Abstract
Loss of bone and muscle with advancing age represent a huge threat to loss of independence in later life. Osteoporosis represents a major public health problem through its association with fragility fractures, primarily of the hip, spine and distal forearm. Sarcopenia, the age related loss of muscle mass and function, may add to fracture risk by increasing falls risk. In the context of muscle aging, it is important to remember that it is not just a decline in muscle mass which contributes to the deterioration of muscle function. Other factors underpinning muscle quality come into play, including muscle composition, aerobic capacity and metabolism, fatty infiltration, insulin resistance, fibrosis and neural activation. Genetic, developmental, endocrine and lifestyle factors, such as physical activity, smoking and poor diet have dual effects on both muscle and bone mass in later life and these will be reviewed here. These include poor nutrition, lack of physical activity and cigarette smoking, comorbidities or medication use. Recent work has highlighted a possible role for the early environment. Inflammaging is an exciting emerging research field that is likely to prove relevant to future work, including interventions designed to retard to reverse bone and muscle loss with age.
Keywords: bone, muscle, aging, determinants
Introduction
Aging is a process that affects both physical abilities and appearance. Loss of bone and muscle with advancing age represent a huge threat to loss of independence in later life, but definition and outcomes in sarcopenia research have until recently lagged behind research in osteoporosis (1), with a particular conundrum being how best to define sarcopenia (2). Osteoporosis represents a major public health problem through its association with fragility fractures, primarily of the hip, spine and distal forearm (3). Sarcopenia, the age related loss of muscle mass and function, may add to fracture risk by increasing falls risk. In addition, the mechanostat hypothesis suggests that bones adapt to mechanical loads generated by voluntary mechanical usage supporting a direct relationship between muscle and bone health (4). In the context of muscle aging, it is important to remember that it is not just a decline in muscle mass which contributes to the deterioration on muscle function. Other factors underpinning muscle quality come into play, including muscle composition, aerobic capacity and metabolism, fatty infiltration, insulin resistance, fibrosis and neural activation. An understanding of these factors may help us to identify those at risk of sarcopenia at an earlier stage in their lives. Genetic, developmental, endocrine and lifestyle factors, such as physical activity, smoking and poor diet have dual effects on both muscle and bone mass in later life and these will be reviewed here, but are summarised in table 1 for ease.
Table 1. Risk factors for muscle and bone aging.
Key: Risk factor for both muscle and bone aging
Risk factor for muscle aging only
Risk factor bone aging only
| Constitutional | Lifestyle |
|---|---|
| Female gender | Low body weight |
| Age | Cigarette smoking |
| Asian or Caucasian race | Excessive alcohol consumption |
| Sex hormone deficiency | Prolonged immobilisation |
| Early environment | Low dietary calcium intake |
| Low protein intake | |
| Co-morbidity | Vitamin D deficiency |
| Genetic Factors | Use of ACE inhibitors |
| Previous fragility fracture | Use of steroids |
| Family history of fragility fracture | Low growth hormone level |
Current approaches to the definition of sarcopenia utilise measurements of muscle mass, muscle strength, and functional capacity. The extent to which the disorder can be characterised on the basis of any one of these variables measured alone, is the source of considerable debate. In recent consensus statements from the International Osteoporosis Foundation and European Society for the Clinical and Economic aspects of Osteoarthritis and Osteoporosis (1,2) the methodology available for assessment of each of these three critical components using dual energy x-ray absorptiometry, conventional isometric dynamometry, and routinely available functional measures such as gait speed, have been outlined. The European and International study group approaches to this definition are itemised in table 2.
Table 2. Diagnostic criteria for sarcopenia: suggested approaches*(reproduced with permission from [2]).
| Study group | Definition | Criteria |
|---|---|---|
| ESPEN Special Interest Groups | “Sarcopenia is a condition characterized by loss of muscle mass and muscle strength. Although sarcopenia is primarily a disease of the elderly, its development may be associated with other conditions that are not exclusively seen in older persons, like disuse, malnutrition and cachexia. Like osteopenia, it can be also be seen in those with inflammatory diseases.” |
|
| European Working Group on Sarcopenia in Older People | “Sarcopenia is a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength with a risk of adverse outcomes such as physical disability, poor quality of life and death.” The condition is called primary sarcopenia when the cause is aging per se, and secondary sarcopenia when disease, inactivity, or malnutrition contribute |
Reference population of healthy young subjects using cutoff points <2 SDs below mean. Criterion 1 and Criterion 2 or 3. |
| International Working Group on Sarcopenia | “Sarcopenia is defined as the age-associated loss of skeletal muscle mass and function. The causes of sarcopenia are multifactorial and can include disuse, altered endocrine function, chronic disease, inflammation, insulin resistance, and nutritional deficiencies. While cachexia may be a component of sarcopenia, the two conditions are not the same.” |
|
| Society of Sarcopenia, Cachexia and Wasting Disorders | “Sarcopenia with limited mobility is a specific condition with clear loss of muscle mass and a clear target for intervention. As such it differs from the more general concept of frailty.” “The limitation in mobility should not be clearly attributable to the direct effect of specific disease, such as peripheral vascular disease with intermittent claudication, or central and peripheral nervous system disorders (such as stroke, Parkinson’s disease, spinal cord disease, or motor neuron disease), dementia, or cachexia.” |
|
Other study groups, such as the Biomarkers Consortium, have convened for the same purpose of developing a consensus statement but have not yet published their findings
Fractures arise through an interaction between bone fragility and trauma (usually falls). There is a clear relationship between skeletal muscle and bone mass throughout the lifecourse. For example, the Sarcopenia and Hip Fracture Study reported that 75% of participants with hip fracture were also sarcopenic. Over one year follow-up, 56% fell at least once, 28% had recurrent falls and 12% sustained a new fracture; 5% of which were hip fractures (5). Furthermore, the Hertfordshire Cohort Study reported an inverse relationship between grip strength and falls within the last year, and Joint American and British Geriatric Society guidelines for the prevention of falls in older people describe muscle weakness as the single biggest intrinsic risk factor for falling with an attributed relative risk of 4.4 (6-8).
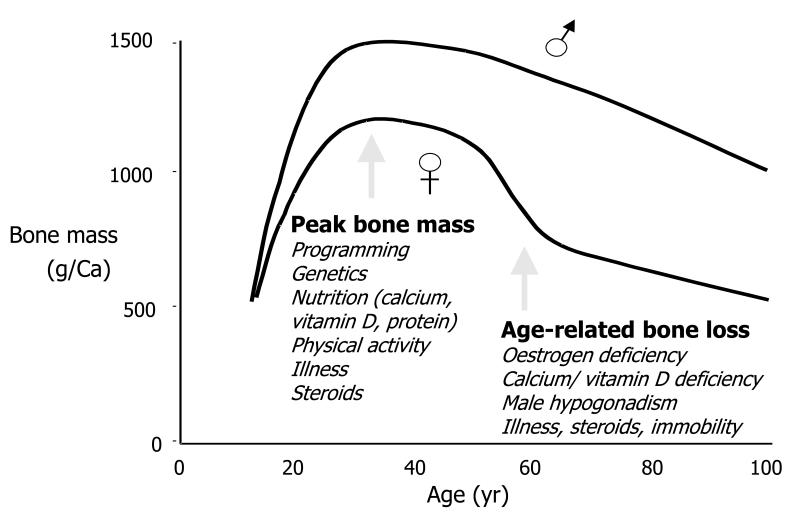
Both peak bone mass and muscle mass and strength peak in early adulthood and subsequently declines with age from approximately the fifth decade. In individuals over the age of 50 years, muscle mass is lost at a rate of 1-2% per year and strength at a rate of 1.5-3% per year (9); in women there is an accelerated period of bone loss perimenopausally superimposed upon bone loss rates of approximately 1-2% annually (10). Determinants of both bone and muscle aging can be considered using a lifecourse approach; these include early life influences that determine maximum mass and strength, in addition to mid- and later life influences that affect rate of decline (Figure 1).
Figure 1.
Change in bone density values over lifetime
Factors that influence later bone or muscle health may exert their effect through peak mass or strength, or rate of loss, or a combination of the two. Genetic factors are thought to be very important in the attainment of peak bone mass: family studies have examined the parent-offspring and sibling-sibling correlations in BMD, giving correlation coefficients of 0.28-59, and twin studies have shown much closer concordance of bone density in monozygotic than dizygotic twins (11, 12). However, environmental factors such as hormonal status, physical activity and calcium intake are also important. Environmental influences are thought to be more important than genetic factors in the determination of bone loss, which begins at about the age of 35 in both sexes, but includes an accelerated phase in the immediate postmenopausal years in women. Likewise, genetic factors are major contributors to muscle strength, and most likely sarcopenia also has a significant heritable component. While few studies have considered individual candidate genes, there has been interest in the myostatin pathway; polymorphisms in the vitamin D receptor gene have been associated with both sarcopenia and low bone mineral density (13).
Age & sex
While most American women under the age of fifty have normal BMD, by the age of 80 years, 27% are osteopenic and 70% are osteoporotic at the hip, lumbar spine or forearm. Sarcopenia is also common with prevalence estimates ranging, according to definitions, from 9% to 18% over the age of 65, rising to 30% in men over 80 and even higher in hospitalised patients (14, 15). As stated above, the definition of sarcopenia has been problematic in recent years; an algorithm to facilitate the diagnosis of sarcopenia in older people has now been developed and has recently been used to demonstrate a UK prevalence of 4.6% and 7.9%, among men and women respectively (mean age 67 years) of the Hertfordshire Cohort Study (16).
Epidemiological studies from North America have estimated the remaining lifetime risk of common fragility fractures to be 17.5% for hip fracture, 15.6% for clinically diagnosed vertebral fracture and 16% for distal forearm fracture among white women aged 50 years. Corresponding risks among men are 6%, 5% and 2.5% (3). This increased frequency of fracture with age in both sexes reflects a combination of lower bone density with an increased tendency to fall in the elderly. While the public health problem associated with low bone mass is fragility fracture, as above, a phenomenon that represents an interplay between bone and muscle health (falls risk), the outcome associated with a diagnosis of sarcopenia can be harder to quantify, though there is a clear relationship with loss of independence.
Race
Hip fracture incidence rates vary considerably according to geographic area and race, and may vary widely within the same country. Within Europe epidemiological studies report rates that vary up to seven-fold from country to country. Age- and sex-adjusted hip fracture rates are generally higher in whites than in black or Asian populations (17). The highest recorded rates of hip fracture, after age-adjustment, come from Sweden and the northern US, intermediate rates are found in Asian populations and African peoples have the lowest rates. Men and women of African origin have similar hip fracture rates (18). Some inter-racial differences may be explained by variations in reversible lifestyle factors such as low milk consumption, cigarette smoking, lack of sunlight exposure, low BMI and physical activity (19), but genetic factors may also be important.
The association between other fragility fractures and falls is more variable; for example vertebral fractures may be spontaneous, and related to heavy lifting or bending rather than a fall. In instances where comparable methods and definitions have been used in studies, the prevalence of vertebral fractures has been more similar across regions than seen for hip fractures (20). For example, vertebral fracture prevalence among women in Hiroshima was 20-80% greater than for white women in Rochester, Minnesota, USA despite lower hip fracture rates in the former (21). Similarly, the risk of vertebral fractures among postmenopausal women in Beijing is only 25% lower than that among women in Rochester, Minnesota, despite much lower hip fracture rates in the former (22).
By contrast, distal forearm fractures typically follow a fall onto an outstretched hand, especially in perimenopausal women (3). Although geographic variation in distal forearm fracture rate exists, a partial explanation may be methodological considerations of case ascertainment as less than 20% of forearm fracture patients are hospitalised, and this figure varies dramatically worldwide.
As discussed above, in addition to a fracture outcome, a loss of muscle mass has been shown in many studies to lead to frailty, and loss of independence. In general, it has been suggested that loss of 30% of reserve capacity limits normal function, whereas loss of 70% results in system failure (23). Hence a higher muscle mass found in some races will be protective against enough muscle loss to result in frailty, and loss of independence, assuming similar rates of muscle loss in all groups. Differences in body composition are well described, with higher muscle mass described in Black populations (24).
Body mass index
Low body mass index (BMI) is a well-documented risk factor for low bone density and future fracture. The risk is most marked for lean individuals with a BMI of <20kg/m2. Above 20 kg/m2 incremental increases in weight have little protective effect; leanness appears to be a risk factor rather than obesity protective. The association of fracture risk with leanness is largely dependent on BMD. For hip fracture, a modest risk persists after adjustment for BMD (25). Several mechanisms have been proposed to explain the protective effect of obesity on bone mass. They include mechanical factors with increased strain on the skeleton and hormonal factors, mainly relating to increased peripheral oestrogen production. After menopause, most of the circulating oestrogens are the result of peripheral conversion of androgens to oestrogens by fat tissue.
With advancing age, body composition changes such that body fat increases and muscle mass decreases, often with relative overall stability in body weight, leading to the term sarcopenic obesity. Although obese or overweight adults often have a higher muscle mass compared to their non-obese peers, their lean mass is low compared to their total weight (26). Obesity is linked to inflammation which may play an important role in the process leading to sarcopenia – a process which will be discussed further in this review. Longitudinal studies have reported that pronounced weight loss is associated with accelerated grip strength decline, possibly reflecting confounding comorbidity (27).
Physical activity
Bone density increases in response to physical loading and mechanical stress; as compressive forces increase, bone mass and bone density increase in response to the increased loading. Muscle contraction and gravity are the two primary mechanical forces applied to bone. A lack of adequate mechanical stimuli results in bone loss, mediated primarily by a proportionately greater increase in bone resorption without an increase in bone formation.
Prolonged immobilisation is a risk factor for bone loss and future fracture. Conversely, physical activity prevents bone loss; a recent meta-analysis (28) found a significant protective effect of physical activity on BMD at the lumbar spine, but effects were not demonstrated at the forearm or femoral neck. The positive effects of mechanical loading on bone mass can be seen in weight lifters and other athletes; this increase may be restricted to the loaded side e.g. tennis player’s arms. Conversely, immobilisation can be associated with rapid bone loss, and if sustained, as in patients with paraplegia or hemiplegia fractures can occur. At a cellular level, the osteocytes which lie embedded within individual lacunae to mineralised bone are believed to be the cellular system that responds to mechanical deformation.
Similarly, physical activity is known to have a profound effect on muscle mass and strength. Inactivity has been shown to lead to loss of muscle mass and strength at any age and bed rest studies have shown that a decrease in muscle strength occurs before a decrease in muscle mass (29). Lifelong physical exercise has been shown to preserve muscle structure and function well trained elderly men such that it is comparable to active men four decades younger (30). Increases in mid-life leisure time physical activity has been shown to reduce the risk of mobility impairment in old age, though interestingly occupational physical activity in mid-life may have a detrimental effect on mobility in old age (31).
A recent systematic review on interventions for sarcopenia investigated the effect of exercise on muscle mass and muscle strength or power. Some studies also assessed physical performance through chair rising, 12 minute walk test, stair climbing or timed up and go. Resistance training was shown to improve muscle mass and strength in two studies (32,33) and muscle strength alone in a further study (35) compared with control (low-intensity home exercise or standard rehabilitation). Resistance training (versus control) also led to improvements in physical performance (chair rise, stair climb or 12 minute walk) in these studies (34-37).
Three other studies assessed the impact of compound exercise interventions (comprising a blend of aerobic, resistance, flexibility and / or balance training) on muscle. One high intensity compound exercise programme over an 18 month period improved muscle mass, strength and physical performance versus control in a study of 246 women (38). The other compound exercise studies produced mixed outcomes however. In another mixed gender study there was no beneficial effect of a compound exercise programme on muscle mass or strength (37).
Overall, most trials of exercise in the elderly showed improved muscle strength and physical performance, but not all found increased muscle mass, suggesting that muscle loss may occur with aging regardless of physical activity level. However, comparison of these studies is difficult as the subjects were identified as frail by different measures and the exercise interventions were not easily comparable.
Cigarette smoking
An inverse relationship between smoking and bone density is well established, and is due to multiple factors including an earlier menopause, reduced body weight and enhanced metabolic breakdown of exogenous oestrogens. In a recent meta-analysis, the results of 48 published studies were combined (38). The authors concluded that although they were able to demonstrate no significant difference in bone density at age 50 between smokers and non-smokers, bone density in women diminished by about an extra 2% for every 10 year increase in age, with a difference of 6% at age 80. Although the confounding effects of body mass index and oestrogen were mentioned, this meta-analysis could not fully address the lifestyle differences between smokers and non-smokers.
Cigarette smoking was recently the subject of a further recent meta-analysis (39) that found current smoking was associated with a significantly increased risk of any fracture compared to non-smokers. Consistent with previous research, adjustment for BMD had little impact on the increased risk. Risk ratios were significantly higher in men than women for all fractures and for osteoporotic fractures, but not for hip fracture. Low BMD accounted for only 23% of the smoking-related risk of hip fracture, while adjustment for BMI had a small downward effect on risk for all fracture outcomes. A smoking history was associated with a significantly increased risk of fracture compared with individuals without a smoking history, but the risk was lower than for current smokers.
Fewer studies have considered the relationship between muscle mass and smoking. In recent data from the Minos study (27) current smokers had lower appendicular muscle mass than non-smokers, and a dose-effect relationship was apparent. By contrast some authors have reported no association (40). Cigarette smoking is often associated with a low BMI, and low levels of physical activity and this may largely underlie the associations described.
Alcohol consumption
High alcohol intake is known to have a detrimental effect on skeletal health, possibly through adverse effects on protein and calcium metabolism, mobility, gonadal function and a direct toxic effect on osteoblasts. High alcohol consumption may also predispose to falls, a risk factor for fracture. A recent meta-analysis (41) suggested that there was no significant increase in fracture risk at intakes of 2 units or less daily, but above this there was an increased risk of any fracture. There was no significant interaction with age or BMD. There was no evidence of a different threshold for effect by gender. While moderate alcohol intake was not associated with muscle mass in one of the few longitudinal studies of muscle loss, the Minos study (42), heavy alcohol consumption is likely to lead to low muscle mass through associated effects on poor nutrition, low levels of physical activity and hormonal abnormalities.
Diet
Diet has a significant effect on bone and muscle health in later life. Dietary calcium intake during growth may play a role in the development and maintenance of peak BMD. It is likely that other environmental and lifestyle factors, particularly exercise, may modulate this effect. Calcium supplementation in growing children produces small increases in BMD that tend not to be maintained. Despite studies showing the benefit of pharmacological supplementation of calcium in the maintenance of skeletal health, the observational evidence relating to dietary calcium and fracture risk suggests there is very little relationship between dietary calcium intake and the risk of osteoporotic fracture (43), with the exception of very low calcium intakes. Vitamin D is necessary for optimal absorption of calcium from the diet. In many countries vitamin D is added to food-stuffs; otherwise adequate skin exposure to ultraviolet light is necessary to maintain vitamin D levels from endogenous synthesis.
There is also some evidence to suggest that dietary protein intake may be important in determining bone mass and fracture risk (44). It has also been suggested that protein derived from vegetable sources may be more beneficial for the skeleton than animal protein (45); this might reflect the different effect of the protein source upon the acid-base balance but this has not yet been demonstrated in prospective studies.
There is little evidence that micronutrients such as zinc, copper and boron have major effects on bone health. Some diets, especially those rich in soy protein, can provide significant amounts of estrogens. A recent study (46) has also suggested that a high soy consumption may be associated with a lower risk of fracture among women from the Shanghai Women’s Health Study. Excessive salt and caffeine may have adverse effects on bone, possibly by increasing urinary calcium excretion directly and hence contribute to a negative calcium balance.
Likewise, nutrition plays a major role in the pathogenesis of muscle loss. The rate of muscle protein synthesis is reported to be reduced by 30% in the elderly, however there is some controversy as to whether this is attributable to poorer nutrition, disease or reduced physical activity rather than aging itself (47-48). In fasting elderly subjects, muscle protein synthesis is also decreased, particularly in specific muscle fractions such as mitochondrial proteins (49). Reduced protein intake, as in the anorexia of aging, is likely to be associated with muscle loss. It has been suggested that elderly people have an increased risk of impaired energy regulation, and that resting metabolic rate increases less in older subjects compared to younger adults during overfeeding, and decreases less during underfeeding, suggesting some age-related disconnection between changes in energy intake and resting metabolic rate (50). Muscle protein synthesis is directly stimulated by amino acid and essential amino acid intake (51)
An insufficient protein intake appears to influence muscle loss primarily by a reduction of synthesis rather than increased degradation of muscle protein. The International Osteoporosis Foundation recently reviewed the impact of protein intake on muscle mass, strength and performance (52), signposting studies that showed that protein intake is positively associated with preservation of lean bone mass in men and women aged 70-79 years. Typically supplementation studies have combined protein supplementation with resistance training, although the results have been mixed (53). Factors that may have contributed to this heterogeneity include participants’ age range; initial degree of sarcopenia; comorbidity burden; supplementation and exercise regimes.
Other dietary constituents and their effect on muscle health were similarly reviewed by Mithal et al (54). Chronic ingestion of acid-producing diets, as is commonly seen in older adults in combination with a low intake of alkalizing fruit and vegetables appears to have a negative impact on both bone and muscle. Decreases in vitamin B12 and folic acid may also impact muscle function through an effect on homocysteine. This is of interest since high homocysteine levels are also associated with fracture in large prospective cohort studies, an association that was independent of bone mineral density in the LASA and Rotterdam studies.
An ESCEO consensus statement has recently been published that recommends an optimal dietary protein intake of 1.0 to 1.2g/ kg body weight/day with at least 20-25g of high-quality protein at each main meal in post-menopausal women for prevention of age-related deterioration of musculoskeletal health (52). This recommendation is made alongside a recommendation for a calcium intake of 1000mg/day, regular physical exercise and recommendations regarding vitamin D intake discussed below.
Hormone deficiency
Hormonal deficiency is associated with bone muscle and bone loss, and leads to an earlier accelerated rate of loss in women compared to men. Oestrogen receptors are present in human muscle and bone cells, and Hormone Replacement Therapy in estrogen deficient women leads to protection of bone and muscle mass. A premature menopause, unopposed by exogenous oestrogen therapy, is a risk factor for fragility fracture, as is primary or secondary amenorrhoea in younger women. The pathogenesis of male osteoporosis is less well understood, but it is thought that estrogens derived by metabolism from androgens play an important role in protecting against bone loss. Testosterone levels gradually decrease in older men at a rate of 1% per year, and age related increases in sex hormone binding globulin levels result in reduced levels of free or bioavailable testosterone. Studies suggest that low testosterone predicts sarcopenia, with low testosterone resulting in lower protein synthesis and a loss of muscle mass (55).
Insulin-like Growth Factor −1 and Growth Hormone also both decline with age, and are potential contributors to muscle and bone loss (56). Likewise 25(OH) D levels decline with age and cross sectional studies have demonstrated an association between low 25(OH)D and low muscle mass and strength, poorer balance and an increased risk of falls (57). These data complement numerous studies that have also highlighted the importance of adequate vitamin D levels with bone (58). Low levels of vitamin D are in themselves associated with a raised PTH level, however high PTH has also been associated with sarcopenia and risk of falling independent of 25(OH)D status (59,60). PTH is purported to modulate muscle function through an increased intracellular calcium concentration or through an induced pro-inflammatory pathway (61).
In their review of dietary supplementation (52), the International Osteoporosis Foundation reviewed the evidence that vitamin D levels are important for muscle health. They concluded that vitamin D supplementation appears to increase number and size of type II muscle fibres in elderly women, and that this effect takes place via binding of vitamin D to specific vitamin D receptors in the muscle, although this remains contentious. For this reason an ESCEO consensus statement recommends an adequate vitamin D intake of 800IU/daily to maintain serum 25(OH)D levels >50 nmol/l in post-menopausal women for prevention of age-related deterioration of musculoskeletal health.
Glucocorticoid therapy
Glucocorticoids are an important cause of osteoporosis and fractures. Bone loss is believed to be most rapid in the first few months of therapy, and affects both axial and appendicular sites, although bone loss is most marked at the spine where cancellous bone predominates. The fracture risk conferred by the use of corticosteroids is not solely mediated through its effect on BMD; in one meta-analysis, the relative risk of hip fracture was increased 4.4 to 2.5 fold with higher risks at younger ages (62). This increased risk was little altered after adjustment for BMD and was independent of prior fracture (63). Similarly, muscle weakness is often observed in clinical practice in patients receiving oral glucocorticoids, although muscle loss in this group has not been well studied.
Co-pathology
Several medical conditions are associated with increased susceptibility to osteoporosis, sarcopenia and fragility fracture. These include rheumatological conditions such as rheumatoid arthritis and ankylosing spondylitis where risk factors such as immobility, low body mass index and corticosteroid medication all play a part, endocrine disorders such as hyperparathyroidism and hyperthyroidism (associated with bone loss), and malabsorption syndromes such as coeliac disease.
Many chronic medical conditions such as COPD, heart failure and cancer are associated with loss of body weight, including lean mass. This can occur in people of all ages and is termed cachexia, though this is more prevalent in the elderly. These conditions lead to increased production of pro-inflammatory cytokines and an acute state of hyper-catabolism. However, production of these pro-inflammatory cytokines, particularly IL-6, IL-1 and TNF, is also increased as part of normal aging and is increased across a spectrum of age-associated diseases (64, 65).
Inflammaging: a possible pathway to muscle and bone loss?
In 2000, Franceschi et al described this phenomenon of ‘inflammaging’ as part of the spectrum of immunosenescence, leading to muscle and potentially bone loss (66). Inflammaging is believed to be a consequence of a cumulative lifetime exposure to antigenic load caused by both clinical and sub-clinical infections as well as from exposure to non-infective antigens (67). The consequence is an inflammatory response, tissue damage and the production of reactive oxygen species (ROS) which result in the release of additional cytokines, primarily from cells of the innate immune system, but also from the acquired immune response (68). The result is a vicious cycle driving immune system remodelling and favouring a chronic pro-inflammatory state.
The Longitudinal Aging Study of Amsterdam showed that high levels of cytokine IL-6 and CRP were associated with increased risk of loss of muscle strength (69). Chronically elevated inflammatory cytokines has been shown to lead to a predisposition to sarcopenia, perhaps through increased activation of the ubiquitine-protease pathway (70, 71). The ubiquitine-proteasome system degrades myofibrillar proteins, but the precise role of these cytokines in sarcopenia is currently a focus of research.
Obesity is also linked to inflammation and this may be the underlying process by which sarcopenic obesity occurs. It has been suggested that this low level chronic inflammatory state leads to accelerated muscle loss through switching on of catabolic rather than anabolic signals. Excess fatty acids in the muscle fibres has also been shown to interfere with normal cellular signalling (72).
Early environment
Epidemiological studies have shown associations between weight at one year of age and adult osteoporotic fractures. The correlation between growth in childhood and risk of hip fracture in later life was demonstrated in a longitudinal study in Helsinki, Finland (73). Data were collected on a total of 7086 men and women born between 1924 and 1933. Body size at birth was recorded and an average of 10 measurements of height and weight were made throughout childhood. The incidence of first hip fractures was assessed using the national hospital discharge register. After adjusting for age and sex two major determinants of hip fracture in later life were identified: tall maternal height (P < 0.001) and a low rate of childhood growth (height, P = 0.006; weight, P = 0.01). The effects of maternal height and slow childhood growth were statistically independent of each other and remained after adjusting for socioeconomic status. In addition, the observation that fracture subjects were shorter at birth but of average height by age 7 years suggests that hip fracture risk might be particularly elevated among children in whom growth of the skeletal envelope is forced ahead of the capacity to mineralise, a phenomenon which is accelerated during pubertal growth.
The association between early life and fragility fracture is likely to reflect dual effects of the early environment on adult bone mass (74) and muscle mass (75). In a conditional analysis of results from the Hertfordshire Cohort Study, 18% of adult bone mass was explained by a model that included birthweight, weight at one year and adult weight, with relative contributions attributable to each being 2.8%, 6.8% and 8.2% respectively (74). The contributions of early growth were greatest for bone area, and clearly apparent for bone mineral content, but not as evident for areal bone mineral density (BMD) or derived volumetric BMD. Early environmental studies have also shown associations between early life and adult grip strength. Kuh and colleagues examined the relation between birth weight and hand grip strength in a prospective national birth cohort of 1,371 men and 1,404 women from the Medical Research Council National Survey of Health and Development aged 53 years and reported a positive relation between birth weight and adult grip strength that remained after adjustment for adult height and weight, and childhood height and weight (75). In findings from the Hertfordshire Cohort Study, grip strength in older adults was most strongly associated with birth weight in men and women. These relationships remained significant after adjustment for adult height and weight. In contrast, the associations with infant growth were weakened after allowing for adult size. Adjustment for age, current social class, physical activity, smoking, and alcohol did not affect these results (76).
Conclusion
Muscle and bone aging is a phenomenon that results in significant morbidity and mortality in older populations, including a gradual erosion in quality of life (77,78). Common risk factors, including poor nutrition, lack of physical activity and cigarette smoking result in accelerated loss that manifest in an ability to live alone, commonly after an osteoporotic fragility fracture. Such fractures usually follow a fall (79, 80), often precipitated by poor musculoskeletal health, comorbidities or medication use (81, 82). A high comorbidity burden, including use of therapeutic agents such as glucocorticoids, is often associated with accelerated muscle and bone loss, and recent work has highlighted a possible role for the early environment. Inflammaging is an exciting emerging research field that is likely to prove relevant to future work, including interventions designed to retard to reverse bone and muscle loss with age. A better knowledge of the risk factors for accelerated musculoskeletal aging may help to reduce the economic burden of this condition, which has recently been estimated at $18.5billion for costs associated with sarcopenia for the USA in 2000, while the economic cost of osteoporosis was 37 billion euros in 2010 (52).
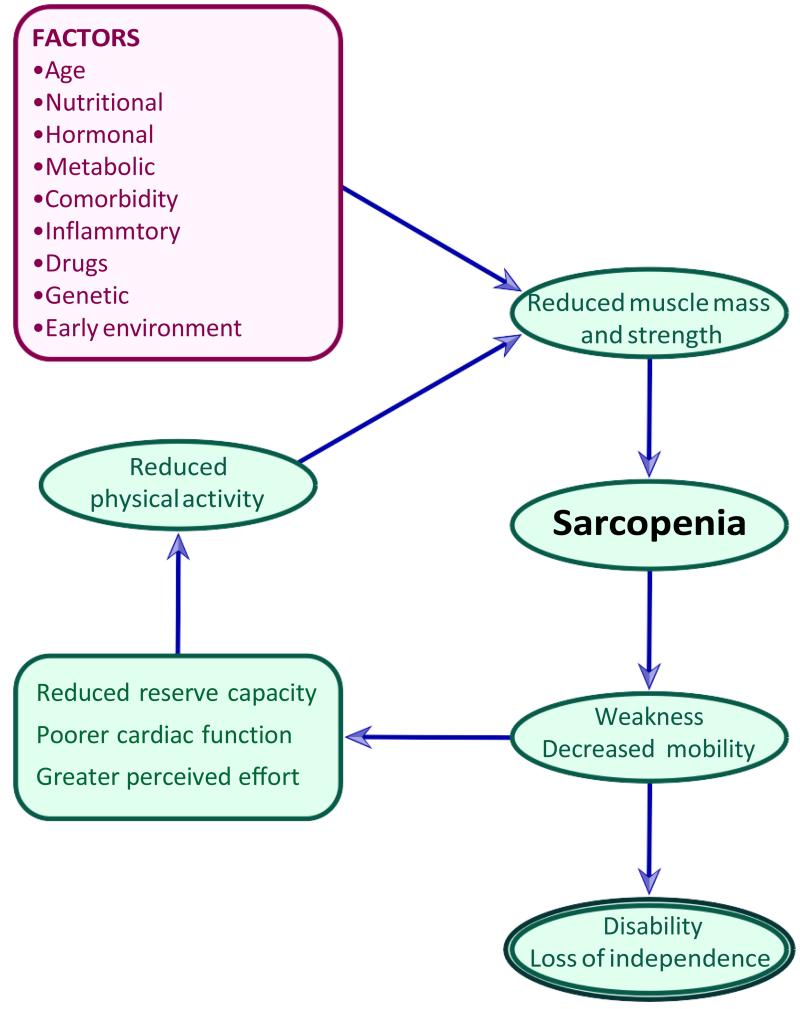
Figure 2.
Sarcopenia: a conceptual framework
References
- 1.Cooper C, Dere W, Evans W, Kanis JA, Rizzoli R, Aihie Sayer A, Sieber CC, Kaufman JM, Abellan van Kan G, Boonen S, Adachi J, Mitlak B, Tsouderos Y, Rolland Y, Reginster JYL. Frailty and sarcopenia: definitions and outcome parameters. Osteoporos Int. 2012;23:1839–1848. doi: 10.1007/s00198-012-1913-1. [DOI] [PubMed] [Google Scholar]
- 2.Cooper C, Fielding R, Visser M, van Loon LJ, Rolland Y, Orwoll E, Reid K, Boonen S, Dere W, Epstein S, Mitlak B, Tsouderos Y, Sayer AA, Rizzoli R, Reginster JY, Kanis JA. Tools in the assessment of sarcopenia. Calcif Tissue Int. 2013;93:201–10. doi: 10.1007/s00223-013-9757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Staa TP, Dennison EM, Leufkens HGM, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517–522. doi: 10.1016/s8756-3282(01)00614-7. [DOI] [PubMed] [Google Scholar]
- 4.Edwards MH, Gregson CL, Patel HP, Jameson KA, Harvey NC, Sayer AA, Dennison EM, Cooper C. Muscle size, strength and physical performance and their associations with bone structure in the Hertfordshire Cohort Study. J Bone Miner Res. 2013 Nov;28(11):2295–304. doi: 10.1002/jbmr.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd BD, Williamson DA, Singh NA, Hansen RD, Diamond TH, Finnegan TP, et al. Recurrent and injurious falls in the year following hip fracture: a prospective study of incidence and risk factors from the Sarcopenia and Hip Fracture study. J Gerontol A Biol Sci Med Sci. 2009;64(5):599–609. doi: 10.1093/gerona/glp003. [DOI] [PubMed] [Google Scholar]
- 6.Sayer AA, Syddall HE, Martin HJ, Dennison EM, Anderson FH, Cooper C. Falls, sarcopenia, and growth in early life: findings from the Hertfordshire cohort study. Am J Epidemiol. 2006;164(7):665–671. doi: 10.1093/aje/kwj255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rose Anne M. Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc. 2001;49(5):664–672.0. [PubMed] [Google Scholar]
- 8.Rose Anne M. Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148–157. doi: 10.1111/j.1532-5415.2010.03234.x. [DOI] [PubMed] [Google Scholar]
- 9.Lang T, Streeper T, Cawthorn P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention and assessment. Osteopor Int. 2010;21:543–559. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, Khosla S. A population- based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23:205–14. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tylavski FA, Bortz AD, Hancock RL, Anderson JJB. Familial resemblance of radial bone mass between premenopausal mothers and their college age daughters. Calcif Tissue Int. 1989;45:265–72. doi: 10.1007/BF02556017. [DOI] [PubMed] [Google Scholar]
- 12.Sambrook PN, Kelly PJ, Morrison NA, Eisman JA. Genetics of osteoporosis. Br J Rheumatol. 1994;33:1007–11. doi: 10.1093/rheumatology/33.11.1007. [DOI] [PubMed] [Google Scholar]
- 13.Rolland Y, Czerwinski S, Abellan Van Kan G, et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12(7):433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mc Gregor R, Cameron-Smith D, Poppitt S. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longevity & Healthspan. 2014;3:9. doi: 10.1186/2046-2395-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sayer AA, Syddall H, Martin H, Patel H, Baylis D, Cooper C. The developmental origins of sarcopenia. J Nutr Health Aging. 2008;12(7):427–432. doi: 10.1007/BF02982703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel HP, Syddall HE, Jameson K, Robinson S, Denison H, Roberts HC, et al. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: findings from the Hertfordshire Cohort Study (HCS) Age Ageing. 2013 doi: 10.1093/ageing/afs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maggi S, Kelsey JL, Litvak J, Heyse SP. Incidence of hip fractures in the elderly: a cross-sectional study. Osteoporosis Int. 1991;1:232–241. doi: 10.1007/BF03187467. [DOI] [PubMed] [Google Scholar]
- 18.Farmer ME, White LR, Brody JA, Bailey KR. Race and sex differences in hip fracture incidence. American Journal of Public Health. 1984;74:1374–138. doi: 10.2105/ajph.74.12.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnell O, Gullberg B, Kanis JA, Allander E, Elffors L, Dequeker J, Dilsen G, Gennari C, Vaz AL, Lyritis G, Mazzuoli G, Miravet L, Passeri M, Cano RP, Rapado A, Ribot C. Risk factors for Hip Fracture in European Women: The MEDOS Study. J Bone Miner Res. 1995;10:1802–1814. doi: 10.1002/jbmr.5650101125. [DOI] [PubMed] [Google Scholar]
- 20.Lunt M, Felesenberg D, Reeve J, Benevolenskaya L, Cannata J, et al. Bone density variation and its effects on risk of vertebral deformity in men and women studied in thirteen European centers: the EVOS study. J Bone Miner Res. 1997;12:1883–1894. doi: 10.1359/jbmr.1997.12.11.1883. [DOI] [PubMed] [Google Scholar]
- 21.Ross PD, Fujiwara S, Huang C, et al. Vertebral fracture prevalence in women in Hiroshima compared to Caucasians or Japanese in the US. International Journal of Epidemiology. 1995;24:1171–77. doi: 10.1093/ije/24.6.1171. [DOI] [PubMed] [Google Scholar]
- 22.Ling X, Cummings SR, Mingwei Q, Xihe Z, Xioashu C, Nevitt M, Stone K. Vertebral fractures in Beijing, China: the Beijing Osteoporosis Project. J Bone Miner Res. 2000;15:2019–25. doi: 10.1359/jbmr.2000.15.10.2019. [DOI] [PubMed] [Google Scholar]
- 23.Bortz WM., II A conceptual framework of frailty: a review. J Gerontol Med Sci. 2002;57:M283–288. doi: 10.1093/gerona/57.5.m283. [DOI] [PubMed] [Google Scholar]
- 24.Araujo AB, Chin GR, Kupelian V, et al. Lean mass, muscle strength and physical function in a diverse population of men: a population-based cross-sectional study. BMC Public Health. 2010;10:508. doi: 10.1186/1471-2458-10-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, Eisman JA, Kroger H, Fujiwara S, Garnero P, McCloskey EV, Mellstrom D, melton LJ, 3rd, Meunier PJ, Pols HA, Reeve J, Silman A, Tenenhouse A. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporosis Int. 2005;16:1330–8. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 26.Marcell TJ. Sarcopenia: Causes, consequences, and preventions. J Gerontol Med Sci. 2003;58A(10):911–916. doi: 10.1093/gerona/58.10.m911. [DOI] [PubMed] [Google Scholar]
- 27.Szulc P, Duboeuf F, Marchand F, Delmas P. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: the MINOS study. Am J Clin Nutr. 2004;80:496–503. doi: 10.1093/ajcn/80.2.496. [DOI] [PubMed] [Google Scholar]
- 28.Berard A, Bravo G, Gauthier P. Meta-analysis of the effectiveness of physical activity for the prevention of bone loss in postmenopausal women. Osteoporosis Int. 1997;7:331–7. doi: 10.1007/BF01623773. [DOI] [PubMed] [Google Scholar]
- 29.Kortebein P, et al. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297(16):1772–4. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 30.Zampieri S, Pietrangelo L, Loefler S, et al. Lifelong physical exercise delays age associated skeletal muscle decline. J Gerontol A Biol Sci Med Sci. 2014 doi: 10.1093/gerona/glu006. [DOI] [PubMed] [Google Scholar]
- 31.Hinrichs T, von Bonsdorff MB, Tormakangas T. Inverse effects of midlife occupational and leisure time physical activity on mobility limitation in old age – a 28-year prospective follow-up study. J Am Geriatr Soc. 2014;62:812–820. doi: 10.1111/jgs.12793. eg al. [DOI] [PubMed] [Google Scholar]
- 32.Binder EF, Yarasheski KE, Steger-May K, et al. Effects of progressive resistance training on body composition in frail older adults: results of a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2005;60:1425–31. doi: 10.1093/gerona/60.11.1425. [DOI] [PubMed] [Google Scholar]
- 33.Suetta C, Andersen JL, Dalgas U, et al. Resistance training induced qualitative changes in muscle morphology, muscle architecture and muscle function in elderly postoperative patients. J Appl Physiol (1985) 2008;105:180–6. doi: 10.1152/japplphysiol.01354.2007. [DOI] [PubMed] [Google Scholar]
- 34.Bunout D, Barrera G, de la Maza P, et al. The impact of nutritional a. supplementation and resistance training on the health functioning of free-living Chilean Elders: results of 18 months of follow-up. J Nutr. 2001;131:2441s–6s. doi: 10.1093/jn/131.9.2441S. [DOI] [PubMed] [Google Scholar]
- 35.Kemmler W, von Stengel S, Engelke K, Haberle L, Mayhew JL, Kalender WA. Exercise, body composition and functional ability: a randomized controlled trial. Am J Prev Med. 2010;38:279–87. doi: 10.1016/j.amepre.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 36.Rydwik E, Lammes E, Frandin K, Akner G. Effects of a physical and nutritional intervention program for frail elderly people over age 75. A randomized controlled pilot treatment trial. Aging Clin Exp Res. 2008;20:159–70. doi: 10.1007/BF03324763. [DOI] [PubMed] [Google Scholar]
- 37.Goodpaster BH, Chomentowski P, Ward BK, et al. Effects of Physical Activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol (1985) 2008;105:1498–503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ. 1997;315:841–6. doi: 10.1136/bmj.315.7112.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, Fujiwara S, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A. Smoking and fracture risk: a meta-analysis. Osteoporosis Int. 2005;16:155–62. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 40.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 41.Kanis JA, Johansson H, Johnell O, Oden A, De Laet C, Eisman JA, Pols H, Tenenhouse A. Alcohol intake as a risk factor for fracture. Osteoporosis Int. 2005;16:737–42. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 42.Renoud A, Ecochard R, Marchand F, Chapurlat R, Szulc P. Predictive Parameters of Accelerated Muscle Loss in Men – MINOS Study. Am J Med. 127(6):554–561. doi: 10.1016/j.amjmed.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Xu L, McElduff P, et al. Does dietary calcium have a protective effect on bone fractures in women? A meta-analysis of observational studies. Br J Nutr. 2004;91:625–34. doi: 10.1079/BJN20031085. [DOI] [PubMed] [Google Scholar]
- 44.Ginty F. Dietary protein and bone health. Proc Nutr Soc. 2003;62:867–76. doi: 10.1079/PNS2003307. [DOI] [PubMed] [Google Scholar]
- 45.Frassetto LA, Todd KM, et al. Worldwide incidence of hip fracture in elderly women: relation to consumption of animal and vegetable foods. J Gerontol A Biol Sci Med Sci. 2000;55:M585–92. doi: 10.1093/gerona/55.10.m585. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, Shu XO, Li H, Yang G, Li Q, Gao YT, Zheng W. Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch Intern Med. 2005;165:1890–5. doi: 10.1001/archinte.165.16.1890. [DOI] [PubMed] [Google Scholar]
- 47.Chaput JP, et al. Relationship between antioxidant intakes and class I sarcopenia in elderly men and women. J Nutr Heath Aging. 2007;11(4):363–9. [PubMed] [Google Scholar]
- 48.Lord C, et al. Dietary animal protein intake: association with muscle mass index in older women. J Nutr Heath Aging. 2007;11(5):383–7. [PubMed] [Google Scholar]
- 49.Rooyackers OE, et al. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci USA. 1996;93(26):15364–9. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson MM, Morley JE. Invited review: Aging and energy balance. J Appl Physiol. 2003;95(4):1728–36. doi: 10.1152/japplphysiol.00313.2003. [DOI] [PubMed] [Google Scholar]
- 51.Volpi E, et al. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78(2):250–8. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rizzoli R, Stevenson JC, Bauer JM, van Loon LJC, Walrand S, Kanis JA, Cooper C, Brandi ML, Diez-Perez A, Reginster JY. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: A consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis. Maturitas. 2014;79:122–132. doi: 10.1016/j.maturitas.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 53.Mithal A, Bonjour JP, Boonen S, Burckhardt P, Degens H, El Hajj Fuleihan G, Josse R, Lips P, Morales Torres J, Rizzoli R, Yoshimura N, Wahl DA, Cooper C, Dawson-Hughes B. Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos Int. 2013;24:1555–1566. doi: 10.1007/s00198-012-2236-y. [DOI] [PubMed] [Google Scholar]
- 54.Morley JE, et al. Potentially predictive and manipulable blood serum correlates of aging in the healthy human male: progressive decreases in bioavailable testosterone, dehydroepiandosterone sulfate, and the ratio of insulin-like growth factor 1 to growth hormone. Proc Natl Acad Sci USA. 1997;94(14):7537–42. doi: 10.1073/pnas.94.14.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amin S, Zhang Y, Sawin CT, Evans SR, Hannan MT, Kiel DP, Wilson PW, Felson DT. Association of hypogonadism and estradiol levels with bone mineral density in elderly men from the Framingham study. Ann Intern Med. 2000;133:1002–4. doi: 10.7326/0003-4819-133-12-200012190-00010. [DOI] [PubMed] [Google Scholar]
- 56.Galvao DA, et al. Exercise can prevent and even reverse adverse effects of androgen suppression treatment in men with prostate cancer. Prostate Cancer Prostatic Dis. 2007 doi: 10.1038/sj.pcan.4500975. [DOI] [PubMed] [Google Scholar]
- 57.Morley JE. Growth hormone: fountain of youth or death hormone? J Am Geriatr Soc. 1999;47(12):1475–6. doi: 10.1111/j.1532-5415.1999.tb01572.x. [DOI] [PubMed] [Google Scholar]
- 58.Stein MS, et al. Falls relate to vitamin D and parathyroid hormone in an Australian nursing home and hostel. J Am Geriatr Soc. 1999;47(10):1195–201. doi: 10.1111/j.1532-5415.1999.tb05199.x. [DOI] [PubMed] [Google Scholar]
- 59.Bischoff-Ferrari HA. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv Exp Med Biol. 2014;810:500–525. doi: 10.1007/978-1-4939-0437-2_28. [DOI] [PubMed] [Google Scholar]
- 60.Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88(12):5766–72. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 61.Jacques PF, et al. Plasma 25-hydroyvitamin D and its determinants in an elderly population sample. Am J Clin Nutr. 1997;66(4):929–36. doi: 10.1093/ajcn/66.4.929. [DOI] [PubMed] [Google Scholar]
- 62.Kanis JA, Johansson H, Oden A, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19:893–9. doi: 10.1359/JBMR.040134. [DOI] [PubMed] [Google Scholar]
- 63.Van Staa TP, Leufkens HGM, Abenhaim L, Zhang B, Cooper C. Use of corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 64.Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, et al. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128(1):83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 65.De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80(3):219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 66.Franceschi C, Bonafe M, Valensin S, Olivieri F, De LM, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 67.De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579(10):2035–2039. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 68.Cannizzo ES, Clement CC, Sahu R, Follo C, Santambrogio L. Oxidative stress, inflamm-aging and immunosenescence. J Proteomics. 2011;74(11):2313–2323. doi: 10.1016/j.jprot.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 69.Schaap LA, et al. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119(6):526, e9–17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 70.Ferrucci L, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47(6):639–46. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 71.Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Eng J Med. 1996;335(25):1897–905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- 72.Corcoran MP, Lamon-Fava S, Fielding RA. Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise. Am J Clin Nutr. 2007;85(3):662–77. doi: 10.1093/ajcn/85.3.662. [DOI] [PubMed] [Google Scholar]
- 73.Cooper C, Eriksson JG, Forsen T, Osmond C, Tuomilehto J, Barker DJP. Maternal height, childhood growth and the risk of hip fracture in later life: a longitudinal study. Osteoporosis Int. 2001;12:623–629. doi: 10.1007/s001980170061. [DOI] [PubMed] [Google Scholar]
- 74.Dennison EM, Syddall HE, Aihie Sayer A, Gilbody HJ, Cooper C. Birthweight and weight at one year are independent determinants of bone mass in the seventh decade: the Hertfordshire Cohort Study. Ped Res. 2005;57:582–6. doi: 10.1203/01.PDR.0000155754.67821.CA. [DOI] [PubMed] [Google Scholar]
- 75.Kuh D, Hardy R, Butterworth S, Okell L, Wadsworth M, Cooper C, Aihie Sayer A. Developmental origins of midlife grip strength: findings from a birth cohort study. J Gerontol A Biol Sci Med Sci. 2006;61:702–706. doi: 10.1093/gerona/61.7.702. [DOI] [PubMed] [Google Scholar]
- 76.Aihie Sayer A, Syddall HE, Martin HJ, Dennison EM, Anderson FH, Cooper C. Falls, sarcopenia and growth in early life: findings from the Hertfordshire Cohort Study. Am J Epidemiol. 2006;164:665–71. doi: 10.1093/aje/kwj255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rizzoli R, Reginster JY, Arnal JF, Bautmans I, Beaudart C, Bischoff-Ferrari H, Biver E, Boonen S, Brandi ML, Chines A, Cooper C, Epstein S, Fielding RA, Goodpaster B, Kanis JA, Kaufman JM, Laslop A, Malafarina V, Rodriguez Manas L, Mitlak BH, Oreffo RO, Petermans J, Reid K, Rolland Y, Sayer AA, Tsouderos Y, Visser M, Bruyere O. Quality of life in sarcopenia and frailty. Calcif Tissue Int. 2013;93:101–120. doi: 10.1007/s00223-013-9758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dennison EM, Jameson KA, Sydall HE, Martin HJ, Cushnaghan J, Aihie Sayer A, Cooper C. Bone health and deterioration in quality of life among participants from the Hertfordshire Cohort Study. Osteoporosis Int. 2009;21(11):1817–24. doi: 10.1007/s00198-009-1147-z. [DOI] [PubMed] [Google Scholar]
- 79.Winner SJ, Morgan CA, Evans JG. Perimenopausal risk of falling and incidence of distal forearm fracture. British Medical Journal. 1989;298:2486–8. doi: 10.1136/bmj.298.6686.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pakkari J, Kannus P, Palvanen M, et al. Majority of hip fractures occur as a result of a fall and impact on the greater trochanter of the femur: a prospective controlled hip fracture study with 206 consecutive patients. Calcified Tissue International. 1999;65:183–187. doi: 10.1007/s002239900679. [DOI] [PubMed] [Google Scholar]
- 81.Liu B, Anderson G, Mittmann N, To T, Axcell T, Shear N. Use of selective Serotonin-reuptake inhibitors or tricyclic antidepressants and risk of hip fractures in elderly people. Lancet. 1998;351:1303–1307. doi: 10.1016/s0140-6736(97)09528-7. [DOI] [PubMed] [Google Scholar]
- 82.Cumming RG. Epidemiology of medication-related falls and fractures in the elderly. Drugs Aging. 1998;12:43–53. doi: 10.2165/00002512-199812010-00005. [DOI] [PubMed] [Google Scholar]