Abstract
The sequence of an invertebrate GABAA receptor subunit is described. This was deduced from a cDNA which was isolated from the mollusc Lymnaea stagnalis and which corresponds to a transcript of extremely low abundance. The cDNA was isolated using short exonic sequences from part of the corresponding gene in combination with a variant of the polymerase chain reaction (PCR) known as RACE (rapid amplification of cDNA ends). The mature polypeptide has a predicted molecular weight of 54,569 Daltons and exhibits approximately 50% identity to vertebrate GABAA receptor beta subunits. The six intron-exon boundaries determined to date in the molluscan gene occur at the same relative positions as those found in vertebrate GABAA receptor genes. Functional expression, in Xenopus oocytes, of the molluscan cDNA alone results in the formation of GABA-activated chloride ion channels that have a finite open probability even in the absence of agonist. These GABA-evoked currents can be reversibly blocked by the vertebrate GABAA receptor antagonist bicuculline. Surprisingly, the molluscan beta subunit is capable of replacing vertebrate beta subunits in co-expression experiments with the bovine GABAA receptor alpha 1 subunit. These findings suggest that invertebrate GABAA receptors exist in vivo as hetero-oligomeric complexes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton G. J., Sternberg M. J. A strategy for the rapid multiple alignment of protein sequences. Confidence levels from tertiary structure comparisons. J Mol Biol. 1987 Nov 20;198(2):327–337. doi: 10.1016/0022-2836(87)90316-0. [DOI] [PubMed] [Google Scholar]
- Bateson A. N., Lasham A., Darlison M. G. gamma-Aminobutyric acidA receptor heterogeneity is increased by alternative splicing of a novel beta-subunit gene transcript. J Neurochem. 1991 Apr;56(4):1437–1440. doi: 10.1111/j.1471-4159.1991.tb11443.x. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blair L. A., Levitan E. S., Marshall J., Dionne V. E., Barnard E. A. Single subunits of the GABAA receptor form ion channels with properties of the native receptor. Science. 1988 Oct 28;242(4878):577–579. doi: 10.1126/science.2845583. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting G. R., Lu L., O'Hara B. F., Kasch L. M., Montrose-Rafizadeh C., Donovan D. M., Shimada S., Antonarakis S. E., Guggino W. B., Uhl G. R. Cloning of the gamma-aminobutyric acid (GABA) rho 1 cDNA: a GABA receptor subunit highly expressed in the retina. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2673–2677. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenningloh G., Pribilla I., Prior P., Multhaup G., Beyreuther K., Taleb O., Betz H. Cloning and expression of the 58 kd beta subunit of the inhibitory glycine receptor. Neuron. 1990 Jun;4(6):963–970. doi: 10.1016/0896-6273(90)90149-a. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Rienitz A., Schmitt B., Methfessel C., Zensen M., Beyreuther K., Gundelfinger E. D., Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987 Jul 16;328(6127):215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Schmieden V., Schofield P. R., Seeburg P. H., Siddique T., Mohandas T. K., Becker C. M., Betz H. Alpha subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J. 1990 Mar;9(3):771–776. doi: 10.1002/j.1460-2075.1990.tb08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacoff E. Y., Jan Y. N., Jan L. Y. Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes. Nature. 1990 Jun 7;345(6275):530–534. doi: 10.1038/345530a0. [DOI] [PubMed] [Google Scholar]
- Jansen R., Kalousek F., Fenton W. A., Rosenberg L. E., Ledley F. D. Cloning of full-length methylmalonyl-CoA mutase from a cDNA library using the polymerase chain reaction. Genomics. 1989 Feb;4(2):198–205. doi: 10.1016/0888-7543(89)90300-5. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990 Sep;15(9):342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Khrestchatisky M., MacLennan A. J., Chiang M. Y., Xu W. T., Jackson M. B., Brecha N., Sternini C., Olsen R. W., Tobin A. J. A novel alpha subunit in rat brain GABAA receptors. Neuron. 1989 Dec;3(6):745–753. doi: 10.1016/0896-6273(89)90243-2. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Schmieden V., Betz H. Identification and functional expression of a novel ligand binding subunit of the inhibitory glycine receptor. J Biol Chem. 1990 Dec 25;265(36):22317–22320. [PubMed] [Google Scholar]
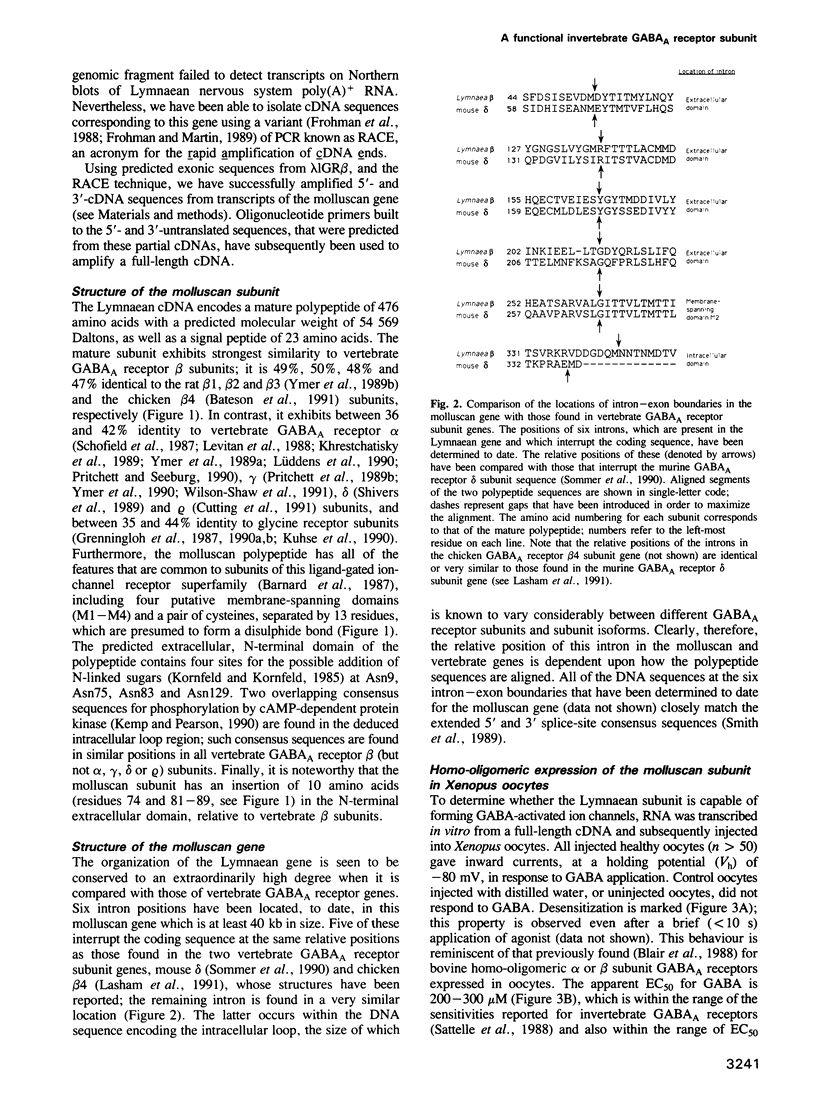
- Lasham A., Vreugdenhil E., Bateson A. N., Barnard E. A., Darlison M. G. Conserved organization of gamma-aminobutyric acidA receptor genes: cloning and analysis of the chicken beta 4-subunit gene. J Neurochem. 1991 Jul;57(1):352–355. doi: 10.1111/j.1471-4159.1991.tb02135.x. [DOI] [PubMed] [Google Scholar]
- Levitan E. S., Schofield P. R., Burt D. R., Rhee L. M., Wisden W., Köhler M., Fujita N., Rodriguez H. F., Stephenson A., Darlison M. G. Structural and functional basis for GABAA receptor heterogeneity. Nature. 1988 Sep 1;335(6185):76–79. doi: 10.1038/335076a0. [DOI] [PubMed] [Google Scholar]
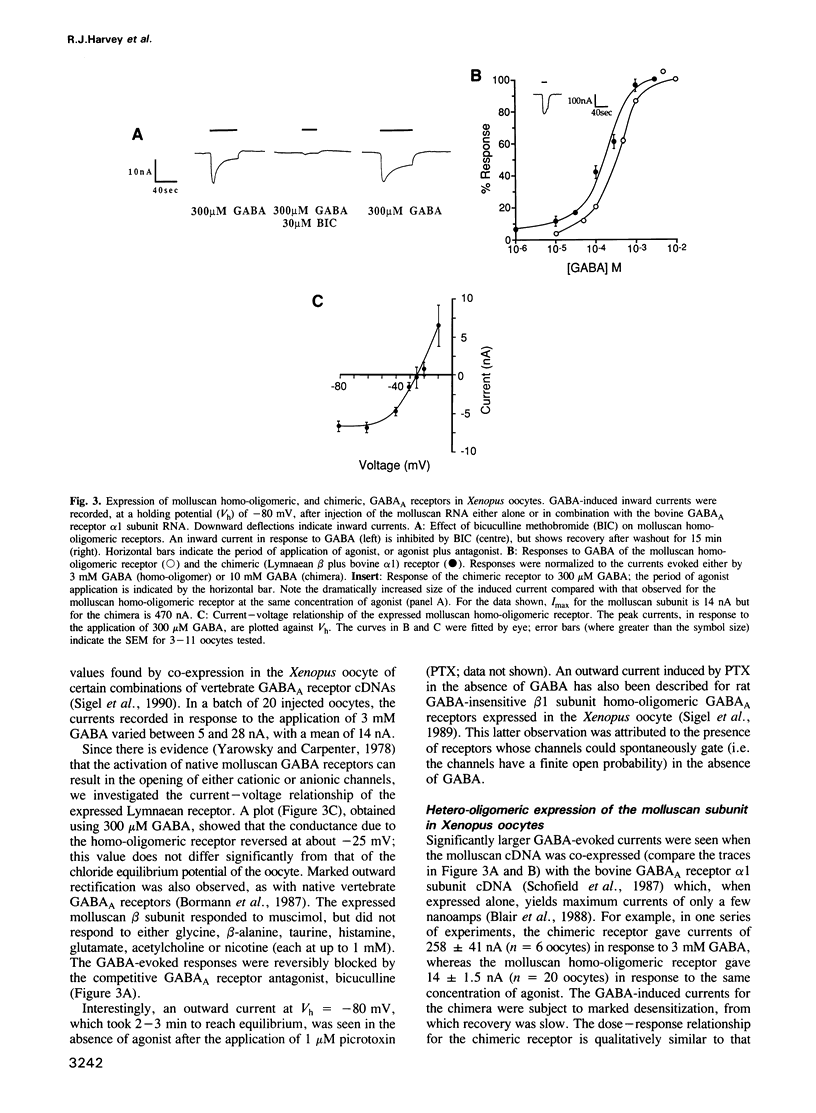
- Lüddens H., Pritchett D. B., Köhler M., Killisch I., Keinänen K., Monyer H., Sprengel R., Seeburg P. H. Cerebellar GABAA receptor selective for a behavioural alcohol antagonist. Nature. 1990 Aug 16;346(6285):648–651. doi: 10.1038/346648a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., Tobin A. J. Molecular biology of GABAA receptors. FASEB J. 1990 Mar;4(5):1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Lüddens H., Seeburg P. H. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989 Sep 22;245(4924):1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Seeburg P. H. Gamma-aminobutyric acidA receptor alpha 5-subunit creates novel type II benzodiazepine receptor pharmacology. J Neurochem. 1990 May;54(5):1802–1804. doi: 10.1111/j.1471-4159.1990.tb01237.x. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Sontheimer H., Shivers B. D., Ymer S., Kettenmann H., Schofield P. R., Seeburg P. H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989 Apr 13;338(6216):582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattelle D. B., Pinnock R. D., Wafford K. A., David J. A. GABA receptors on the cell-body membrane of an identified insect motor neuron. Proc R Soc Lond B Biol Sci. 1988 Jan 22;232(1269):443–456. doi: 10.1098/rspb.1988.0006. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Shivers B. D., Killisch I., Sprengel R., Sontheimer H., Köhler M., Schofield P. R., Seeburg P. H. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989 Sep;3(3):327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- Sigel E., Baur R., Malherbe P., Möhler H. The rat beta 1-subunit of the GABAA receptor forms a picrotoxin-sensitive anion channel open in the absence of GABA. FEBS Lett. 1989 Nov 6;257(2):377–379. doi: 10.1016/0014-5793(89)81576-5. [DOI] [PubMed] [Google Scholar]
- Sigel E., Baur R., Trube G., Möhler H., Malherbe P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990 Nov;5(5):703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Patton J. G., Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- Sommer B., Poustka A., Spurr N. K., Seeburg P. H. The murine GABAA receptor delta-subunit gene: structure and assignment to human chromosome 1. DNA Cell Biol. 1990 Oct;9(8):561–568. doi: 10.1089/dna.1990.9.561. [DOI] [PubMed] [Google Scholar]
- Syed N. I., Bulloch A. G., Lukowiak K. In vitro reconstruction of the respiratory central pattern generator of the mollusk Lymnaea. Science. 1990 Oct 12;250(4978):282–285. doi: 10.1126/science.2218532. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Draguhn A., Ymer S., Seeburg P. H., Sakmann B. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron. 1990 Jun;4(6):919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil E., Jackson J. F., Bouwmeester T., Smit A. B., Van Minnen J., Van Heerikhuizen H., Klootwijk J., Joosse J. Isolation, characterization, and evolutionary aspects of a cDNA clone encoding multiple neuropeptides involved in the stereotyped egg-laying behavior of the freshwater snail Lymnaea stagnalis. J Neurosci. 1988 Nov;8(11):4184–4191. doi: 10.1523/JNEUROSCI.08-11-04184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. M., Mayne K. M., Lester H. A., Davidson N. Mouse-Torpedo hybrid acetylcholine receptors: functional homology does not equal sequence homology. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4852–4856. doi: 10.1073/pnas.82.14.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Shaw D., Robinson M., Gambarana C., Siegel R. E., Sikela J. M. A novel gamma subunit of the GABAA receptor identified using the polymerase chain reaction. FEBS Lett. 1991 Jun 24;284(2):211–215. doi: 10.1016/0014-5793(91)80687-x. [DOI] [PubMed] [Google Scholar]
- Wisden W., Morris B. J., Darlison M. G., Hunt S. P., Barnard E. A. Distinct GABAA receptor alpha subunit mRNAs show differential patterns of expression in bovine brain. Neuron. 1988 Dec;1(10):937–947. doi: 10.1016/0896-6273(88)90151-1. [DOI] [PubMed] [Google Scholar]
- Wisden W., Morris B. J., Darlison M. G., Hunt S. P., Barnard E. A. Localization of GABAA receptor alpha-subunit mRNAs in relation to receptor subtypes. Brain Res Mol Brain Res. 1989 Jun;5(4):305–310. doi: 10.1016/0169-328x(89)90065-x. [DOI] [PubMed] [Google Scholar]
- Yarowsky P. J., Carpenter D. O. Receptors for gamma-aminobutyric acid (GABA) on Aplysia neurons. Brain Res. 1978 Apr 7;144(1):75–94. doi: 10.1016/0006-8993(78)90436-5. [DOI] [PubMed] [Google Scholar]
- Ymer S., Draguhn A., Köhler M., Schofield P. R., Seeburg P. H. Sequence and expression of a novel GABAA receptor alpha subunit. FEBS Lett. 1989 Nov 20;258(1):119–122. doi: 10.1016/0014-5793(89)81630-8. [DOI] [PubMed] [Google Scholar]
- Ymer S., Draguhn A., Wisden W., Werner P., Keinänen K., Schofield P. R., Sprengel R., Pritchett D. B., Seeburg P. H. Structural and functional characterization of the gamma 1 subunit of GABAA/benzodiazepine receptors. EMBO J. 1990 Oct;9(10):3261–3267. doi: 10.1002/j.1460-2075.1990.tb07525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ymer S., Schofield P. R., Draguhn A., Werner P., Köhler M., Seeburg P. H. GABAA receptor beta subunit heterogeneity: functional expression of cloned cDNAs. EMBO J. 1989 Jun;8(6):1665–1670. doi: 10.1002/j.1460-2075.1989.tb03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]



