Abstract
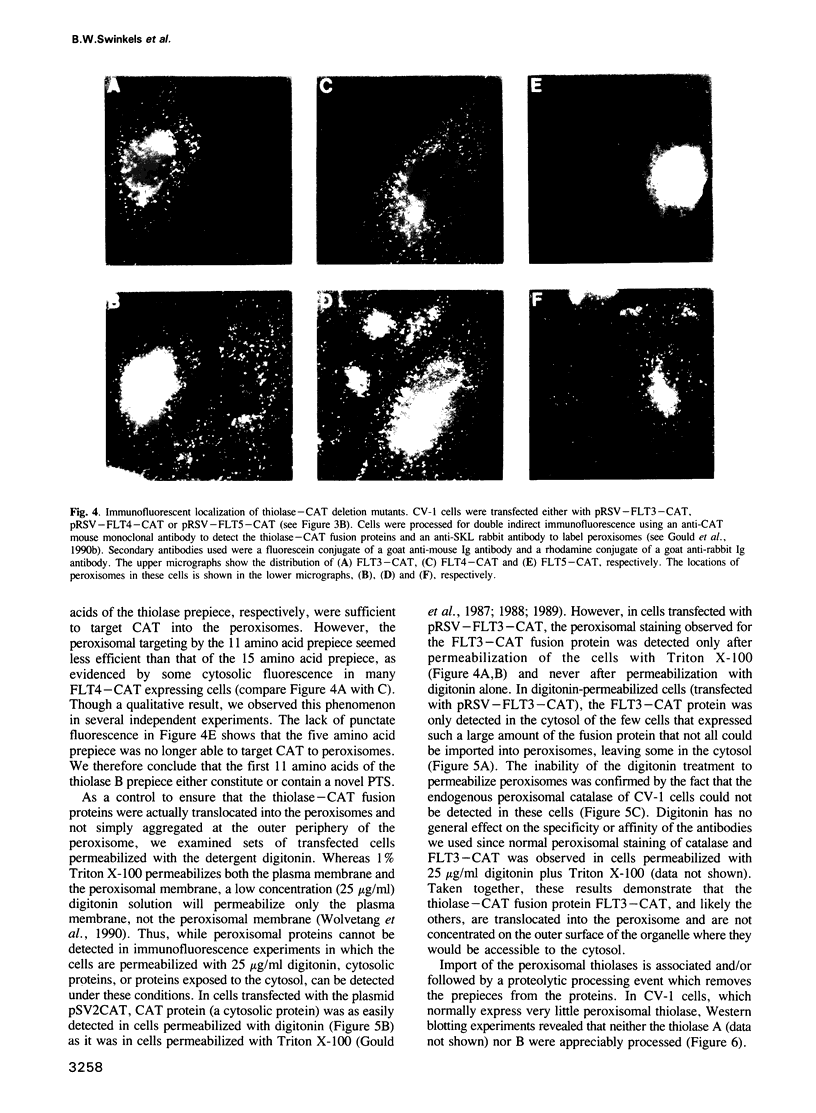
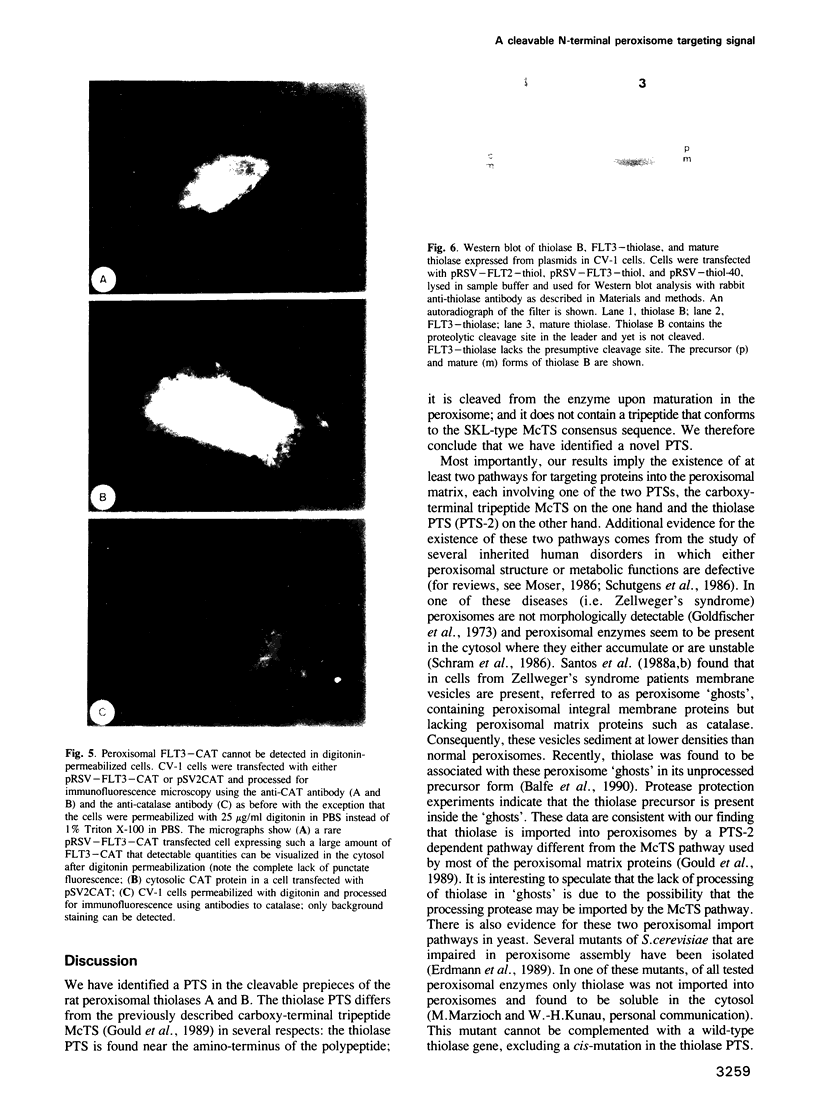
Several peroxisomal proteins do not contain the previously identified tripeptide peroxisomal targeting signal (PTS) at their carboxy-termini. One such protein is the peroxisomal 3-ketoacyl CoA thiolase, of which two types exist in rat [Hijikata et al. (1990) J. Biol. Chem., 265, 4600-4606]. Both rat peroxisomal thiolases are synthesized as larger precursors with an amino-terminal prepiece of either 36 (type A) or 26 (type B) amino acids, that is cleaved upon translocation of the enzyme into the peroxisome. The prepieces are necessary for import of the thiolases into peroxisomes because expression of an altered cDNA encoding only the mature thiolase, which lacks any prepiece, results in synthesis of a cytosolic enzyme. When appended to an otherwise cytosolic passenger protein, the bacterial chloramphenicol acetyltransferase (CAT), the prepieces direct the fusion proteins into peroxisomes, demonstrating that they encode sufficient information to act as peroxisomal targeting signals. Deletion analysis of the thiolase B prepiece shows that the first 11 amino acids are sufficient for peroxisomal targeting. We conclude that we have identified a novel PTS that functions at amino-terminal or internal locations and is distinct from the C-terminal PTS. These results imply the existence of two different routes for targeting proteins into the peroxisomal matrix.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa H., Takiguchi M., Amaya Y., Nagata S., Hayashi H., Mori M. cDNA-derived amino acid sequence of rat mitochondrial 3-oxoacyl-CoA thiolase with no transient presequence: structural relationship with peroxisomal isozyme. EMBO J. 1987 May;6(5):1361–1366. doi: 10.1002/j.1460-2075.1987.tb02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfe A., Hoefler G., Chen W. W., Watkins P. A. Aberrant subcellular localization of peroxisomal 3-ketoacyl-CoA thiolase in the Zellweger syndrome and rhizomelic chondrodysplasia punctata. Pediatr Res. 1990 Mar;27(3):304–310. doi: 10.1203/00006450-199003000-00023. [DOI] [PubMed] [Google Scholar]
- Bodnar A. G., Rachubinski R. A. Cloning and sequence determination of cDNA encoding a second rat liver peroxisomal 3-ketoacyl-CoA thiolase. Gene. 1990 Jul 16;91(2):193–199. doi: 10.1016/0378-1119(90)90088-9. [DOI] [PubMed] [Google Scholar]
- Borst P. How proteins get into microbodies (peroxisomes, glyoxysomes, glycosomes). Biochim Biophys Acta. 1986 May 5;866(4):179–203. doi: 10.1016/0167-4781(86)90044-8. [DOI] [PubMed] [Google Scholar]
- Bout A., Teunissen Y., Hashimoto T., Benne R., Tager J. M. Nucleotide sequence of human peroxisomal 3-oxoacyl-CoA thiolase. Nucleic Acids Res. 1988 Nov 11;16(21):10369–10369. doi: 10.1093/nar/16.21.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann R., Veenhuis M., Mertens D., Kunau W. H. Isolation of peroxisome-deficient mutants of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5419–5423. doi: 10.1073/pnas.86.14.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung K., Clayton C. Recognition of a peroxisomal tripeptide entry signal by the glycosomes of Trypanosoma brucei. Mol Biochem Parasitol. 1991 Apr;45(2):261–264. doi: 10.1016/0166-6851(91)90093-l. [DOI] [PubMed] [Google Scholar]
- Gietl C. Glyoxysomal malate dehydrogenase from watermelon is synthesized with an amino-terminal transit peptide. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5773–5777. doi: 10.1073/pnas.87.15.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfischer S., Moore C. L., Johnson A. B., Spiro A. J., Valsamis M. P., Wisniewski H. K., Ritch R. H., Norton W. T., Rapin I., Gartner L. M. Peroxisomal and mitochondrial defects in the cerebro-hepato-renal syndrome. Science. 1973 Oct 5;182(4107):62–64. doi: 10.1126/science.182.4107.62. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. G., Keller G. A., Subramani S. Identification of a peroxisomal targeting signal at the carboxy terminus of firefly luciferase. J Cell Biol. 1987 Dec;105(6 Pt 2):2923–2931. doi: 10.1083/jcb.105.6.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Hosken N., Wilkinson J., Subramani S. A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol. 1989 May;108(5):1657–1664. doi: 10.1083/jcb.108.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Schneider M., Howell S. H., Garrard L. J., Goodman J. M., Distel B., Tabak H., Subramani S. Peroxisomal protein import is conserved between yeast, plants, insects and mammals. EMBO J. 1990 Jan;9(1):85–90. doi: 10.1002/j.1460-2075.1990.tb08083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Subramani S. Identification of peroxisomal targeting signals located at the carboxy terminus of four peroxisomal proteins. J Cell Biol. 1988 Sep;107(3):897–905. doi: 10.1083/jcb.107.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Krisans S., Keller G. A., Subramani S. Antibodies directed against the peroxisomal targeting signal of firefly luciferase recognize multiple mammalian peroxisomal proteins. J Cell Biol. 1990 Jan;110(1):27–34. doi: 10.1083/jcb.110.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijikata M., Ishii N., Kagamiyama H., Osumi T., Hashimoto T. Structural analysis of cDNA for rat peroxisomal 3-ketoacyl-CoA thiolase. J Biol Chem. 1987 Jun 15;262(17):8151–8158. [PubMed] [Google Scholar]
- Hijikata M., Wen J. K., Osumi T., Hashimoto T. Rat peroxisomal 3-ketoacyl-CoA thiolase gene. Occurrence of two closely related but differentially regulated genes. J Biol Chem. 1990 Mar 15;265(8):4600–4606. [PubMed] [Google Scholar]
- Keller G. A., Gould S., Deluca M., Subramani S. Firefly luciferase is targeted to peroxisomes in mammalian cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3264–3268. doi: 10.1073/pnas.84.10.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S., Mori M., Takiguchi M., Tatibana M., Furuta S., Miyazawa S., Hashimoto T. Biosynthesis and intracellular transport of enzymes of peroxisomal beta-oxidation. J Biol Chem. 1984 May 25;259(10):6397–6402. [PubMed] [Google Scholar]
- Miyazawa S., Osumi T., Hashimoto T., Ohno K., Miura S., Fujiki Y. Peroxisome targeting signal of rat liver acyl-coenzyme A oxidase resides at the carboxy terminus. Mol Cell Biol. 1989 Jan;9(1):83–91. doi: 10.1128/mcb.9.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser H. W. Peroxisomal disorders. J Pediatr. 1986 Jan;108(1):89–91. doi: 10.1016/s0022-3476(86)80774-0. [DOI] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M. J., Imanaka T., Shio H., Lazarow P. B. Peroxisomal integral membrane proteins in control and Zellweger fibroblasts. J Biol Chem. 1988 Jul 25;263(21):10502–10509. [PubMed] [Google Scholar]
- Santos M. J., Imanaka T., Shio H., Small G. M., Lazarow P. B. Peroxisomal membrane ghosts in Zellweger syndrome--aberrant organelle assembly. Science. 1988 Mar 25;239(4847):1536–1538. doi: 10.1126/science.3281254. [DOI] [PubMed] [Google Scholar]
- Schram A. W., Strijland A., Hashimoto T., Wanders R. J., Schutgens R. B., van den Bosch H., Tager J. M. Biosynthesis and maturation of peroxisomal beta-oxidation enzymes in fibroblasts in relation to the Zellweger syndrome and infantile Refsum disease. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6156–6158. doi: 10.1073/pnas.83.16.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutgens R. B., Heymans H. S., Wanders R. J., van den Bosch H., Tager J. M. Peroxisomal disorders: a newly recognised group of genetic diseases. Eur J Pediatr. 1986 Feb;144(5):430–440. doi: 10.1007/BF00441734. [DOI] [PubMed] [Google Scholar]
- Sugden B., Marsh K., Yates J. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol Cell Biol. 1985 Feb;5(2):410–413. doi: 10.1128/mcb.5.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner K., Schatz G. Protein translocation across membranes. Science. 1988 Sep 9;241(4871):1307–1313. doi: 10.1126/science.2842866. [DOI] [PubMed] [Google Scholar]
- Wolvetang E. J., Tager J. M., Wanders R. J. Latency of the peroxisomal enzyme acyl-CoA:dihydroxyacetonephosphate acyltransferase in digitonin-permeabilized fibroblasts: the effect of ATP and ATPase inhibitors. Biochem Biophys Res Commun. 1990 Aug 16;170(3):1135–1143. doi: 10.1016/0006-291x(90)90511-k. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Nishikawa K. Chloroplast transit peptides. The perfect random coil? FEBS Lett. 1991 Jan 14;278(1):1–3. doi: 10.1016/0014-5793(91)80069-f. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Steppuhn J., Herrmann R. G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989 Apr 1;180(3):535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. The signal peptide. J Membr Biol. 1990 May;115(3):195–201. doi: 10.1007/BF01868635. [DOI] [PubMed] [Google Scholar]