greater in moderate and severe lesions and correlated with IL-23 and CAL | (97) |
| Healthy and PD | Gingival biopsies analyzed for gene expression | IL-17 mRNA levels significantly higher in periodontal disease than gingivitis | (76) |
| Healthy and PD | Gingival and alveolar bone biopsies for mRNA and protein expression | PD tissues had elevated IL- 17, TGF-β, IL-1β, IL-6 and IL-23 mRNA while bone had elevated IL-17 and RANKL | (19) |
| Healthy and PD | PD biopsies analyzed for mRNA expression of IL-17 and other inflammatory mediators | FoxP3, IL-17, IL-1β and RANKL significantly increased in PD; IL-17 and RANKL and IL-17 correlated with RORC2 (RORγt) | (40) |
| Healthy and PD | PD biopsies analyzed for mRNA expression of Th17- related cytokines | Upregulation of IL-21, IL- 1β, IL-6, IL-17, and IL- 23p19, but downregulation of IL-10 and TGF-β1 in PD. IL-21 correlated with CAL | (41) |
| Healthy and PD | Gingival biopsies examined for IL-17, IL-12, IL-23, and receptors | IL-17, IL-12 and IL-23 all increased in periodontal diseased tissue, with IL- 23R greater relative expression than IL-12 receptor β2 | (118) |
| Healthy and PD | Gingival tissue examined for Th17-related cytokine mRNA and protein expression by IHC | mRNA and protein levels of IL-17 and RORC2 (RORγt) elevated in PD | (3) |
| Healthy and GAgP | GCF examined for IL-17 by ELISA | IL-17 levels not significantly different between the groups | (7) |
| Gingivitis and PD | Gingival tissue was examined for FoxP3 and IL-17 by IHC and FC | PD had higher cell numbers with FoxP3+IL- 17-, FoxP3-IL-17+ and double positive FoxP3+IL- 17+ cells | (119) |
| Healthy and PD | Gingival tissues were analyzed for Th2 and Th17 cytokine mRNA | Higher gene expression of Th17 markers(RORC2 and IL-17) than Th2 markers (GATA-3, IL-4) in PD | (10) |
| Healthy, PD and GAgP | GCF examined for IL-17 levels by ELISA. Plaque analyzed by PCR for bacteria | GAgP had highest levels of IL-17; P. gingivalis presence correlated with significantly increased IL- 17 in GPD and GAgP | (136) |
| PD patients before and after periodontal therapy | GCF examined for Th1, Th2, and Th17 cytokines by ELISA | IL-17 and IL-21 decreased, IL-4 increased, and IFN-γ unaltered upon therapy | (163) |
| PD and healthy | Biopsies examined for immune cells and cytokines by IHC, IF, and FC | IL-23–expressing macrophages correlated with inflammation and IL- 17–expressing T cells | (5) |
| PD and gingivitis | Biospsies examined by IHC and for mRNA expression | Abundance of IL-17- expressing CD3+ T cells in PD; IL-17 mRNA elevated in PD relative to gingivitis | (113) |
It is currently uncertain whether the chronic nature of periodontitis represents a constant pathologic process or a persistent series of brief acute insults (bursts) (55). In the context of the burst model, it is tempting to speculate that IL-17–producing cells with inflammatory or regulatory functions (see above) might be involved in the mechanisms by which ‘inflammatory bursts’ could occur. In view of the plasticity by which Tregs can convert into IL-17-producing (Th17) cells, a recent study has identified IL-17+/Foxp3+ double-positive cells in human periodontal lesions, which is suggestive of an intermediate T cell-stage in this process (119). This conversion may be facilitated by the presence of IL-23 in the periodontal tissue, which was shown to restrain Treg development in favor of effector Th17 cells (125). Moreover, IL-23 can induce the clonal expansion of Th17 cells and stimulate their IL-17 production (157). In this regard, a recent study has shown that the number of IL-23–expressing macrophages correlated positively with both inflammation and the abundance of IL-17–expressing T cells, which was the predominant T cell subset in the lesions (5).
Conclusion and perspectives
Interleukin-17 plays a central role in innate immunity, inflammation, and osteoclastogenesis and links T cell activation to neutrophil mobilization and activation. Although it is likely that IL-17 exerts both protective and destructive effects in periodontitis, the burden of evidence from human and animal model studies suggests that the net effect of IL-17 signaling leads to disease. In the absence of definitive clinical evidence (i.e., anti-IL-17 intervention in human periodontitis), however, this notion remains a plausible but unproven hypothesis. Several IL-17 inhibitors (e.g., the anti-IL-17A monoclonal antibodies secukinumab and ixekizumab, and the anti-IL-17RA monoclonal antibody brodalumab) have been tested in clinical trials for other diseases and encouraging results have been obtained in rheumatoid arthritis, ankylosing spondylitis, and psoriasis, despite occasional adverse effects involving mostly fungal infections (8, 24, 51, 79, 87, 107). Since systemic treatment with IL-17 blockers is generally well tolerated, local treatment for local inflammatory diseases, such as periodontitis, should present increased safety. As such clinical trials have not been yet undertaken, it would be interesting to know the impact of on-going systemic therapies with IL-17 inhibitors on a relatively common disease such as periodontitis. Systemic anti-IL-17 intervention, as already performed for rheumatoid arthritis, ankylosing spondylitis, and psoriasis (8, 24, 51, 79, 87, 107), could potentially shed light on the true effects of IL-17 responses in human periodontitis.
Acknowledgments
We thank Debbie Maizels (Zoobotanica Scientific Illustration) for redrawing the figures in this paper. The authors’ research is supported by NIH/NIDCR grants; DE15254, DE17138, and DE21685 (GH).
Footnotes
The authors declare no financial/commercial conflicts of interests.
References
- 1.Abe T, Hosur KB, Hajishengallis E, Reis ES, Ricklin D, Lambris JD, Hajishengallis G. Local complement-targeted intervention in periodontitis: proof-of-concept using a C5a receptor (CD88) antagonist. J Immunol. 2012;189:5442–5448. doi: 10.4049/jimmunol.1202339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 3.Adibrad M, Deyhimi P, Ganjalikhani Hakemi M, Behfarnia P, Shahabuei M, Rafiee L. Signs of the presence of Th17 cells in chronic periodontal disease. J Periodontal Res. 2012;47:525–531. doi: 10.1111/j.1600-0765.2011.01464.x. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- 5.Allam JP, Duan Y, Heinemann F, Winter J, Gotz W, Deschner J, Wenghoefer M, Bieber T, Jepsen S, Novak N. IL-23-producing CD68(+) macrophage-like cells predominate within an IL-17-polarized infiltrate in chronic periodontitis lesions. J Clin Periodontol. 2011;38:879–886. doi: 10.1111/j.1600-051X.2011.01752.x. [DOI] [PubMed] [Google Scholar]
- 6.Anderson DC, Springer TA. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150, 95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- 7.Ay ZY, Yilmaz G, Ozdem M, Kocak H, Sutcu R, Uskun E, Tonguc MO, Kirzioglu FY. The gingival crevicular fluid levels of interleukin-11 and interleukin-17 in patients with aggressive periodontitis. J Periodontol. 2012;83:1425–1431. doi: 10.1902/jop.2012.110585. [DOI] [PubMed] [Google Scholar]
- 8.Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, van der Heijde D, McInnes I, van Laar JM, Landewe R, Wordsworth P, Wollenhaupt J, Kellner H, Paramarta J, Wei J, Brachat A, Bek S, Laurent D, Li Y, Wang YA, Bertolino AP, Gsteiger S, Wright AM, Hueber W. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382:1705–1713. doi: 10.1016/S0140-6736(13)61134-4. [DOI] [PubMed] [Google Scholar]
- 9.Barksby HE, Lea SR, Preshaw PM, Taylor JJ. The expanding family of interleukin-1 cytokines and their role in destructive inflammatory disorders. Clin Exp Immunol. 2007;149:217–225. doi: 10.1111/j.1365-2249.2007.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behfarnia P, Birang R, Pishva SS, Hakemi MG, Khorasani MM. Expression levels of th-2 and th-17 characteristic genes in healthy tissue versus periodontitis. J Dent (Tehran) 2013;10:23–31. [PMC free article] [PubMed] [Google Scholar]
- 11.Beklen A, Ainola M, Hukkanen M, Gurgan C, Sorsa T, Konttinen YT. MMPs, IL-1, and TNF are regulated by IL-17 in periodontitis. J Dent Res. 2007;86:347–351. doi: 10.1177/154405910708600409. [DOI] [PubMed] [Google Scholar]
- 12.Belibasakis GN, Bostanci N. The RANKL-OPG system in clinical periodontology. J Clin Periodontol. 2012;39:239–248. doi: 10.1111/j.1600-051X.2011.01810.x. [DOI] [PubMed] [Google Scholar]
- 13.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 14.Bosmann M, Haggadone MD, Zetoune FS, Sarma JV, Ward PA. The interaction between C5a and both C5aR and C5L2 receptors is required for production of G-CSF during acute inflammation. Eur J Immunol. 2013;43:1907–1913. doi: 10.1002/eji.201243075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosmann M, Sarma JV, Atefi G, Zetoune FS, Ward PA. Evidence for anti-inflammatory effects of C5a on the innate IL-17A/IL-23 axis. FASEB J. 2011;26:1640–1651. doi: 10.1096/fj.11-199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosshardt DD, Lang NP. The junctional epithelium: from health to disease. J Dent Res. 2005;84:9–20. doi: 10.1177/154405910508400102. [DOI] [PubMed] [Google Scholar]
- 17.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 18.Bulek K, Liu C, Swaidani S, Wang L, Page RC, Gulen MF, Herjan T, Abbadi A, Qian W, Sun D, Lauer M, Hascall V, Misra S, Chance MR, Aronica M, Hamilton T, Li X. The inducible kinase IKKi is required for IL-17-dependent signaling associated with neutrophilia and pulmonary inflammation. Nat Immunol. 2011;12:844–852. doi: 10.1038/ni.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardoso CR, Garlet GP, Crippa GE, Rosa AL, Junior WM, Rossi MA, Silva JS. Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol Immunol. 2009;24:1–6. doi: 10.1111/j.1399-302X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 20.Cevenini E, Caruso C, Candore G, Capri M, Nuzzo D, Duro G, Rizzo C, Colonna-Romano G, Lio D, Di Carlo D, Palmas MG, Scurti M, Pini E, Franceschi C, Vasto S. Age-related inflammation: the contribution of different organs, tissues and systems. How to face it for therapeutic approaches. Curr Pharm Des. 2010;16:609–618. doi: 10.2174/138161210790883840. [DOI] [PubMed] [Google Scholar]
- 21.Chabaud M, Lubberts E, Joosten L, van Den Berg W, Miossec P. IL-17 derived from juxta-articular bone and synovium contributes to joint degradation in rheumatoid arthritis. Arthritis Res. 2001;3:168–177. doi: 10.1186/ar294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chai L, Song YQ, Zee KY, Leung WK. Single nucleotide polymorphisms of complement component 5 and periodontitis. J Periodontal Res. 2010;45:301–308. doi: 10.1111/j.1600-0765.2009.01234.x. [DOI] [PubMed] [Google Scholar]
- 23.Chakravarti A, Raquil MA, Tessier P, Poubelle PE. Surface RANKL of Toll-like receptor 4-stimulated human neutrophils activates osteoclastic bone resorption. Blood. 2009;114:1633–1644. doi: 10.1182/blood-2008-09-178301. [DOI] [PubMed] [Google Scholar]
- 24.Chiricozzi A, Krueger JG. IL-17 targeted therapies for psoriasis. Expert Opin Investig Drugs. 2013;22:993–1005. doi: 10.1517/13543784.2013.806483. [DOI] [PubMed] [Google Scholar]
- 25.Choi EY, Chavakis E, Czabanka MA, Langer HF, Fraemohs L, Economopoulou M, Kundu RK, Orlandi A, Zheng YY, Prieto DA, Ballantyne CM, Constant SL, Aird WC, Papayannopoulou T, Gahmberg CG, Udey MC, Vajkoczy P, Quertermous T, Dimmeler S, Weber C, Chavakis T. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science. 2008;322:1101–1104. doi: 10.1126/science.1165218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Correa JD, Madeira MF, Resende RG, de Correia-Silva JF, Gomez RS, da de Souza DG, Teixeira MM, Queiroz-Junior CM, da Silva TA. Association between polymorphisms in interleukin-17A and -17F genes and chronic periodontal disease. Mediators Inflamm. 2012;2012:846052. doi: 10.1155/2012/846052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Courts FJ, Boackle RJ, Fudenberg HH, Silverman MS. Detection of functional complement components in gingival crevicular fluid from humans with periodontal diseases. J Dent Res. 1977;56:327–331. doi: 10.1177/00220345770560032001. [DOI] [PubMed] [Google Scholar]
- 29.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 30.Dababneh R, Al-Wahadneh AM, Hamadneh S, Khouri A, Bissada NF. Periodontal manifestation of leukocyte adhesion deficiency type I. J Periodontol. 2008;79:764–768. doi: 10.1902/jop.2008.070323. [DOI] [PubMed] [Google Scholar]
- 31.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 33.de Pablo P, Chapple IL, Buckley CD, Dietrich T. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 2009;5:218–224. doi: 10.1038/nrrheum.2009.28. [DOI] [PubMed] [Google Scholar]
- 34.Deas DE, Mackey SA, McDonnell HT. Systemic disease and periodontitis: manifestations of neutrophil dysfunction. Periodontol 2000. 2003;32:82–104. doi: 10.1046/j.0906-6713.2003.03207.x. [DOI] [PubMed] [Google Scholar]
- 35.Delima AJ, Van Dyke TE. Origin and function of the cellular components in gingival crevice fluid. Periodontol 2000. 2003;31:55–76. doi: 10.1034/j.1600-0757.2003.03105.x. [DOI] [PubMed] [Google Scholar]
- 36.Diamond G, Beckloff N, Ryan LK. Host defense peptides in the oral cavity and the lung: similarities and differences. J Dent Res. 2008;87:915–927. doi: 10.1177/154405910808701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 38.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, Fabien N, Cochat P, Pouteil-Noble C, Trolliet P, Durieu I, Tebib J, Kassai B, Ansieau S, Puisieux A, Eliaou JF, Bonnefoy-Berard N. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 39.Dragon S, Rahman MS, Yang J, Unruh H, Halayko AJ, Gounni AS. IL-17 enhances IL-1beta-mediated CXCL-8 release from human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1023–1029. doi: 10.1152/ajplung.00306.2006. [DOI] [PubMed] [Google Scholar]
- 40.Dutzan N, Gamonal J, Silva A, Sanz M, Vernal R. Over-expression of forkhead box P3 and its association with receptor activator of nuclear factor-kappa B ligand, interleukin (IL) -17, IL-10 and transforming growth factor-beta during the progression of chronic periodontitis. J Clin Periodontol. 2009;36:396–403. doi: 10.1111/j.1600-051X.2009.01390.x. [DOI] [PubMed] [Google Scholar]
- 41.Dutzan N, Vernal R, Vaque JP, Garcia-Sesnich J, Hernandez M, Abusleme L, Dezerega A, Gutkind JS, Gamonal J. Interleukin-21 expression and its association with proinflammatory cytokines in untreated chronic periodontitis patients. J Periodontol. 2012;83:948–954. doi: 10.1902/jop.2011.110482. [DOI] [PubMed] [Google Scholar]
- 42.Eskan MA, Jotwani R, Abe T, Chmelar J, Lim JH, Liang S, Ciero PA, Krauss JL, Li F, Rauner M, Hofbauer LC, Choi EY, Chung KJ, Hashim A, Curtis MA, Chavakis T, Hajishengallis G. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13:465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, O’Connor W, Jr, Rongvaux A, Van Rooijen N, Haberman AM, Iwakura Y, Kuchroo VK, Kolls JK, Bluestone JA, Herold KC, Flavell RA. Control of TH17 cells occurs in the small intestine. Nature. 2011;475:514–518. doi: 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forlow SB, Schurr JR, Kolls JK, Bagby GJ, Schwarzenberger PO, Ley K. Increased granulopoiesis through interleukin-17 and granulocyte colony-stimulating factor in leukocyte adhesion molecule-deficient mice. Blood. 2001;98:3309–3314. doi: 10.1182/blood.v98.12.3309. [DOI] [PubMed] [Google Scholar]
- 45.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaddis DE, Maynard CL, Weaver CT, Michalek SM, Katz J. Role of TLR2 dependent-IL-10 production in the inhibition of the initial IFN-γ T cell response to Porphyromonas gingivalis. J Leukoc Biol. 2012;93:21–31. doi: 10.1189/jlb.0512220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J Dent Res. 2008;87:817–828. doi: 10.1177/154405910808700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gemmell E, Yamazaki K, Seymour GJ. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontology 2000. 2007;43:14–40. doi: 10.1111/j.1600-0757.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 50.Gemmell E, Yamazaki K, Seymour GJ. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontol 2000. 2007;43:14–40. doi: 10.1111/j.1600-0757.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 51.Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Mazurov V, Aelion JA, Lee SH, Codding CE, Kellner H, Ikawa T, Hugot S, Mpofu S. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis. 2013;72:863–869. doi: 10.1136/annrheumdis-2012-201601. [DOI] [PubMed] [Google Scholar]
- 52.Gomez CR, Acuna-Castillo C, Perez C, Leiva-Salcedo E, Riquelme DM, Ordenes G, Oshima K, Aravena M, Perez VI, Nishimura S, Sabaj V, Walter R, Sierra F. Diminished acute phase response and increased hepatic inflammation of aged rats in response to intraperitoneal injection of lipopolysaccharide. J Gerontol A Biol Sci Med Sci. 2008;63:1299–1306. doi: 10.1093/gerona/63.12.1299. [DOI] [PubMed] [Google Scholar]
- 53.Gorr SU. Antimicrobial peptides of the oral cavity. Periodontol 2000. 2009;51:152–180. doi: 10.1111/j.1600-0757.2009.00310.x. [DOI] [PubMed] [Google Scholar]
- 54.Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. 2008;79:1585–1591. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- 55.Graves DT, Oates T, Garlet GP. Review of osteoimmunology and the host response in endodontic and periodontal lesions. J Oral Microbiol. 2011:3. doi: 10.3402/jom.v3403i3400.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grayson R, Douglas CW, Heath J, Rawlinson A, Evans GS. Activation of human matrix metalloproteinase 2 by gingival crevicular fluid and Porphyromonas gingivalis. J Clin Periodontol. 2003;30:542–550. doi: 10.1034/j.1600-051x.2003.00301.x. [DOI] [PubMed] [Google Scholar]
- 57.Griffin GK, Newton G, Tarrio ML, Bu DX, Maganto-Garcia E, Azcutia V, Alcaide P, Grabie N, Luscinskas FW, Croce KJ, Lichtman AH. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol. 2012;188:6287–6299. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gu C, Wu L, Li X. IL-17 family: Cytokines, receptors and signaling. Cytokine. 2013;64:477–485. doi: 10.1016/j.cyto.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guentsch A, Kramesberger M, Sroka A, Pfister W, Potempa J, Eick S. Comparison of gingival crevicular fluid sampling methods in patients with severe chronic periodontitis. J Periodontol. 2011;82:1051–1060. doi: 10.1902/jop.2011.100565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hajishengallis E, Hajishengallis G. Neutrophil homeostasis and periodontal health in children and adults. J Dent Res. 2013;93:231–237. doi: 10.1177/0022034513507956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hajishengallis G. Complement and periodontitis. Biochem Pharmacol. 2010;80:1992–2001. doi: 10.1016/j.bcp.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hajishengallis G. Aging and its impact on innate immunity and inflammation: Implications for periodontitis. J Oral Biosci. 2013;56:30–37. doi: 10.1016/j.job.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35:3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hajishengallis G, Chavakis T. Endogenous modulators of inflammatory cell recruitment. Trends Immunol. 2013;34:1–6. doi: 10.1016/j.it.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hajishengallis G, Lambris JD. Microbial manipulation of receptor crosstalk in innate immunity. Nat Rev Immunol. 2011;11:187–200. doi: 10.1038/nri2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hajishengallis G, Lamont RJ. Breaking bad: Manipulation of the host response by Porphyromonas gingivalis. Eur J Immunol. 2014;44:328–338. doi: 10.1002/eji.201344202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, McIntosh ML, Alsam A, Kirkwood KL, Lambris JD, Darveau RP, Curtis MA. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hanna S, Etzioni A. Leukocyte adhesion deficiencies. Ann N Y Acad Sci. 2012;1250:50–55. doi: 10.1111/j.1749-6632.2011.06389.x. [DOI] [PubMed] [Google Scholar]
- 69.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 70.Hart TC, Atkinson JC. Mendelian forms of periodontitis. Periodontol 2000. 2007;45:95–112. doi: 10.1111/j.1600-0757.2007.00233.x. [DOI] [PubMed] [Google Scholar]
- 71.Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J Immunol. 2007;179:4135–4141. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- 72.Hebel K, Rudolph M, Kosak B, Chang HD, Butzmann J, Brunner-Weinzierl MC. IL-1beta and TGF-beta act antagonistically in induction and differentially in propagation of human proinflammatory precursor CD4+ T cells. J Immunol. 2011;187:5627–5635. doi: 10.4049/jimmunol.1003998. [DOI] [PubMed] [Google Scholar]
- 73.Hernandez-Santos N, Gaffen SL. Th17 cells in immunity to Candida albicans. Cell Host Microbe. 2012;11:425–435. doi: 10.1016/j.chom.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, Sakaguchi N, Sakaguchi S. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ho AW, Gaffen SL. IL-17RC: a partner in IL-17 signaling and beyond. Semin Immunopathol. 2010;32:33–42. doi: 10.1007/s00281-009-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Honda T, Aoki Y, Takahashi N, Maekawa T, Nakajima T, Ito H, Tabeta K, Okui T, Kajita K, Domon H, Yamazaki K. Elevated expression of IL-17 and IL-12 genes in chronic inflammatory periodontal disease. Clin Chim Acta. 2008;395:137–141. doi: 10.1016/j.cca.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Honorati MC, Meliconi R, Pulsatelli L, Cane S, Frizziero L, Facchini A. High in vivo expression of interleukin-17 receptor in synovial endothelial cells and chondrocytes from arthritis patients. Rheumatology (Oxford) 2001;40:522–527. doi: 10.1093/rheumatology/40.5.522. [DOI] [PubMed] [Google Scholar]
- 78.Honorati MC, Neri S, Cattini L, Facchini A. Interleukin-17, a regulator of angiogenic factor release by synovial fibroblasts. Osteoarthritis Cartilage. 2006;14:345–352. doi: 10.1016/j.joca.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 79.Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, Wehkamp J, Feagan BG, Yao MD, Karczewski M, Karczewski J, Pezous N, Bek S, Bruin G, Mellgard B, Berger C, Londei M, Bertolino AP, Tougas G, Travis SP. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ito H, Honda T, Domon H, Oda T, Okui T, Amanuma R, Nakajima T, Yamazaki K. Gene expression analysis of the CD4+ T-cell clones derived from gingival tissues of periodontitis patients. Oral Microbiol Immunol. 2005;20:382–386. doi: 10.1111/j.1399-302X.2005.00241.x. [DOI] [PubMed] [Google Scholar]
- 81.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 82.Jauregui CE, Wang Q, Wright CJ, Takeuchi H, Uriarte SM, Lamont RJ. Suppression of T-cell chemokines by Porphyromonas gingivalis. Infect Immun. 2013;81:2288–2295. doi: 10.1128/IAI.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnson RB, Wood N, Serio FG. Interleukin-11 and IL-17 and the pathogenesis of periodontal disease. J Periodontol. 2004;75:37–43. doi: 10.1902/jop.2004.75.1.37. [DOI] [PubMed] [Google Scholar]
- 84.Jones SA, Sutton CE, Cua D, Mills KH. Therapeutic potential of targeting IL-17. Nat Immunol. 2012;13:1022–1025. doi: 10.1038/ni.2450. [DOI] [PubMed] [Google Scholar]
- 85.Kadkhodazadeh M, Baghani Z, Ebadian AR, Youssefi N, Mehdizadeh AR, Azimi N. IL-17 gene polymorphism is associated with chronic periodontitis and peri-implantitis in Iranian patients: a cross-sectional study. Immunol Invest. 2013;42:156–163. doi: 10.3109/08820139.2012.746697. [DOI] [PubMed] [Google Scholar]
- 86.Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, Miranda LA, Ernst CW, Izumi Y, Taubman MA. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006;169:987–998. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kellner H. Targeting interleukin-17 in patients with active rheumatoid arthritis: rationale and clinical potential. Ther Adv Musculoskelet Dis. 2013;5:141–152. doi: 10.1177/1759720X13485328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2:403–411. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 90.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 92.Kotake S, Yago T, Kawamoto M, Nanke Y. Role of osteoclasts and interleukin-17 in the pathogenesis of rheumatoid arthritis: crucial ‘human osteoclastology’. J Bone Miner Metab. 2012;30:125–135. doi: 10.1007/s00774-011-0321-5. [DOI] [PubMed] [Google Scholar]
- 93.Kwan WH, van der Touw W, Paz-Artal E, Li MO, Heeger PS. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med. 2013;210:257–268. doi: 10.1084/jem.20121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11:928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Laugisch O, Schacht M, Guentsch A, Kantyka T, Sroka A, Stennicke HR, Pfister W, Sculean A, Potempa J, Eick S. Periodontal pathogens affect the level of protease inhibitors in gingival crevicular fluid. Mol Oral Microbiol. 2012;27:45–56. doi: 10.1111/j.2041-1014.2011.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, Sobel RA, Regev A, Kuchroo VK. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lester SR, Bain JL, Johnson RB, Serio FG. Gingival concentrations of interleukin-23 and -17 at healthy sites and at sites of clinical attachment loss. J Periodontol. 2007;78:1545–1550. doi: 10.1902/jop.2007.060458. [DOI] [PubMed] [Google Scholar]
- 98.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD. IL-17 produced by neutrophils regulates IFN-γ–mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liang S, Krauss JL, Domon H, McIntosh ML, Hosur KB, Qu H, Li F, Tzekou A, Lambris JD, Hajishengallis G. The C5a receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J Immunol. 2011;186:869–877. doi: 10.4049/jimmunol.1003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liang SC, Nickerson-Nutter C, Pittman DD, Carrier Y, Goodwin DG, Shields KM, Lambert AJ, Schelling SH, Medley QG, Ma HL, Collins M, Dunussi-Joannopoulos K, Fouser LA. IL-22 induces an acute-phase response. J Immunol. 2010;185:5531–5538. doi: 10.4049/jimmunol.0904091. [DOI] [PubMed] [Google Scholar]
- 101.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu B, Wei L, Meyerle C, Tuo J, Sen HN, Li Z, Chakrabarty S, Agron E, Chan CC, Klein ML, Chew E, Ferris F, Nussenblatt RB. Complement component C5a promotes expression of IL-22 and IL-17 from human T cells and its implication in age-related macular degeneration. J Transl Med. 2011;9:1–12. doi: 10.1186/1479-5876-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lubberts E. IL-17/Th17 targeting: on the road to prevent chronic destructive arthritis? Cytokine. 2008;41:84–91. doi: 10.1016/j.cyto.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 104.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 105.Mei J, Liu Y, Dai N, Hoffmann C, Hudock KM, Zhang P, Guttentag SH, Kolls JK, Oliver PM, Bushman FD, Worthen GS. Cxcr2 and Cxcl5 regulate the IL-17/G-CSF axis and neutrophil homeostasis in mice. J Clin Invest. 2012;122:974–986. doi: 10.1172/JCI60588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mills KH, Dungan LS, Jones SA, Harris J. The role of inflammasome-derived IL-1 in driving IL-17 responses. J Leukoc Biol. 2013;93:489–497. doi: 10.1189/jlb.1012543. [DOI] [PubMed] [Google Scholar]
- 107.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 108.Mizutani N, Goshima H, Nabe T, Yoshino S. Complement C3a-induced IL-17 plays a critical role in an IgE-mediated late-phase asthmatic response and airway hyperresponsiveness via neutrophilic inflammation in mice. J Immunol. 2012;188:5694–5705. doi: 10.4049/jimmunol.1103176. [DOI] [PubMed] [Google Scholar]
- 109.Moon MR, Parikh AA, Pritts TA, Kane C, Fischer JE, Salzman AL, Hasselgren PO. Interleukin-1beta induces complement component C3 and IL-6 production at the basolateral and apical membranes in a human intestinal epithelial cell line. Shock. 2000;13:374–378. doi: 10.1097/00024382-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 110.Moran EM, Mullan R, McCormick J, Connolly M, Sullivan O, Fitzgerald O, Bresnihan B, Veale DJ, Fearon U. Human rheumatoid arthritis tissue production of IL-17A drives matrix and cartilage degradation: synergy with tumour necrosis factor-alpha, Oncostatin M and response to biologic therapies. Arthritis Res Ther. 2009;11:R113. doi: 10.1186/ar2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 112.Moutsopoulos N, Konkel J, Sarmadi M, Eskan MA, Wild T, Dutzan N, Abusleme L, Zenobia C, Hosur KB, Abe T, Uzel G, Chen W, Chavakis T, Holland SM, Hajishengallis G. Defective neutrophil recruitment in LAD Type I causes local IL-17–driven inflammatory bone loss. Sci Transl Med. 2014;6:229ra40. doi: 10.1126/scitranslmed.3007696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moutsopoulos NM, Kling HM, Angelov N, Jin W, Palmer RJ, Nares S, Osorio M, Wahl SM. Porphyromonas gingivalis promotes Th17 inducing pathways in chronic periodontitis. J Autoimmun. 2012;39:294–303. doi: 10.1016/j.jaut.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood. 2013;121:2402–2414. doi: 10.1182/blood-2012-09-378653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nussbaum G, Shapira L. How has neutrophil research improved our understanding of periodontal pathogenesis? J Clin Periodontol. 2011;38:49–59. doi: 10.1111/j.1600-051X.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- 116.O’Connor W, Jr, Zenewicz LA, Flavell RA. The dual nature of T(H)17 cells: shifting the focus to function. Nat Immunol. 2010;11:471–476. doi: 10.1038/ni.1882. [DOI] [PubMed] [Google Scholar]
- 117.Oda T, Yoshie H, Yamazaki K. Porphyromonas gingivalis antigen preferentially stimulates T cells to express IL-17 but not receptor activator of NF-kappaB ligand in vitro. Oral Microbiol Immunol. 2003;18:30–36. doi: 10.1034/j.1399-302x.2003.180105.x. [DOI] [PubMed] [Google Scholar]
- 118.Ohyama H, Kato-Kogoe N, Kuhara A, Nishimura F, Nakasho K, Yamanegi K, Yamada N, Hata M, Yamane J, Terada N. The involvement of IL-23 and the Th17 pathway in periodontitis. J Dent Res. 2009;88:633–638. doi: 10.1177/0022034509339889. [DOI] [PubMed] [Google Scholar]
- 119.Okui T, Aoki Y, Ito H, Honda T, Yamazaki K. The presence of IL-17+/FOXP3+ double-positive cells in periodontitis. J Dent Res. 2012;91:574–579. doi: 10.1177/0022034512446341. [DOI] [PubMed] [Google Scholar]
- 120.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Paintlia MK, Paintlia AS, Singh AK, Singh I. Synergistic activity of interleukin-17 and tumor necrosis factor-alpha enhances oxidative stress-mediated oligodendrocyte apoptosis. J Neurochem. 2011;116:508–521. doi: 10.1111/j.1471-4159.2010.07136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park YD, Kim YS, Jung YM, Lee SI, Lee YM, Bang JB, Kim EC. Porphyromonas gingivalis lipopolysaccharide regulates interleukin (IL)-17 and IL-23 expression via SIRT1 modulation in human periodontal ligament cells. Cytokine. 2012;60:284–293. doi: 10.1016/j.cyto.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 124.Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato F, Romagnani S, Cassatella MA. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115:335–343. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- 125.Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S, Prinz I, Hemmer B, Kuchroo VK, Oukka M, Korn T. γδ T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Potempa J, Pike RN. Corruption of innate immunity by bacterial proteases. J Innate Immun. 2009;1:70–87. doi: 10.1159/000181144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rabiet MJ, Huet E, Boulay F. The N-formyl peptide receptors and the anaphylatoxin C5a receptors: an overview. Biochimie. 2007;89:1089–1106. doi: 10.1016/j.biochi.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, Hackney J, Kim J, Zhou M, Lai J, Modrusan Z, Sai T, Lee W, Xu M, Caplazi P, Diehl L, de Voss J, Balazs M, Gonzalez L, Jr, Singh H, Ouyang W, Pappu R. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol. 2011;12:1159–1166. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- 129.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rong Z, Wang A, Li Z, Ren Y, Cheng L, Li Y, Wang Y, Ren F, Zhang X, Hu J, Chang Z. IL-17RD (Sef or IL-17RLM) interacts with IL-17 receptor and mediates IL-17 signaling. Cell Res. 2009;19:208–215. doi: 10.1038/cr.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150:5445–5456. [PubMed] [Google Scholar]
- 132.Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 133.Sadik CD, Kim ND, Iwakura Y, Luster AD. Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and FcgammaR signaling. Proc Natl Acad Sci U S A. 2012;109:E3177–3185. doi: 10.1073/pnas.1213797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schenkein HA. The role of complement in periodontal diseases. Crit Rev Oral Biol Med. 1991;2:65–81. doi: 10.1177/10454411910020010501. [DOI] [PubMed] [Google Scholar]
- 135.Schmidt S, Moser M, Sperandio M. The molecular basis of leukocyte recruitment and its deficiencies. Mol Immunol. 2012;55:49–58. doi: 10.1016/j.molimm.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 136.Shaker OG, Ghallab NA. IL-17 and IL-11 GCF levels in aggressive and chronic periodontitis patients: relation to PCR bacterial detection. Mediators Inflamm. 2012;2012:174764. doi: 10.1155/2012/174764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol. 2005;77:388–399. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- 138.Shin J, Hosur KB, Pyaram K, Jotwani R, Liang S, Chavakis T, Hajishengallis G. Expression and function of the homeostatic molecule Del-1 in endothelial cells and the periodontal tissue. Clin Dev Immunol. 2013;2013:Article ID 617809. doi: 10.1155/2013/617809617809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Silva TA, Garlet GP, Fukada SY, Silva JS, Cunha FQ. Chemokines in oral inflammatory diseases: apical periodontitis and periodontal disease. J Dent Res. 2007;86:306–319. doi: 10.1177/154405910708600403. [DOI] [PubMed] [Google Scholar]
- 140.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 141.Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-beta1 signaling and induction of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14:162–171. doi: 10.1038/ni.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun D, Novotny M, Bulek K, Liu C, Li X, Hamilton T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF) Nat Immunol. 2011;12:853–860. doi: 10.1038/ni.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sutton CE, Mielke LA, Mills KH. IL-17-producing gammadelta T cells and innate lymphoid cells. Eur J Immunol. 2012;42:2221–2231. doi: 10.1002/eji.201242569. [DOI] [PubMed] [Google Scholar]
- 145.Takahashi K, Azuma T, Motohira H, Kinane DF, Kitetsu S. The potential role of interleukin-17 in the immunopathology of periodontal disease. J Clin Periodontol. 2005;32:369–374. doi: 10.1111/j.1600-051X.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 146.Tan Z, Jiang R, Wang X, Wang Y, Lu L, Liu Q, Zheng SG, Sun B, Ryffel B. RORγt+IL-17+ neutrophils play a critical role in hepatic ischemia-reperfusion injury. J Mol Cell Biol. 2013;5:143–146. doi: 10.1093/jmcb/mjs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Taylor PR, Roy S, Leal SM, Jr, Sun Y, Howell SJ, Cobb BA, Li X, Pearlman E. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat Immunol. 2013 doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thornton RD, Lane P, Borghaei RC, Pease EA, Caro J, Mochan E. Interleukin 1 induces hypoxia-inducible factor 1 in human gingival and synovial fibroblasts. Biochem J. 2000;350(Pt 1):307–312. [PMC free article] [PubMed] [Google Scholar]
- 149.Turner M, Chantry D, Katsikis P, Berger A, Brennan FM, Feldmann M. Induction of the interleukin 1 receptor antagonist protein by transforming growth factor-beta. Eur J Immunol. 1991;21:1635–1639. doi: 10.1002/eji.1830210708. [DOI] [PubMed] [Google Scholar]
- 150.Van bezooijen RL, Farih-Sips HC, Papapoulos SE, Lowik CW. Interleukin-17: A new bone acting cytokine in vitro. J Bone Miner Res. 1999;14:1513–1521. doi: 10.1359/jbmr.1999.14.9.1513. [DOI] [PubMed] [Google Scholar]
- 151.Vanaudenaerde BM, Verleden SE, Vos R, De Vleeschauwer SI, Willems-Widyastuti A, Geenens R, Van Raemdonck DE, Dupont LJ, Verbeken EK, Meyts I. Innate and adaptive interleukin-17-producing lymphocytes in chronic inflammatory lung disorders. Am J Respir Crit Care Med. 2011;183:977–986. doi: 10.1164/rccm.201007-1196PP. [DOI] [PubMed] [Google Scholar]
- 152.Vernal R, Dutzan N, Chaparro A, Puente J, Antonieta Valenzuela M, Gamonal J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J Clin Periodontol. 2005;32:383–389. doi: 10.1111/j.1600-051X.2005.00684.x. [DOI] [PubMed] [Google Scholar]
- 153.von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J Immunol. 2008;181:5183–5188. doi: 10.4049/jimmunol.181.8.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Voo KS, Wang YH, Santori FR, Boggiano C, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, Zheng B, Littman DR, Liu YJ. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wahl SM, Hunt DA, Wong HL, Dougherty S, McCartney-Francis N, Wahl LM, Ellingsworth L, Schmidt JA, Hall G, Roberts AB, et al. Transforming growth factor-beta is a potent immunosuppressive agent that inhibits IL-1-dependent lymphocyte proliferation. J Immunol. 1988;140:3026–3032. [PubMed] [Google Scholar]
- 156.Wang M, Krauss JL, Domon H, Hosur KB, Liang S, Magotti P, Triantafilou M, Triantafilou K, Lambris JD, Hajishengallis G. Microbial hijacking of complement-toll-like receptor crosstalk. Sci Signal. 2010;3:ra11. doi: 10.1126/scisignal.2000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol. 2013;8:477–512. doi: 10.1146/annurev-pathol-011110-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 159.Xu R, Wang R, Han G, Wang J, Chen G, Wang L, Li X, Guo R, Shen B, Li Y. Complement C5a regulates IL-17 by affecting the crosstalk between DC and gammadelta T cells in CLP-induced sepsis. Eur J Immunol. 2010;40:1079–1088. doi: 10.1002/eji.200940015. [DOI] [PubMed] [Google Scholar]
- 160.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 161.Yu JJ, Ruddy MJ, Wong GC, Sfintescu C, Baker PJ, Smith JB, Evans RT, Gaffen SL. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood. 2007;109:3794–3802. doi: 10.1182/blood-2005-09-010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zenobia C, Luo XL, Hashim A, Abe T, Jin L, Chang Y, Jin ZC, Sun JX, Hajishengallis G, Curtis MA, Darveau RP. Commensal bacteria-dependent select expression of CXCL2 contributes to periodontal tissue homeostasis. Cell Microbiol. 2013;15:1419–1426. doi: 10.1111/cmi.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao L, Zhou Y, Xu Y, Sun Y, Li L, Chen W. Effect of non-surgical periodontal therapy on the levels of Th17/Th1/Th2 cytokines and their transcription factors in Chinese chronic periodontitis patients. J Clin Periodontol. 2011;38:509–516. doi: 10.1111/j.1600-051X.2011.01712.x. [DOI] [PubMed] [Google Scholar]
- 164.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]