Abstract
Background:
The clinical significance of diffusion tensor (DT) magnetic resonance imaging (MRI) parameters was analyzed to predict postoperative functional recovery in patients with cervical myelopathy.
Materials and Methods:
Sixteen patients with cervical myelopathy caused by cervical spondylosis, disk herniation or ossification of the posterior longitudinal ligament who underwent surgical intervention in our institute were enrolled in this retrospective study. There were 7 men and 9 women, with a mean age of 62.8 years. Clinical assessment was done before surgery and at least 3 months after surgery. All patients underwent whole-body 3.0-Tesla MRI before surgery. DT images (DTIs) were obtained using a single-shot fast spin-echo-based sequence. Mean values of mean diffusivity (MD) and fractional anisotropy (FA) at 6 disk levels of the cervical spine were measured using manual setting of regions of interest. The MD and FA values at the most compressed part were analyzed. Absolute MD and FA values at the most compressed spinal level in patients were transformed into the normalized values with a z-score analysis.
Results:
MD-z may decrease with the severity of cervical myelopathy. Receiver operating characteristic analysis of MD-z and FA-z suggested that both MD-z and FA-z have clinical validity for predicting the efficacy of surgical intervention, but MD-z was considered to be the most appropriate value to predict the efficacy of surgery.
Conclusions:
DTIs may be a promising modality to predict functional recovery after surgery. MD changes may reflect spinal cord condition and its reversibility.
Keywords: Cervical myelopathy, diffusion tensor parameters, fractional anisotropy, functional recovery, mean diffusivity
INTRODUCTION
Magnetic resonance imaging (MRI) has become clinically important for evaluating spinal cord condition. The spinal cord can be well visualized on T1- and T2-weighted images, as well as other MR sequences. T2-weighted images are considered to reflect damage to the spinal cord, which can be expressed in the form of an intramedullary high signal. [1] High signal change on T2-weighted images does not fully explain spinal cord condition, and it is still controversial in terms of whether these changes reflect reversible damage. After diffusion tensor image (DTI) techniques were developed, [2] mean diffusivity (MD) and fractional anisotropy (FA) were highlighted as being useful for assessing spinal cord condition more concisely in the form of an objective index. Our previous study examined 26 patients with cervical spondylosis and 30 normal subjects using 3.0-Tesla DTI techniques and demonstrated that MD and FA were significantly different between spinal levels, and MD increase or FA decrease was detected in most patients. [3,4] Furthermore, the presence of cervical myelopathy can be determined with high accuracy with DTI parameters, especially with MD z-score analysis at the most compressed spinal level. [4] This study focused on the clinical significance of DTI parameters to predict postoperative functional recovery in patients with cervical myelopathy.
MATERIALS AND METHODS
Patients
Sixteen patients with cervical myelopathy caused by cervical spondylosis, disk herniation or ossification of the posterior longitudinal ligament who underwent surgical intervention in our institute were enrolled in this retrospective study. The patients who showed only radiculopathy, had undergone other surgery of the cervical spine before the present analysis or showed neurological manifestations after trauma were excluded. There were 7 men and 9 women, with a mean age of 62.8 years (range 41–82 years). The surgical method was classified into anterior cervical discectomy and fusion in 10 patients, posterior decompression with or without cervical laminoplasty in 5 patients, and anterior and posterior combined surgery in 1 patient. Although the definition of cervical myelopathy was not straightforward, the clinical diagnosis of cervical myelopathy was defined as significant symptoms with long tract signs. Board-certified neurosurgeons (T.T., K.N., T.Y.) examined all patients before and after surgery and diagnosed the presence of clinical myelopathy. Clinical assessment was done using the Neurosurgical Cervical Spine Scale (NCSS). [5] The NCSS evaluation was performed before surgery and at least 3 months after surgery. The percentage of the recovery rate (RR) was calculated using the following formula: (Postoperative score − preoperative score)/(14 − preoperative score) × 100.
Magnetic resonance imaging protocol
All patients in this study underwent whole-body 3.0-Tesla MRI (Achieva; Philips Medical Systems, Best, The Netherlands) using a 16-element phased-array coil before surgery. For anatomical and diagnostic imaging of the spine, T1-weighted (echo time [TE]/repetition time [TR], 7/600 ms) and T2-weighted (TE/TR, 90/3680 ms) images were acquired in the sagittal and axial planes. Then, DTIs were obtained using a single-shot fast spin-echo-based sequence with the following parameters: TE/TR, 80/6000 ms; number of excitations, 1; field of view, 240 mm2; matrix size, 160; voxel size, 1.5 × 1.5 mm2 in-plane; slice thickness, 3 mm; gradient directions, 15; and b values, 0 and 1000 s/mm2. [6,7] Thirty DTI slices in the axial plane were obtained from the C2/3 to C7/Th1 spinal levels without interslice gaps, parallel to the inferior line of the C5 vertebral body on the T2-weighted midsagittal plane. DTIs were acquired in a total of 4 min and 54 s.
Analysis and evaluation
For the reconstruction of the MD and FA maps from the DTIs, a Philips MRI workstation was used. The mean values of MD and FA at six-disk levels of the cervical spine (C2/3, C3/4, C4/5, C5/6, C6/7, and C7/Th1) were measured. After the appropriate axial slice was selected using the sagittal T2-weighted images for anatomic reference, regions of interest (ROIs) were manually set to enclose the whole spinal cord in the slice. ROIs were drawn carefully to exclude cerebrospinal fluid, which would contribute an unwanted partial volume effect to the DT results. The MD and FA values at the most compressed part were analyzed [Figure 1]. Absolute MD and FA values at the most compressed spinal level in patients were transformed into normalized values with a z-score ([patient's value − mean value in normal database]/standard deviation [SD] in normal database), as previously shown.[3,4] The mean value and SD of normal subjects in our previous study were used in the present study. The MD, z-score of MD (MD-z), FA, and z-score-FA (FA-z) values were used for statistical analysis. The first author was not aware of the clinical status of each patient before analysis and determined the ROI setting at the maximally compressed level.
Figure 1.
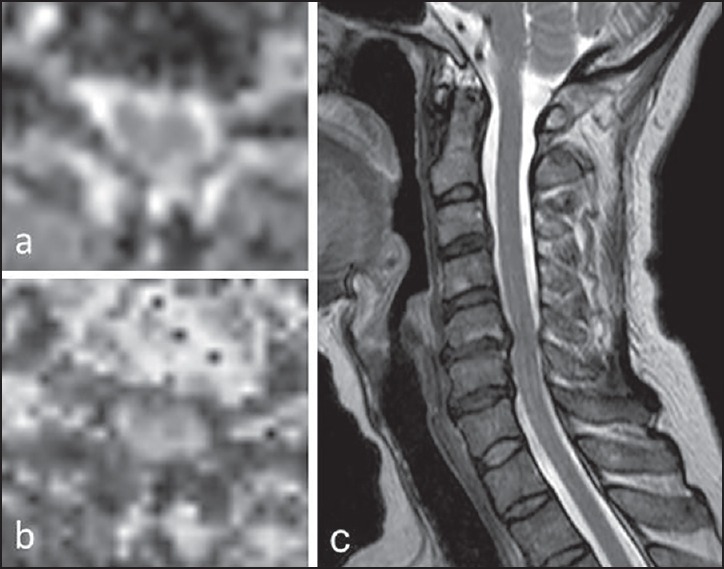
The mean diffusivity (a) and fractional anisotropy (b) scores at the most compressed part are measured using manual setting of regions of interest. The appropriate axial slice is selected using the sagittal T2-weighted images for anatomic reference (c)
Statistical analysis
Statistical analysis was conducted using JMP 9.0 (SAS Institute, Inc., Cary, NC, USA). The Spearman correlation test was used to determine the statistical relationships between the preoperative values of DTI parameters and functional status or recovery estimated using the NCSS. Significant differences were accepted at P < 0.05 in all analyses. The diagnostic validities of MD and FA values were analyzed using receiver operating characteristic (ROC) analysis. The optimal cut-off value for the prediction was determined to be the value with the maximum Youden index.
Statement of ethics
The authors certify that all applicable institutional and governmental regulations concerning the ethical use of clinical data were followed in the present study. This comprehensive analysis of surgery-related outcomes was approved by the ethics committee of Osaka City University Graduate School of Medicine.
RESULTS
Clinical correlations between diffusion tensor image parameters and preoperative functional status
There was a significant correlation between MD-z and preoperative NCSS scores, whereas there was no significant correlation between FA-z and preoperative NCSS scores [Figure 2]. A higher MD-z correlated well with higher NCSS scores, suggesting that MD-z may decrease in accordance with the severity of cervical myelopathy.
Figure 2.
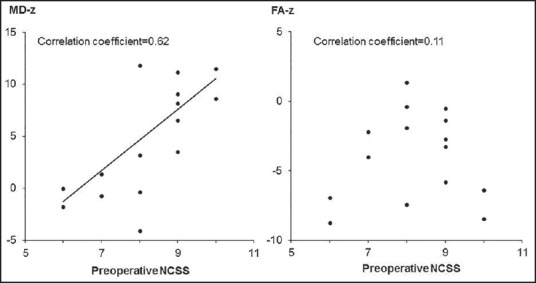
Clinical correlations between diffusion tensor image parameters and preoperative neurosurgical cervical spine scale (NCSS) scores. Higher mean diffusivity-z correlates well with higher NCSS scores (correlation coefficient = 0.62, P = 0.01), but the correlation between fractional anisotropy-z and preoperative NCSS scores is not significant (correlation coefficient = 0.11, P = 0.68)
Clinical correlations between diffusion tensor image parameters and functional recovery
There was a significant correlation between MD-z and the RR, whereas there was no significant correlation between FA-z and the RR [Figure 3]. A higher MD-z correlated well with a higher percent RR, suggesting that a higher MD-z may predict possible functional recovery after surgery in patients with cervical myelopathy.
Figure 3.
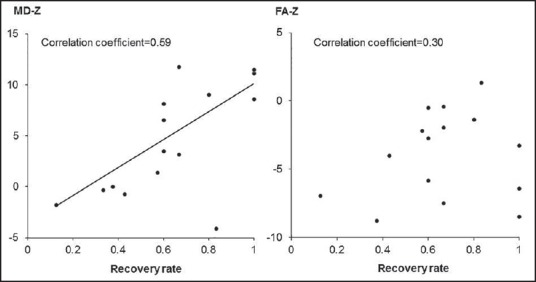
Clinical correlations between diffusion tensor image parameters and postoperative functional recovery. Higher mean diffusivity-z correlates well with higher percent recovery rate (RR) (correlation coefficient = 0.59, P = 0.015), but the correlation between fractional anisotropy-z and RR is not significant (correlation coefficient = 0.30, P = 0.26)
Receiver operating characteristic analyses
Receiver operating characteristic analyses of MD-z and FA-z are shown in Figure 4. Both MD-z and FA-z have clinical validity for predicting the efficacy of the surgical intervention. The areas under the curves of MD-z and FA-z were 0.91 and 0.78, respectively. MD-z was considered to be the most appropriate value to predict the efficacy of surgery. The optimal cut-off value of MD-z was 3.2, with a sensitivity of 91%, and specificity of 100%. The predictive probability of a positive test was 100% (10 of 10), and the predictive probability of a negative test was 83% (5 of 6).
Figure 4.
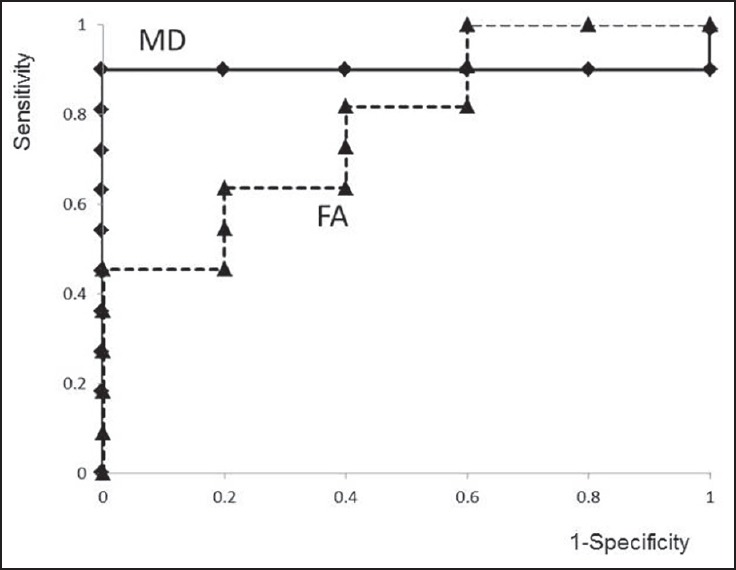
Receiver operating characteristic curves of mean diffusivity (MD) and fractional anisotropy (FA). The areas under the curves of MD-z and FA-z are 0.91 and 0.78, respectively. The optimal cut-off value of MD-z is 3.2, with a sensitivity of 91%, and specificity of 100%. The predictive probability of a positive test is 100% (10 of 10), and the predictive probability of a negative test is 83% (5 of 6)
DISCUSSION
Assessment of spinal cord condition in cervical myelopathy using magnetic resonance imaging
Although spinal cord compression is well visualized on MRI, its correlation with clinical symptoms is still controversial. As the DTI technique has developed, movement of water molecules can be well measured by DTI parameters, such as MD and FA values. MD represents the degree of diffusional motion of water molecules and FA represents the direction of diffusivity. [2] These values have been found to have the capability to analyze the spinal cord condition in greater detail than conventional MRI. Recently, several clinical studies focusing on MD or FA values in patients with cervical myelopathy have been reported. It has been suggested that acute spinal cord compression may result in a focal decrease in MD, as well as a focal increase in FA. [6,7,8] Ford and Hackney assumed that spinal cord axons are infinite cylinders and suggested that compressed axon fibers result in a decrease in transverse MD, [9,10] which may lead to a slight increase in FA. On the other hand, clinical studies have clearly documented that progressive and chronic compression of the spinal cord results in increased MD and decreased FA. [4,8,11,12,13,14,15,16] Kerkovský et al. demonstrated that significant differences in DTI parameters measured at the maximal compression level of the cervical spine were found between patients with spondylotic spinal cord compression and healthy volunteers. [12] These changes are likely to be the result of chronic ischemic damage to the spinal cord, which causes histopathological changes such as gliosis, edema, necrosis, and cavitation. [17,18,19,20] Actually, an animal model of chronic compression demonstrated increased MD and decreased FA 9 months after the occurrence of compression. [21] However, MD and FA values in normal subjects are still not fully understood. In our previous study, we found significant differences in MD and FA at each spinal level in normal subjects; we also reported that a significant negative correlation was observed between aging and MD. [3] In a further analysis of patients with cervical myelopathy, we reported that cervical myelopathy can be well predicted with high accuracy using a DTI parameter, with an MD z-score analysis at the most compressed spinal level. [4] In the present study, there was a significant correlation between MD-z and preoperative ADL, whereas there was no significant correlation between FA-z and preoperative ADL. MD-z may decrease in accordance with the severity of cervical myelopathy. It can be presumed that MD values may become higher in the early stage of cervical myelopathy, so that patients develop slight or mild symptoms, but the MD value may decrease in accordance with progression of cervical myelopathy. Although reversibility of spinal cord dysfunction has not been determined, DTI technique may be helpful in its determination.
Diffusion tensor image may be a promising modality to predict functional recovery after surgery
Although a close relationship between clinical symptoms and DTI parameters in patients with cervical myelopathy has been strongly suggested, whether it can be used to predict functional recovery after surgery has not been determined. Nakamura et al. analyzed the fiber tract ratio in patients with cervical myelopathy using DTI technique, and they found that the preoperative fiber tract ratio based on FA values correlated significantly with the RRs. [22] Jones et al. reported that preoperative FA at the level of stenosis correlated with improvement in the neck disability index (NDI) after surgery. [23] Wen et al. also reported that FA at the C2 level correlated with improvement of the modified Japanese Orthopedic Association score. [24] In the present study, there was a significant correlation between MD-z and the postoperative functional RR, whereas there was no significant correlation between FA-z and the postoperative functional RR. Higher MD-z may predict possible functional recovery after surgery in cervical myelopathy. Although ROC analysis of MD-z and FA-z suggested that both MD-z and FA-z have clinical validity for predicting the efficacy of surgical intervention, MD-z was considered to be the most appropriate value for predicting the efficacy of surgery. MD changes may reflect spinal cord condition and its reversibility.
LIMITATIONS OF THE PRESENT STUDY
There are several limitations in the present study. First, the number of patients was small, and further prospective analysis needs to be done with a larger number of patients to confirm its reproducibility. Second, analysis of DTI parameters was based on the careful setting of ROIs that were placed manually. Resolution and accuracy of the image analysis should be further explored. Third, the clinical relationship between MD and FA is still unclear. Comprehensive evaluation using DTI parameters would be more desirable. Finally, the z-score analysis was based on the values in normal subjects. A larger database of DTI parameters in normal subjects is very important to perform a more accurate analysis.
CONCLUSIONS
Diffusion tensor parameters like MD or FA appeared to be promising for evaluating the spinal condition. MD-z may decrease in accordance with the severity of cervical myelopathy. ROC analysis of MD-z and FA-z suggested that both MD-z and FA-z have clinical validity for predicting the efficacy of surgical intervention, but MD-z was considered to be the most appropriate value to predict the efficacy of surgery. MD changes may reflect spinal cord condition and its reversibility.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Eiji W, Munehisa O, Kazuo Y. Intramedullary changes of the spinal cord in cervical spondylotic myelopathy. Spine (Phila Pa 1976) 1995;20:2226–32. doi: 10.1097/00007632-199510001-00009. [DOI] [PubMed] [Google Scholar]
- 2.Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, et al. Diffusion tensor imaging: Concepts and applications. J Magn Reson Imaging. 2001;13:534–46. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 3.Uda T, Takami T, Sakamoto S, Tsuyuguchi N, Yamagata T, Ohata K. Normal variation of diffusion tensor parameters of the spinal cord in healthy subjects at 3.0-Tesla. J Craniovertebr Junction Spine. 2011;2:77–81. doi: 10.4103/0974-8237.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uda T, Takami T, Tsuyuguchi N, Sakamoto S, Yamagata T, Ikeda H, et al. Assessment of cervical spondylotic myelopathy using diffusion tensor magnetic resonance imaging parameter at 3.0 tesla. Spine (Phila Pa 1976) 2013;38:407–14. doi: 10.1097/BRS.0b013e31826f25a3. [DOI] [PubMed] [Google Scholar]
- 5.Kadoya S. Grading and scoring system for neurological function in degenerative cervical spine disease - Neurosurgical Cervical Spine Scale. Neurol Med Chir (Tokyo) 1992;32:40–1. doi: 10.2176/nmc.32.40. [DOI] [PubMed] [Google Scholar]
- 6.Facon D, Ozanne A, Fillard P, Lepeintre JF, Tournoux-Facon C, Ducreux D. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol. 2005;26:1587–94. [PMC free article] [PubMed] [Google Scholar]
- 7.Nilsson M, Lätt J, Ståhlberg F, van Westen D, Hagslätt H. The importance of axonal undulation in diffusion MR measurements: A Monte Carlo simulation study. NMR Biomed. 2012;25:795–805. doi: 10.1002/nbm.1795. [DOI] [PubMed] [Google Scholar]
- 8.Mamata H, Jolesz FA, Maier SE. Apparent diffusion coefficient and fractional anisotropy in spinal cord: Age and cervical spondylosis-related changes. J Magn Reson Imaging. 2005;22:38–43. doi: 10.1002/jmri.20357. [DOI] [PubMed] [Google Scholar]
- 9.Ford JC, Hackney DB. Numerical model for calculation of apparent diffusion coefficients (ADC) in permeable cylinders - comparison with measured ADC in spinal cord white matter. Magn Reson Med. 1997;37:387–94. doi: 10.1002/mrm.1910370315. [DOI] [PubMed] [Google Scholar]
- 10.Ford JC, Hackney DB, Lavi E, Phillips M, Patel U. Dependence of apparent diffusion coefficients on axonal spacing, membrane permeability, and diffusion time in spinal cord white matter. J Magn Reson Imaging. 1998;8:775–82. doi: 10.1002/jmri.1880080405. [DOI] [PubMed] [Google Scholar]
- 11.Kara B, Celik A, Karadereler S, Ulusoy L, Ganiyusufoglu K, Onat L, et al. The role of DTI in early detection of cervical spondylotic myelopathy: A preliminary study with 3-T MRI. Neuroradiology. 2011;53:609–16. doi: 10.1007/s00234-011-0844-4. [DOI] [PubMed] [Google Scholar]
- 12.Kerkovský M, Bednarík J, Dušek L, Sprláková-Puková A, Urbánek I, Mechl M, et al. Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: Correlations between clinical and electrophysiological findings. Spine (Phila Pa 1976) 2012;37:48–56. doi: 10.1097/BRS.0b013e31820e6c35. [DOI] [PubMed] [Google Scholar]
- 13.Budzik JF, Balbi V, Le Thuc V, Duhamel A, Assaker R, Cotten A. Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur Radiol. 2011;21:426–33. doi: 10.1007/s00330-010-1927-z. [DOI] [PubMed] [Google Scholar]
- 14.Ellingson BM, Ulmer JL, Kurpad SN, Schmit BD. Diffusion tensor MR imaging in chronic spinal cord injury. AJNR Am J Neuroradiol. 2008;29:1976–82. doi: 10.3174/ajnr.A1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JW, Kim JH, Park JB, Park KW, Yeom JS, Lee GY, et al. Diffusion tensor imaging and fiber tractography in cervical compressive myelopathy: Preliminary results. Skeletal Radiol. 2011;40:1543–51. doi: 10.1007/s00256-011-1161-z. [DOI] [PubMed] [Google Scholar]
- 16.Rajasekaran S, Yerramshetty JS, Chittode VS, Kanna RM, Balamurali G, Shetty AP. The assessment of neuronal status in normal and cervical spondylotic myelopathy using diffusion tensor imaging. Spine (Phila Pa 1976) 2014;39:1183–9. doi: 10.1097/BRS.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 17.Hukuda S, Wilson CB. Experimental cervical myelopathy: Effects of compression and ischemia on the canine cervical cord. J Neurosurg. 1972;37:631–52. doi: 10.3171/jns.1972.37.6.0631. [DOI] [PubMed] [Google Scholar]
- 18.Harkey HL, al-Mefty O, Marawi I, Peeler DF, Haines DE, Alexander LF. Experimental chronic compressive cervical myelopathy: Effects of decompression. J Neurosurg. 1995;83:336–41. doi: 10.3171/jns.1995.83.2.0336. [DOI] [PubMed] [Google Scholar]
- 19.DeGirolami U, Zivin JA. Neuropathology of experimental spinal cord ischemia in the rabbit. J Neuropathol Exp Neurol. 1982;41:129–49. doi: 10.1097/00005072-198203000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Mair WG, Druckman R. The pathology of spinal cord lesions and their relation to the clinical features in protrusion of cervical intervertebral discs; a report of four cases. Brain. 1953;76:70–91. doi: 10.1093/brain/76.1.70. [DOI] [PubMed] [Google Scholar]
- 21.Cheung MM, Li DT, Hui ES, Fan S, Ding AY, Hu Y, et al. In vivo diffusion tensor imaging of chronic spinal cord compression in rat model. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:2715–8. doi: 10.1109/IEMBS.2009.5333389. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura M, Fujiyoshi K, Tsuji O, Konomi T, Hosogane N, Watanabe K, et al. Clinical significance of diffusion tensor tractography as a predictor of functional recovery after laminoplasty in patients with cervical compressive myelopathy. J Neurosurg Spine. 2012;17:147–52. doi: 10.3171/2012.5.SPINE1196. [DOI] [PubMed] [Google Scholar]
- 23.Jones JG, Cen SY, Lebel RM, Hsieh PC, Law M. Diffusion tensor imaging correlates with the clinical assessment of disease severity in cervical spondylotic myelopathy and predicts outcome following surgery. AJNR Am J Neuroradiol. 2013;34:471–8. doi: 10.3174/ajnr.A3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen CY, Cui JL, Liu HS, Mak KC. Is diffusion anisotropy a biomarker for disease severity and surgical prognosis of cervical spondylotic myelopathy? Neuroradiology. 2014;270:197–204. doi: 10.1148/radiol.13121885. [DOI] [PubMed] [Google Scholar]


