Abstract
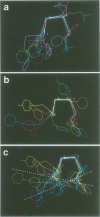
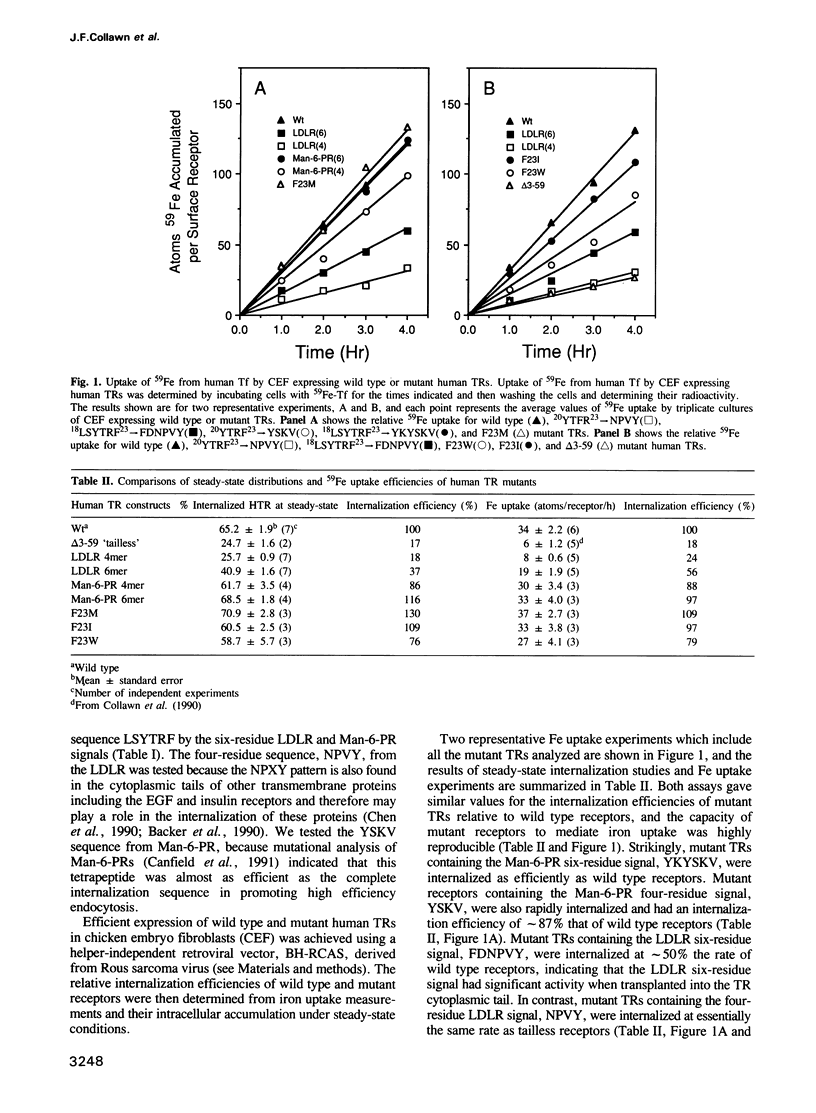
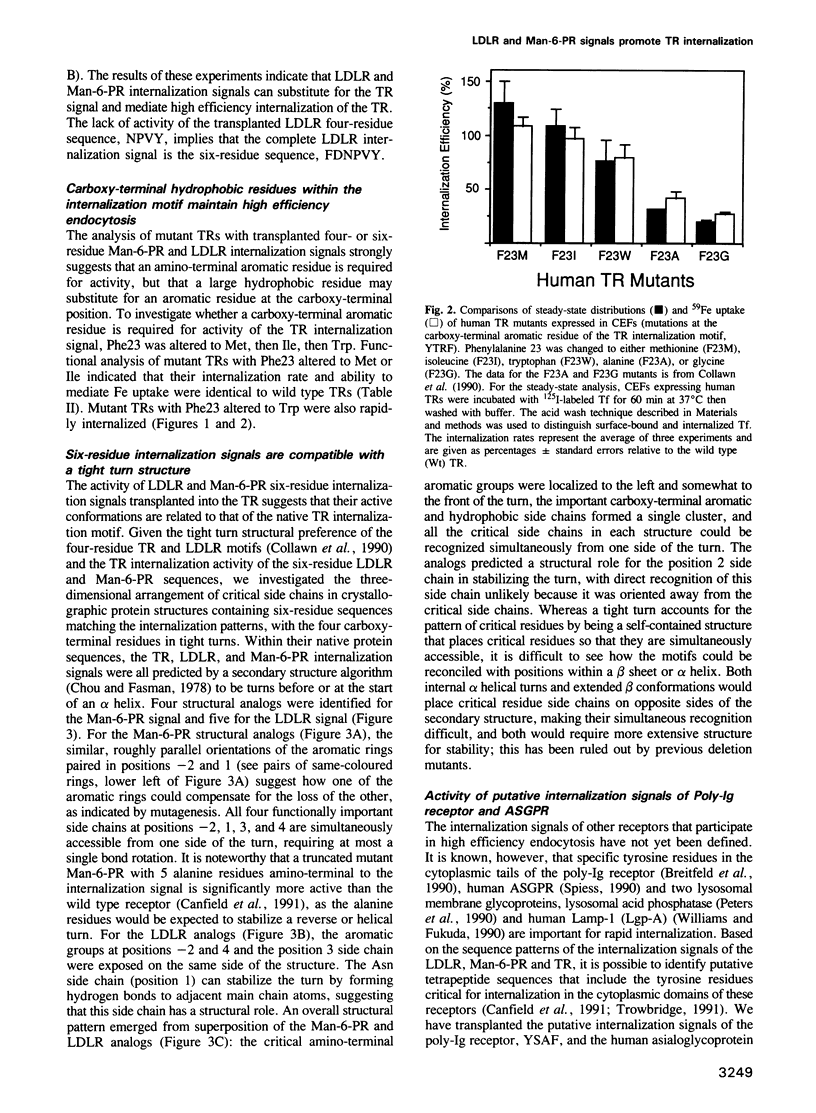
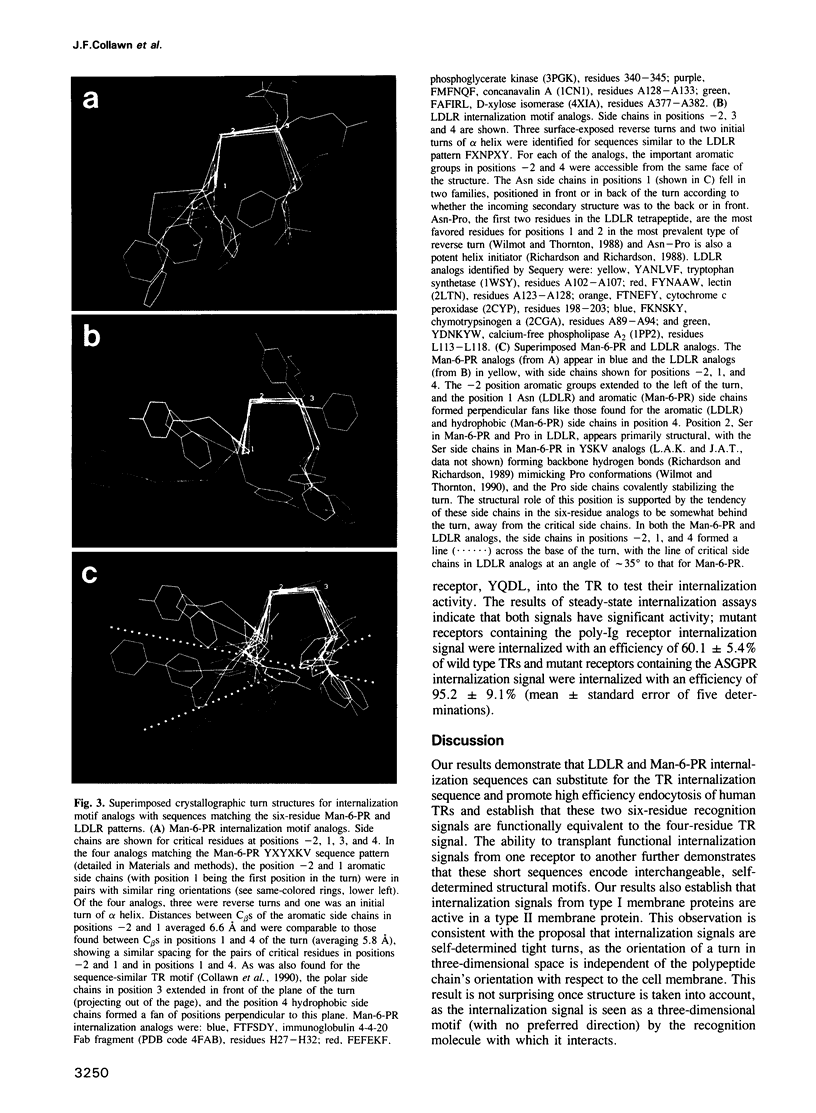
The internalization signals of several constitutively recycling receptors have recently been identified as regions of four or six amino acids that include an aromatic residue, usually tyrosine. Here, we show that transplanted signals from the low density lipoprotein receptor (LDLR) and cation-independent mannose-6-phosphate receptor (Man-6-PR) promote rapid internalization of the transferrin receptor (TR), directly establishing that recognition signals are interchangeable, self-determined structural motifs and that signals from type I membrane proteins are active in a type II receptor. We also show that the chemical and spatial patterns of critical residues in both four- and six-residue internalization motifs are consistent with a tight turn structure. A six-residue LDLR signal is needed for activity in TR, suggesting that an amino-terminal aromatic side chain is obligatory. In contrast, the carboxy-terminal aromatic side chain in the TR signal can be replaced by a large hydrophobic residue. Thus, internalization signals apparently require an aromatic amino-terminal residue and either an aromatic or large hydrophobic carboxy-terminal residue rather than a conserved tyrosine per se. Consistent with this conclusion, predicted internalization signals from the poly-Ig receptor, YSAF, and asialoglycoprotein receptor (ASGPR) subunit H1, YQDL, also promote internalization of TR.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backer J. M., Kahn C. R., Cahill D. A., Ullrich A., White M. F. Receptor-mediated internalization of insulin requires a 12-amino acid sequence in the juxtamembrane region of the insulin receptor beta-subunit. J Biol Chem. 1990 Sep 25;265(27):16450–16454. [PubMed] [Google Scholar]
- Bates G. W., Schlabach M. R. The reaction of ferric salts with transferrin. J Biol Chem. 1973 May 10;248(9):3228–3232. [PubMed] [Google Scholar]
- Breitfeld P. P., Casanova J. E., McKinnon W. C., Mostov K. E. Deletions in the cytoplasmic domain of the polymeric immunoglobulin receptor differentially affect endocytotic rate and postendocytotic traffic. J Biol Chem. 1990 Aug 15;265(23):13750–13757. [PubMed] [Google Scholar]
- Canfield W. M., Johnson K. F., Ye R. D., Gregory W., Kornfeld S. Localization of the signal for rapid internalization of the bovine cation-independent mannose 6-phosphate/insulin-like growth factor-II receptor to amino acids 24-29 of the cytoplasmic tail. J Biol Chem. 1991 Mar 25;266(9):5682–5688. [PubMed] [Google Scholar]
- Chen W. J., Goldstein J. L., Brown M. S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990 Feb 25;265(6):3116–3123. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Collawn J. F., Stangel M., Kuhn L. A., Esekogwu V., Jing S. Q., Trowbridge I. S., Tainer J. A. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990 Nov 30;63(5):1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- Davis C. G., Lehrman M. A., Russell D. W., Anderson R. G., Brown M. S., Goldstein J. L. The J.D. mutation in familial hypercholesterolemia: amino acid substitution in cytoplasmic domain impedes internalization of LDL receptors. Cell. 1986 Apr 11;45(1):15–24. doi: 10.1016/0092-8674(86)90533-7. [DOI] [PubMed] [Google Scholar]
- Davis C. G., van Driel I. R., Russell D. W., Brown M. S., Goldstein J. L. The low density lipoprotein receptor. Identification of amino acids in cytoplasmic domain required for rapid endocytosis. J Biol Chem. 1987 Mar 25;262(9):4075–4082. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R., Trowbridge I. S. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983 Aug;97(2):508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Greenhouse J. J., Petropoulos C. J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987 Oct;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing S. Q., Spencer T., Miller K., Hopkins C., Trowbridge I. S. Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J Cell Biol. 1990 Feb;110(2):283–294. doi: 10.1083/jcb.110.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. F., Chan W., Kornfeld S. Cation-dependent mannose 6-phosphate receptor contains two internalization signals in its cytoplasmic domain. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10010–10014. doi: 10.1073/pnas.87.24.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Nishizawa M. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 1984 Jun;4(6):1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ktistakis N. T., Thomas D., Roth M. G. Characteristics of the tyrosine recognition signal for internalization of transmembrane surface glycoproteins. J Cell Biol. 1990 Oct;111(4):1393–1407. doi: 10.1083/jcb.111.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarovits J., Roth M. A single amino acid change in the cytoplasmic domain allows the influenza virus hemagglutinin to be endocytosed through coated pits. Cell. 1988 Jun 3;53(5):743–752. doi: 10.1016/0092-8674(88)90092-x. [DOI] [PubMed] [Google Scholar]
- Livneh E., Prywes R., Kashles O., Reiss N., Sasson I., Mory Y., Ullrich A., Schlessinger J. Reconstitution of human epidermal growth factor receptors and its deletion mutants in cultured hamster cells. J Biol Chem. 1986 Sep 25;261(27):12490–12497. [PubMed] [Google Scholar]
- Lobel P., Fujimoto K., Ye R. D., Griffiths G., Kornfeld S. Mutations in the cytoplasmic domain of the 275 kd mannose 6-phosphate receptor differentially alter lysosomal enzyme sorting and endocytosis. Cell. 1989 Jun 2;57(5):787–796. doi: 10.1016/0092-8674(89)90793-9. [DOI] [PubMed] [Google Scholar]
- McGraw T. E., Maxfield F. R. Human transferrin receptor internalization is partially dependent upon an aromatic amino acid on the cytoplasmic domain. Cell Regul. 1990 Mar;1(4):369–377. doi: 10.1091/mbc.1.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw T. E., Pytowski B., Arzt J., Ferrone C. Mutagenesis of the human transferrin receptor: two cytoplasmic phenylalanines are required for efficient internalization and a second-site mutation is capable of reverting an internalization-defective phenotype. J Cell Biol. 1991 Mar;112(5):853–861. doi: 10.1083/jcb.112.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B. M., Robinson M. S. Clathrin, adaptors, and sorting. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- Peters C., Braun M., Weber B., Wendland M., Schmidt B., Pohlmann R., Waheed A., von Figura K. Targeting of a lysosomal membrane protein: a tyrosine-containing endocytosis signal in the cytoplasmic tail of lysosomal acid phosphatase is necessary and sufficient for targeting to lysosomes. EMBO J. 1990 Nov;9(11):3497–3506. doi: 10.1002/j.1460-2075.1990.tb07558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. S., Richardson D. C. Amino acid preferences for specific locations at the ends of alpha helices. Science. 1988 Jun 17;240(4859):1648–1652. doi: 10.1126/science.3381086. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess M. The asialoglycoprotein receptor: a model for endocytic transport receptors. Biochemistry. 1990 Oct 30;29(43):10009–10018. doi: 10.1021/bi00495a001. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S. Endocytosis and signals for internalization. Curr Opin Cell Biol. 1991 Aug;3(4):634–641. doi: 10.1016/0955-0674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Williams M. A., Fukuda M. Accumulation of membrane glycoproteins in lysosomes requires a tyrosine residue at a particular position in the cytoplasmic tail. J Cell Biol. 1990 Sep;111(3):955–966. doi: 10.1083/jcb.111.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmot C. M., Thornton J. M. Analysis and prediction of the different types of beta-turn in proteins. J Mol Biol. 1988 Sep 5;203(1):221–232. doi: 10.1016/0022-2836(88)90103-9. [DOI] [PubMed] [Google Scholar]
- Wilmot C. M., Thornton J. M. Beta-turns and their distortions: a proposed new nomenclature. Protein Eng. 1990 May;3(6):479–493. doi: 10.1093/protein/3.6.479. [DOI] [PubMed] [Google Scholar]