Abstract
Objective
Normal pressure hydrocephalus is characterized by gait impairment, cognitive impairment, and urinary incontinence, and is associated with disproportionate ventricular dilation. Here we report the distribution of ventricular volume relative to sulcal cerebrospinal fluid (CSF) volume, and the association of increasing ventricular volume relative to sulcal CSF volume with a cluster of gait impairment, cognitive impairment, and urinary incontinence in a stroke-free cohort of elderly persons from the general population.
Methods
Data are based on 858 persons (35.4% men; age range, 66–92 years) who participated in the Age, Gene/Environment Susceptibility–Reykjavik Study. Gait was evaluated with an assessment of gait speed. Composite scores representing speed of processing, memory, and executive function were constructed from a neuropsychological battery. Bladder function was assessed with a questionnaire. Magnetic resonance brain imaging was followed by semiautomated segmentation of intracranial CSF volume. White matter hyperintensity (WMH) volume was assessed with a semiquantitative scale. For the analysis of ventricular dilation relative to the sulcal spaces, ventricular volume was divided by sulcal CSF volume (VV/SV).
Results
Disproportion between ventricular and sulcal CSF volume, defined as the highest quartile of the VV/SV z score, was associated with gait impairment (odds ratio [OR], 1.9; 95% confidence interval [CI], 1.1–3.3) and cognitive impairment (OR, 1.8; 95% CI, 1.1–3.0). We did not find an association between the VV/SV z score and bladder dysfunction.
Interpretation
The prevalence and severity of gait impairment and cognitive impairment increases with ventricular dilation in persons without stroke from the general population, independent of WMH volume.
Cerebral atrophy is a pathologic diagnosis indicating an irreversible loss of brain substance.1,2 It appears as progressive dilation of the ventricles and cortical sulci on magnetic resonance imaging (MRI).1 Global cerebral atrophy is often classified into subcortical atrophy, reflecting ventricular dilation, and cortical atrophy, reflecting the dilation of cortical sulci.3
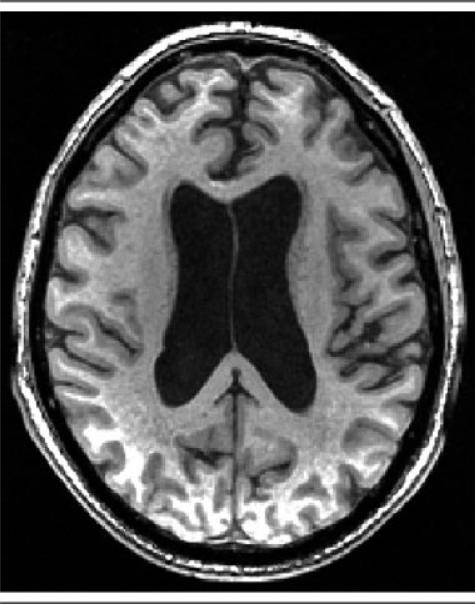
Subcortical atrophy and cortical atrophy may not be in proportion with each other (Fig 1). When the amount of subcortical atrophy corresponds with the amount of cortical atrophy, this may be indicative of global cerebral atrophy, as seen with increasing age. However, when a disproportion between ventricular dilation and the dilation of sulcal cerebrospinal fluid (CSF) volume is noted (Fig 1), normal pressure hydrocephalus (NPH) may be suggested, when these MRI findings are associated with gait or cognitive impairment, or urinary urgency or incontinence.4–7 Suspicion of NPH is increased when gait imbalance predominates and when cognitive deficit is only slight, moderate, or even absent.8 When dementia is the most severe symptom, the probability of NPH is very low.9,10 Bladder dysfunction occurs only at later stages of NPH and is present in approximately 55% of patients with NPH.9,10 Thus, based on previous work, the complete triad can be observed in nearly half of NPH patients.9
Fig 1.
In this example, the volume of the ventricles is out of proportion to the volume of the sulcal spaces.
The different clinical components of the NPH triad are each highly prevalent in older persons. Gait disorders affect 20 to 50% of elderly persons.11,12 Prevalence studies of dementing illnesses suggest an overall prevalence rate of about 6 to 8% among individuals aged >65 years and a prevalence rate of >30% among individuals aged >85 years.13,14 The prevalence of at least some degree of bladder dysfunction is estimated at 11 to 31% of men aged ≥60 years15 and at 30 to 50% of elderly women.16 It is not known to what extent gait impairment, cognitive impairment, and bladder dysfunction cluster together in the general population of older adults. Furthermore, patients with white matter hyperintensities (WMHs) or subcortical arteriosclerotic encephalopathy often present with an enlarged ventricular system and symptoms and signs similar to those seen in NPH.17–19
Here we report on the prevalence of clusters of gait impairment, cognitive impairment, and bladder dysfunction and their association with ventricular dilation, independent of WMH volume.
Materials and Methods
Persons participating in this study were a sample of the Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study; a population-based study initiated to examine the contribution of genetic susceptibility and gene/environment interaction to conditions common in old age. The AGES-Reykjavik Study is an extension of the Reykjavik Study (1967–1994), a prospective study of cardiovascular disease based on a cohort of men and women born 1907–1935 and living in Reykjavik at the time of baseline measurement in 1967.20,21 All participants in the AGES-Reykjavik Study underwent extensive evaluation, including MRI of the brain, neuropsychological testing, physical performance, a standard clinical evaluation, and an in-person questionnaire. This report is based on those participants examined from September 2002 until March 2004 (n = 2,300). The protocol was approved by the Icelandic National Bioethics Committee (VSN 00-063), the Icelandic Data Protection Authority, and by the institutional review board of the US National Institutes of Health.22
Measurement of Gait
Gait was evaluated as the time in seconds needed to walk 6m. There were 2 measurements at usual pace and separately 2 at quick pace.23–25 The 2 measurements for usual pace and quick pace were averaged separately for 1 estimate of normal gait and 1 for fast gait. Since there are no standardized cut-points for impaired gait speed in the 6m walk test, the upper quartile in either normal or fast gait was defined as gait impairment, similar to previous studies.26,27
Measurement of Cognitive Function
Composite scores of executive function, memory, and speed of processing were constructed from a battery of neuropsychological tests. The fit of these theoretical composites has been described in a previous publication.28 The executive function composite consisted of the Digits Backward,29 the Cambridge Neuropsychological Test Automated Battery (CANTAB) spatial working memory task,30 and the Stroop Test Part III.31 The memory composite consisted of the California Verbal Learning Test immediate and delayed recall.32 The speed of processing composite consisted of the Digit Symbol Substitution Test (DSST),29 Figure Comparison,33 and the Stroop Test Parts I and II.31 All tests were normally distributed, thus composite measures were computed by averaging z scores. A diagnosis of dementia—established in a multidisciplinary consensus meeting—was used to control for the possibility that cognitive impairment was partly due to comorbidities, such as Alzheimer disease.
Measurement of Bladder Function
Bladder function was assessed with a standard questionnaire. Dysfunction, when present, was categorized into 1) urinary incontinence associated with an activity like coughing, lifting, standing up, or exercise; 2) urge incontinence where the subject cannot get to the toilet fast enough; and 3) urinary incontinence unrelated to coughing, sneezing, lifting, or urge. NPH type bladder dysfunction was defined as positive answers to questions 2 or 3, corresponding with descriptions in literature.4,10,34
MRI Acquisition and Postprocessing
The current analyses required dual fast spin-echo (proton density [PD] and T2-weighted) and fluid-attenuated inversion recovery (FLAIR) images, which were acquired at a field strength of 1.5T (GE Medical Systems, Milwaukee, WI). The PD- and T2-weighted scans were performed with a field of view of 220mm, a 256 × 256 matrix size, section thickness of 3.0mm, and no slice gap. The FLAIR scans were performed with a field of view of 220mm, a 256 × 256 matrix size, section thickness of 3.0mm, and no slice gap.
Cerebral infarcts were defined as lesions ≥4mm in the maximum diameter over a vascular distribution with typical MRI characteristics (eg, a signal intensity that was isointense to that of cerebrospinal fluid), and that were distinguished from WMHs. Cerebellar infarcts had no size criteria, because lesions in this area can be very small. A neuroradiologist first examined the images for presence of cortical, subcortical, and cerebellar infarcts. Then, radiographers characterized the infarcts in more detail. WMH volume was assessed with a semiquantitative scale with known reliability and validity.35
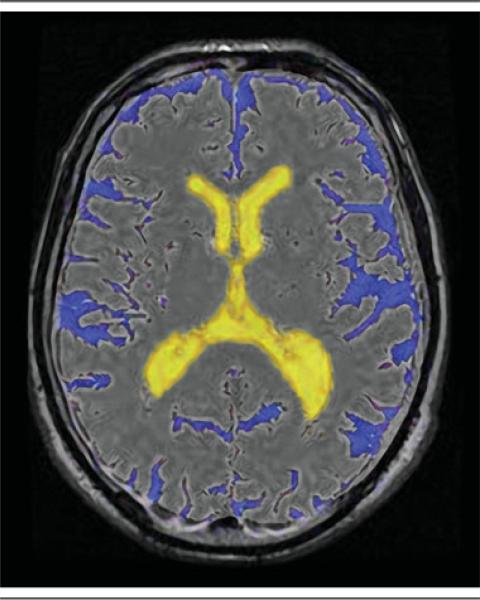
To analyze the scans, we used previously described automated segmentation software (Software for Neuro-Image Processing in Experimental Research [SNIPER], Leiden University Medical Center, Leiden, The Netherlands) that combines knowledge-based fuzzy clustering and region-growing techniques.35 The dual fast spin-echo and FLAIR images were coregistered using Oxford Centre for Functional MRI of the Brain's Linear Imaging Regression Tool36 prior to processing by SNIPER. Brain extraction was followed by an automated segmentation procedure that assigned CSF within the cranium. CSF belonging to the lateral and third ventricles was manually labeled as ventricular volume by an experienced reader (W.M.P.). The volumetric assessment was repeated for 43 of 834 persons (5%) to analyze the intrarater reliability in our sample. The assessment yielded an intraclass correlation coefficient >0.99, indicating high intrarater reliability. The program estimated ventricular volume (VV), sulcal CSF volume (SV), total brain volume (TBV), and total intracranial volume (TICV). To express ventricular dilation relative to the volume of the sulcal spaces, VV was divided by SV (Fig 2). TBV/TICV was used as a measure of corrected brain volume.
Fig 2.
Intracranial semiautomated segmentation was based on dual spin-echo (proton attenuation and T2-weighted) and fluid-attenuated inversion recovery images. Using Software for Neuro-Image Processing in Experimental Research, we estimated ventricular volume, sulcal cerebrospinal fluid volume, total brain volume (TBV), and total intracranial volume (TICV). TBV/TICV was used as a measure of corrected brain volume.
Covariates
Based on previous studies, we adjusted for a number of potentially confounding demographic and health history factors. Education (primary, secondary, college, and university), and smoking status (categorized in this analysis as current or previous smoker versus nonsmoker) were assessed with a questionnaire.37,38 The presence of depressive symptoms was assessed using the 15-item, shortened version of the Geriatric Depression Scale-Shortened39; a score of ≥6 was classified as high depressive symptomatology.40 We adjusted for cardiovascular risk factors and disease, as they are reported to be associated with gait and cognition.41,42 Systolic and diastolic blood pressures, measured in supine position, were defined as the first and fifth Korotkoff sounds, respectively. History of hypertension was defined as the use of antihypertensive medication, self-reported physician's diagnosis of hypertension, or a systolic blood pressure ≥140mmHg or a diastolic blood pressure ≥90mmHg. History of coronary heart disease and peripheral arterial disease (intermittent claudication) were assessed with a questionnaire. Diabetes was defined as a self-reported doctor's diagnosis of diabetes, the use of diabetic medications (glucose lowering medications and insulin), which was noted from medication vials brought to the clinic, or a fasting blood glucose level ≥7.0mmol/l (equivalent to a fasting blood glucose level of 126mg/dl, defined as diabetes by the American Diabetes Association). Body mass index (BMI) was calculated from measured height and weight.
Analytical Sample
Of the first 2,300 participants, 435 were excluded because no or incomplete MRI images were acquired. The reasons for this were: participation only in a home visit, contraindications for MRI (such as a pacemaker, ocular foreign body, or artificial heart valve), claustrophobia, or equipment failure. Of the remaining 1,865 participants, 697 persons were excluded because of the presence of infarcts (parenchymal defects ≥4mm, including Virchow-Robin spaces, and cerebellar infarcts), acute hematomas, or mass occupying lesions. The exclusion for the presence of infarcts was based on 2 reasons. Infarcts may be associated with gait or cognitive impairment as well as bladder dysfunction. Furthermore, infarcts cause an overestimation of SV. An additional 310 persons were excluded due to failed MRI pre- and postprocessing with the SNIPER program, leaving a final sample of 858 persons with complete MRI postprocessed data. Compared with those with complete MRI postprocessing data, the group of excluded persons was older and contained a higher percentage of men and persons with coronary heart disease, diabetes mellitus, and cognitive impairment (in either executive function, memory, or speed of processing), all significantly different at p < 0.05, adjusted for age and sex. There was no significant difference in education, smoking status, BMI, depressive symptomatology, hypertension, bladder dysfunction, or gait speed between the included and excluded persons.
Statistical Analysis
The higher the value of VV/SV, the more the disproportion between ventricular and sulcal CSF volume. The VV/SV was transformed into standard deviation units (the VV/SV z score) and divided into quartiles; the first quartile of the VV/SV z score, that is, the group with the least disproportion, served as the reference group. To discriminate persons with a low performance in the gait test, persons were divided into quartiles of the gait variables. The upper quartile in either normal or fast gait was defined as gait impairment. The lowest quartile of each of the cognitive composites was classified as impairment in that ability. Impairment in executive function, memory, or speed of processing was classified as cognitive impairment. Bladder dysfunction was classified as urinary urgency or incontinence unrelated to coughing, sneezing, lifting, or urge.
We used logistic regressions to examine the association of the VV/SV z score with gait impairment, cognitive impairment, and bladder dysfunction. We also examined the association of combinations of overall gait impairment, cognitive impairment, and bladder dysfunction with quartiles of the VV/SV z score. Two models were tested; the first model was adjusted for age and sex; the second model had additional adjustments for education, smoking status, BMI, depressive symptomatology, coronary heart disease, hypertension, diabetes mellitus, peripheral arterial disease, white matter hyperintensity volume, and corrected brain volume (SPSS, version 11.5; SPSS Inc., Chicago, IL).
Results
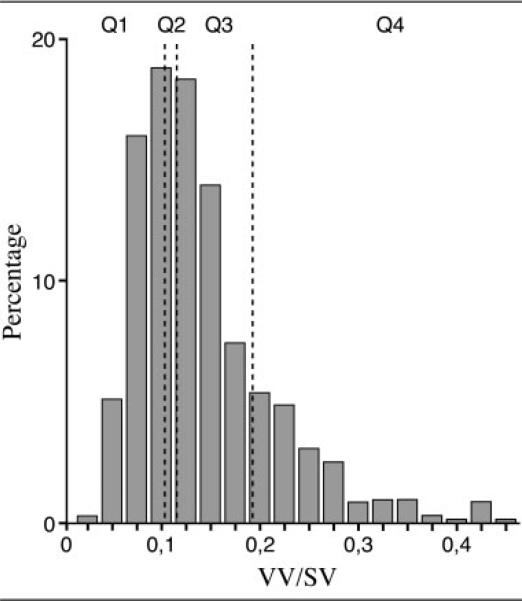
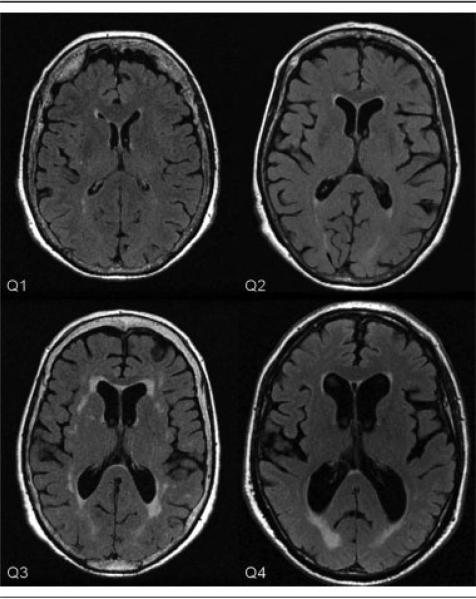
The mean, raw VV/SV for the entire population of 858 was 0.16 (standard deviation, 0.07; range, 0.04–0.71). The distribution of VV/SV is presented in Figure 3, with image examples from each of the 4 quartiles in Figure 4. Persons in the lowest quartile of VV/SV z score were younger and consisted of relatively fewer men, but otherwise were not significantly different in the characteristics shown in Table 1.
Fig 3.
This histogram shows the distribution of ventricular volume (VV)/sulcal cerebrospinal fluid volume (SV) in our study population. The vertical lines indicate borders between quartiles (Q).
Fig 4.
Examples of magnetic resonance images for persons in the first, second, third, and fourth quartile (Q) of the ventricular volume/sulcal cerebrospinal fluid volume z score.
Table 1.
Participant Characteristics by Quartile of the VV/SV z Score: AGES-Reykjavik Study
| Quartiles of the VV/SV z Score |
||||
|---|---|---|---|---|
| 1 n = 214 | 2 n = 215 | 3 n = 215 | 4 n= 214 | |
| Age, mean y (SD) | 73.4 (5.0) | 74.8 (5.1) | 75.4 (5.4) | 76.4 (5.5) |
| Men, % | 27 | 37 | 37 | 42 |
| Education, % only primary education | 22 | 22 | 22 | 22 |
| Ever smokers, % | 60 | 61 | 61 | 58 |
| Body mass index, mean (SD) | 27.5 (4.4) | 26.5 (3.9) | 27.4 (4.6) | 26.3 (4.5) |
| Depression symptomatology, % | 4 | 7 | 6 | 6 |
| History of coronary heart disease, % | 18 | 24 | 16 | 18 |
| Hypertension, % | 69 | 68 | 64 | 67 |
| Diabetes mellitus, % | 7 | 7 | 9 | 8 |
| Peripheral arterial disease, % | 4 | 4 | 5 | 3 |
VV = ventricular volume; SV = sulcal cerebrospinal fluid volume; AGES = Age, Gene/Environment Susceptibility; SD = standard deviation.
Compared to the lowest quartile, those in the top 3 quartiles were older and more likely to be male. Depression symptomatology is defined as Geriatric Depression Scale-Shortened score of ≥6. There are no significant trends across quartiles after adjustment for age and sex.
Of the 858 persons, 16.1% had both gait impairment and cognitive impairment, 7% had both gait impairment and bladder dysfunction, 7% had both cognitive impairment and bladder dysfunction, and 4% had the complete cluster of symptoms that, as well as in other diseases, typically appear combined in NPH. For all except the bladder dysfunction, the prevalence of impaired individuals increased with increasing quartile of VV/SV (Table 2).
Table 2.
Percent Distribution of Impaired Cases by Quartile of VV/SV z Score: AGES-Reykjavik Study
| Quartiles of the VV/SV z Score |
||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Mean VV/SV score | 0.09 | 0.12 | 0.16 | 0.26 |
| Mean VV/SV z score | 1.2 | 1.7 | 2.3 | 3.6 |
| Gait impairment | 40 (19%)a | 51 (24%)a | 55 (26%)a | 77 (37%)a |
| Executive function impairment | 27 (13%) | 43 (21%) | 47 (23%) | 51 (26%) |
| Memory impairment | 27 (14%) | 32 (16%) | 42 (22%) | 51 (28%) |
| Speed of processing impairment | 26 (13%)a | 31 (15%)a | 45 (21%)a | 49 (25%)a |
| Cognitive impairment | 58 (30%)a | 70 (36%)a | 86 (45%)a | 101 (52%)a |
| Bladder dysfunction | 40 (19%) | 42 (20%) | 59 (28%) | 40 (19%) |
| Gait impairment and cognitive impairment | 18 (9%)a | 29 (14%)a | 39 (19%)a | 48 (24%)a |
| Gait impairment and bladder dysfunction | 9 (4%) | 11 (5%) | 28 (13%) | 14 (7%) |
| Cognitive impairment and bladder dysfunction | 11 (5%) | 9 (4%) | 26 (13%) | 17 (8%) |
| Triad | 4 (2%) | 4 (2%) | 20 (10%) | 9 (4%) |
VV = ventricular volume; SV = sulcal cerebrospinal fluid volume; AGES = Age, Gene/Environment Susceptibility.
Percentages in parentheses represent the percentage of the included sample (n = 858).
Significant trend across quartiles after adjustment for age and sex (p < 0.05).
Mean scores for each of the individual cognitive tests by quartile of the VV/SV ratio are presented in Table 3. Trend tests (p = 0.05) showed that normal gait speed, fast gait speed, CANTAB Spatial Working Memory, Stroop 3, Immediate Recall, Delayed Recall, DSST, Figure Comparison, Stroop 1, and Stroop 2 had a greater impairment with increasing VV/SV ratio. Table 3 also shows the prevalence of dementia for each quartile of the z score for the VV/SV ratio. The prevalence of dementia moderately increased with an increase of the VV/SV ratio.
Table 3.
Sample Characteristics of Individual Gait and Cognitive Tests According to Quartiles of the VV/SV z Score
| Quartiles of the VV/SV z Score |
p trend | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Gait speeda (time in s) | |||||||||
| Normalb | –0.30 | 0.67 | –0.21 | 0.64 | –0.16 | 0.78 | 0.12 | 1.00 | <0.001 |
| Fastb | –0.23 | 0.83 | –0.14 | 0.85 | –0.16 | 0.92 | 0.08 | 1.03 | 0.002 |
| Executive functiona | |||||||||
| Digits backward | 0.23 | 0.99 | 0.04 | 0.98 | 0.09 | 1.04 | 0.02 | 1.08 | 0.060 |
| Spatial working memoryb | –0.26 | 0.89 | –0.11 | 1.02 | 0.10 | 0.90 | 0.02 | 0.96 | <0.001 |
| Stroop 3b | –0.26 | 0.76 | –0.09 | 0.95 | –0.05 | 1.02 | 0.01 | 0.96 | 0.003 |
| Memorya | |||||||||
| Immediate recall | 0.40 | 0.97 | 0.14 | 0.96 | 0.06 | 1.00 | –0.10 | 0.95 | <0.001 |
| Delayed recall | 0.39 | 0.98 | 0.15 | 0.98 | 0.11 | 0.97 | –0.14 | 0.98 | <0.001 |
| Speed of processinga | |||||||||
| DSST | 0.41 | 0.91 | 0.25 | 1.00 | 0.11 | 0.98 | 0.01 | 1.02 | <0.001 |
| Figure comparison | 0.37 | 0.91 | 0.20 | 1.04 | 0.11 | 1.00 | –0.06 | 1.00 | <0.001 |
| Stroop 1b | –0.23 | 0.79 | –0.12 | 1.01 | –0.12 | 0.77 | –0.05 | 0.74 | 0.037 |
| Stroop 2b | –0.27 | 0.77 | –0.14 | 1.01 | –0.08 | 0.80 | –0.02 | 0.88 | 0.002 |
| Dementia (%) | 0.47 | 3.26 | 3.72 | 3.27 | |||||
VV = ventricular volume; SV = sulcal cerebrospinal fluid volume; SD = standard deviation; DSST = Digit Symbol Substitution Test.
z Scores (mean for total sample = 0.0, standard deviation for total sample = 1.0).
Lower scores represent better performance.
Compared to the lowest quartile, the highest quartile of the VV/SV z score, was associated with overall gait impairment (odds ratio [OR], 1.9; 95% confidence interval [CI], 1.1–3.3; Model 2; Table 4). The trend of increasing overall gait impairment with increasing VV/SV z score was significant (p = 0.04; Model 2; Table 4). Compared with the lowest quartile, the highest quartile of the VV/SV z score was associated with cognitive impairment (OR, 1.8; 95% CI, 1.1–3.0; Model 2; Table 4), but not a specific type of cognitive function. In addition, there was a significant trend of increasing cognitive impairment with increasing VV/SV z score (p = 0.008; Model 2; Table 4). There was an association of bladder dysfunction with the second quartile of VV/SV z score; but there was no trend across quartiles (Table 4).
Table 4.
Quartiles of the VV/SV z Score and Individual Impairments: AGES-Reykjavik Study
| Model 1 |
Model 2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartiles of the VV/SV Z-score |
p trend | Quartiles of the VV/SV Z-score |
p trend | |||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |||
| Gait | 1.0 | 1.3 (0.8–2.2) | 1.3 (0.8–2.1) | 2.2a (1.4–3.6) | 0.002a | 1.0 | 1.2 (0.7–2.0) | 1.0 (0.6–1.8) | 1.9a (1.1–3.3) | 0.04a |
| Executive function | 1.0 | 1.6 (0.9–2.7) | 1.6 (0.9–2.8) | 1.6 (0.96–2.8) | 0.1 | 1.0 | 1.2 (0.7–2.2) | 1.4 (0.8–2.6) | 1.3 (0.8–2.4) | 0.4 |
| Memory | 1.0 | 0.9 (0.5–1.6) | 1.3 (0.7–2.3) | 1.5 (0.9–2.6) | 0.07 | 1.0 | 0.9 (0.5–1.8) | 1.3 (0.7–2.4) | 1.4 (0.7–2.7) | 0.2 |
| Speed of processing | 1.0 | 1.1 (0.6–1.9) | 1.5 (0.9–2.6) | 1.6 (0.9–2.7) | 0.048a | 1.0 | 1.0 (0.5–1.9) | 1.3 (0.7–2.4) | 1.6 (0.8–3.0) | 0.1 |
| Cognition | 1.0 | 1.0 (0.6–1.6) | 1.4 (0.9–2.2) | 1.7a (1.1–2.7) | 0.006a | 1.0 | 0.9 (0.6–1.5) | 1.4 (0.8–2.3) | 1.8a (1.1–3.0) | 0.008a |
| Bladder dysfunction | 1.0 | 1.2 (0.7–1.9) | 1.8a (1.2–2.9) | 1.2 (0.7–1.9) | 0.2 | 1.0 | 1.3 (0.7–2.1) | 1.7 (0.99–2.8) | 1.2(0.7–2.1) | 0.3 |
VV = ventricular volume; SV = sulcal cerebrospinal fluid volume; AGES = Age, Gene/Environment Susceptibility.
The first quartile of the VV/SV z score, indicated in the table as 1.0, served as the reference group. Model 1 was adjusted for age and sex. Model 2, the fully adjusted model, was adjusted for age, sex, education, smoking, body mass index, depression, hypertension, coronary heart disease, degenerative myelopathy, total white matter lesions volume, history of peripheral artery disease, and brain volume corrected for intracranial volume.
Indicates a significant relationship (p < 0.05).
Compared with the lowest quartile, the highest quartile of the VV/SV z score was associated with a combination of gait impairment and cognitive impairment (OR, 2.6; 95% CI 1.3–5.3; Model 2; Table 5). There was a significant trend for a combination of gait impairment and cognitive impairment with increasing VV/SV z score (p = 0.006; Model 2; Table 5).
Table 5.
Quartiles of the VV/SV z Score and Impairment Combinations: AGES-Reykjavik Study
| Model 1 |
Model 2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartiles of the VV/SV z Score |
p trend | Quartiles of the VV/SV z Score |
p trend | |||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |||
| Gait + cognition | 1.0 | 1.5 (0.8–2.8) | 2.0a (1.1–3.7) | 2.5a (1.3–4.6) | 0.002a | 1.0 | 1.5 (0.7–3.2) | 1.9 (0.9–3.9) | 2.6a (1.3–5.3) | 0.006a |
| Gait + bladder dysfunction | 1.0 | 1.2 (0.5–3.1) | 3.3a (1.5–7.4) | 1.5 (0.6–3.7) | 0.09 | 1.0 | 1.3 (0.5–3.4) | 3.0a (1.2–7.4) | 1.6 (0.6–4.3) | 0.1 |
| Cognition + bladder dysfunction | 1.0 | 0.8 (0.3–1.9) | 2.5a (1.2–5.2) | 1.5 (0.7–3.3) | 0.06 | 1.0 | 0.7 (0.3–1.8) | 1.9 (0.8–4.3) | 1.3 (0.6–3.3) | 0.2 |
| Triad (gait + cognition + bladder dysfunction) | 1.0 | 0.9 (0.2–3.8) | 4.9a (1.6–14.8) | 1.9 (0.6–6.6) | 0.05 | 1.0 | 0.9 (0.2–4.3) | 4.9a (1.4–18.0) | 2.2 (0.5–8.8) | 0.07 |
VV = ventricular volume; SV = sulcal cerebrospinal fluid volume; AGES = Age, Gene/Environment Susceptibility.
The first quartile of the VV/SV z score, indicated in the table as 1.0, served as the reference group. Model 1 was adjusted for age and sex. Model 2, the fully adjusted model, was adjusted for age, sex, education, smoking, body mass index, depression, hypertension, coronary heart disease, degenerative myelopathy, total white matter lesions volume, history of peripheral artery disease, and brain volume corrected for total intracranial volume.
Indicates a significant relationship (p < 0.05).
In general, having a combination of bladder dysfunction with either gait impairment or cognitive impairment was not associated with increasing quartiles of VV/SV z score (Table 5). Compared with the lowest quartile, only the third quartile of the VV/SV z score was significantly associated with the triad of gait impairment, cognitive impairment, and bladder dysfunction (OR, 4.9; 95% CI, 1.4–18.0). There was a modest trend (p = 0.07; Model 2; Table 5) for more persons with the complete cluster of symptoms to have a higher VV/SV index.
Discussion
This study examined the association of ventricular dilation relative to SV with gait, cognitive function, and bladder dysfunction in infarct-free older persons from a population-based sample. We found that those in the highest quartile of the VV/SV z score, reflecting ventricular dilation, were more likely to have impaired gait and cognition. Those in the highest quartile of the VV/SV z score were also more likely to have both impaired gait and impaired cognition. There was no direct relationship between ventricular dilation and bladder dysfunction. The combination of gait impairment, cognitive impairment, and bladder dysfunction, the 3 symptoms that typically appear combined in NPH, was present in 4.4% of our study sample. Presence of the complete cluster of symptoms was moderately more frequent as the quartile of VV/SV z score increased. These effects were present after adjustment for brain volume corrected for total intracranial volume. All associations were independent of WMH volume, and no differences were found in the prevalence of cardiovascular risk factors between the different quartiles of VV/SV z scores.
This study is based on a well-characterized population-based cohort of men and women originally identified in the Reykjavik Study who participated in the follow-up, the AGES-Reykjavik Study; they were not recruited into the study based on any particular characteristic, such as gait or cognitive impairment. Further, we had a standardized neuropsychological test battery of key cognitive functions and an automatic and highly reproducible segmentation of intracranial CSF volumes. However, it is noted that this analytical sample tended to exclude those who were older, who were male, and who had cerebral infarcts on MRI, potentially limiting the generalizability of our study sample. NPH-associated bladder dysfunction is described as urinary urgency or complete disinhibition of bladder function. Previous studies have employed many different questionnaires to classify the NPH type of bladder dysfunction, with the number of questions ranging between 2 and 18. However, an international group of experts recommended the use of a self-administered 3-question incontinence questionnaire with questions similar to ours.16 Our assessment of bladder dysfunction captured a defined set of symptoms similar to those recommended; it is possible that questions on additional symptoms may identify a different group than was identified here.
The association between ventricular dilation and gait impairment was known to exist in persons with NPH, and was also found in our study. In the pathogenesis of NPH, the mechanism by which distended ventricles affect gait, cognition, and bladder function is unclear.43 One theory is that pressure effects of the distended ventricles are exerted on critical cerebral sites.43 The fibers of the corticospinal tract that supply motor function to the legs pass closest to the lateral ventricles in the corona radiata, which may explain why gait disturbance is usually the first and leading symptom to appear in NPH.4,9,44 Of the 3 impairments, the association between ventricular dilation and gait impairment was the strongest in our study population, consistent with the NPH syndrome.
We also found an association between ventricular dilation and cognitive impairment. Cognitive impairment is usually the second symptom to appear in NPH, and does not occur in all patients with NPH.9,10 When there is impairment, the cognitive deficit consists principally of memory impairment, decreased speed of complex information processing, or poor executive function.4,10,45 Although we did not find an association between ventricular dilation and impairment in a specific cognitive ability, ventricular dilation was associated with each of the functions we measured.
We did not find an individual association between the VV/SV z score and bladder dysfunction. However, participants in the third quartile of the VV/SV z score were more likely to have both gait impairment and bladder dysfunction. This may be due to an uneven distribution of persons with both gait impairment and bladder dysfunction among the quartiles (Table 2). In addition, it may be possible that persons in the highest quartile of the VV/SV z score had difficulties reporting bladder dysfunction due to cognitive impairment. Bladder dysfunction has been described as a late stage symptom in NPH,10 suggesting that it would only be associated with VV/SV z score when both gait impairment and cognitive impairment are present.
The exact incidence and prevalence of NPH in the general population is not known.9 This is partly explained by inconsistent definitions of NPH, which rely on clinical and neuroimaging criteria in some series, and is confirmed by improvement after ventricular shunting in others.10 We found a 4.4% prevalence of a combination of gait impairment, cognitive impairment, and bladder dysfunction. This number is limited by the inability to control for other causes of gait impairment, such as lumbar canal stenosis, or other causes of bladder dysfunction.8
In conclusion, in our population-based sample of infarct-free men and women, ventricular dilation, the radiological hallmark of NPH, can be observed frequently and, similar to NPH, this phenomenon is associated with cognitive and gait impairment, and sometimes bladder dysfunction. Whether and how often symptomatic individuals with ventricular dilation from the general population could benefit from ventricular shunting remains to be determined. The quantitative measure of ventricular dilation presented in this study would be a useful tool for future studies addressing this question.
Acknowledgments
This study has been funded by National Institutes of Health contract N01-AG-12100, the National Institute on Aging Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament).
We thank K. Siggeirsdóttir and M. Jónsdóttir for their contributions to the assessment of gait and cognition, T. Aspelund and M. Chang for their support in data management, and J.A.L. Vanneste for reviewing the manuscript.
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Drayer BP. Imaging of the aging brain. Part I. Normal findings. Radiology. 1988;166:785–796. doi: 10.1148/radiology.166.3.3277247. [DOI] [PubMed] [Google Scholar]
- 2.Kurihara Y, Simonson TM, Nguyen HD, et al. MR imaging of ventriculomegaly—a qualitative and quantitative comparison of communicating hydrocephalus, central atrophy, and normal studies. J Magn Reson Imaging. 1995;5:451–456. doi: 10.1002/jmri.1880050415. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt R, Launer LJ, Nilsson LG, et al. Magnetic resonance imaging of the brain in diabetes: the Cardiovascular Determinants of Dementia (CASCADE) Study. Diabetes. 2004;53:687–692. doi: 10.2337/diabetes.53.3.687. [DOI] [PubMed] [Google Scholar]
- 4.Bradley WG. Normal pressure hydrocephalus: new concepts on etiology and diagnosis. AJNR Am J Neuroradiol. 2000;21:1586–1590. [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley WG, Jr, Scalzo D, Queralt J, et al. Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology. 1996;198:523–529. doi: 10.1148/radiology.198.2.8596861. [DOI] [PubMed] [Google Scholar]
- 6.Fishman RA, Dillon WP. Normal pressure hydrocephalus: new findings and old questions. AJNR Am J Neuroradiol. 2001;22:1640–1641. [PMC free article] [PubMed] [Google Scholar]
- 7.Jack CR, Jr, Lexa FJ, Trojanowski JQ, et al. Normal aging, dementia, and neurodegenerative disease. In: Atlas SW, editor. Magnetic Resonance Imaging of the Brain and Spine. 3rd ed. Vol. 1. Lippincott Williams & Wilkins; Philadelphia, PA: 2002. pp. 1177–1240. [Google Scholar]
- 8.Factora R, Luciano M. Normal pressure hydrocephalus: diagnosis and new approaches to treatment. Clin Geriatr Med. 2006;22:645–657. doi: 10.1016/j.cger.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Meier U, Zeilinger FS, Kintzel D. Signs, symptoms and course of normal pressure hydrocephalus in comparison with cerebral atrophy. Acta Neurochir (Wien ) 1999;141:1039–1048. doi: 10.1007/s007010050480. [DOI] [PubMed] [Google Scholar]
- 10.Vanneste JA. Diagnosis and management of normal-pressure hydrocephalus. J Neurol. 2000;247:5–14. doi: 10.1007/s004150050003. [DOI] [PubMed] [Google Scholar]
- 11.Rubenstein LZ, Josephson KR, Robbins AS. Falls in the nursing home. Ann Intern Med. 1994;121:442–451. doi: 10.7326/0003-4819-121-6-199409150-00009. [DOI] [PubMed] [Google Scholar]
- 12.Sudarsky L. Geriatrics: gait disorders in the elderly. N Engl J Med. 1990;322:1441–1446. doi: 10.1056/NEJM199005173222007. [DOI] [PubMed] [Google Scholar]
- 13.Hofman A, Ott A, Breteler MM, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 14.Knopman DS, Boeve BF, Petersen RC. Essentials of the proper diagnoses of mild cognitive impairment, dementia, and major subtypes of dementia. Mayo Clin Proc. 2003;78:1290–1308. doi: 10.4065/78.10.1290. [DOI] [PubMed] [Google Scholar]
- 15.Anger JT, Saigal CS, Stothers L, et al. The prevalence of uri-nary incontinence among community dwelling men: results from the National Health and Nutrition Examination survey. J Urol. 2006;176:2103–2108. doi: 10.1016/j.juro.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 16.Brown JS, Nyberg LM, Kusek JW, et al. Proceedings of the National Institute of Diabetes and Digestive and Kidney Diseases International Symposium on Epidemiologic Issues in Uri-nary Incontinence in Women. Am J Obstet Gynecol. 2003;188:S77–S88. doi: 10.1067/mob.2003.353. [DOI] [PubMed] [Google Scholar]
- 17.Babikian V, Ropper AH. Binswanger's disease: a review. Stroke. 1987;18:2–12. doi: 10.1161/01.str.18.1.2. [DOI] [PubMed] [Google Scholar]
- 18.Bennett DA, Wilson RS, Gilley DW, et al. Clinical diagnosis of Binswanger's disease. J Neurol Neurosurg Psychiatry. 1990;53:961–965. doi: 10.1136/jnnp.53.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pantoni L, Garcia JH. The significance of cerebral white matter abnormalities 100 years after Binswanger's report. A review. Stroke. 1995;26:1293–1301. doi: 10.1161/01.str.26.7.1293. [DOI] [PubMed] [Google Scholar]
- 20.Andresdottir MB, Sigfusson N, Sigvaldason H, et al. Erythrocyte sedimentation rate, an independent predictor of coronary heart disease in men and women: the Reykjavik Study. Am J Epidemiol. 2003;158:844–851. doi: 10.1093/aje/kwg222. [DOI] [PubMed] [Google Scholar]
- 21.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 22.Saczynski JS, Jonsdottir MK, Garcia ME, et al. Cognitive impairment: an increasingly important complication of type 2 diabetes: the age, gene/environment susceptibility—Reykjavik study. Am J Epidemiol. 2008;168:1132–1139. doi: 10.1093/aje/kwn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 24.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 25.Ostir GV, Markides KS, Black SA, et al. Lower body functioning as a predictor of subsequent disability among older Mexican Americans. J Gerontol A Biol Sci Med Sci. 1998;53:M491–M495. doi: 10.1093/gerona/53a.6.m491. [DOI] [PubMed] [Google Scholar]
- 26.Fitzpatrick AL, Buchanan CK, Nahin RL, et al. Associations of gait speed and other measures of physical function with cognition in a healthy cohort of elderly persons. J Gerontol A Biol Sci Med Sci. 2007;62:1244–1251. doi: 10.1093/gerona/62.11.1244. [DOI] [PubMed] [Google Scholar]
- 27.Inzitari M, Newman AB, Yaffe K, et al. Gait speed predicts decline in attention and psychomotor speed in older adults: the health aging and body composition study. Neuroepidemiology. 2007;29:156–162. doi: 10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saczynski JS, Jonsdottir MK, Sigurdsson S, et al. White matter lesions and cognitive performance: the role of cognitively complex leisure activity. J Gerontol A Biol Sci Med Sci. 2008;63:848–854. doi: 10.1093/gerona/63.8.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wechsler D. Manual for the Wechsler Adult Intelligence Scale. Psychological Corporation; New York: 1955. The measurement and appraisal of adult intelligence. [Google Scholar]
- 30.Robbins TW, James M, Owen AM, et al. Cambridge Neuro-psychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- 31.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 32.Delis DC, Kramer JH, Kaplan E, et al. The California Verbal Learning Test—Research Edition. Psychological Corporation; New York, NY: 1987. [Google Scholar]
- 33.Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Dev Psychol. 1991;27:763–776. [Google Scholar]
- 34.Corkill RG, Cadoux-Hudson TA. Normal pressure hydrocephalus: developments in determining surgical prognosis. Curr Opin Neurol. 1999;12:671–677. doi: 10.1097/00019052-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Admiraal-Behloul F, van den Heuvel DM, Olofsen H, et al. Fully automatic segmentation of white matter hyperintensities in MR images of the elderly. Neuroimage. 2005;28:607–617. doi: 10.1016/j.neuroimage.2005.06.061. [DOI] [PubMed] [Google Scholar]
- 36.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 37.Aevarsson O, Skoog I. A longitudinal population study of the mini-mental state examination in the very old: relation to dementia and education. Dement Geriatr Cogn Disord. 2000;11:166–175. doi: 10.1159/000017231. [DOI] [PubMed] [Google Scholar]
- 38.Anstey K, Christensen H. Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: a review. Gerontology. 2000;46:163–177. doi: 10.1159/000022153. [DOI] [PubMed] [Google Scholar]
- 39.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–711. [PubMed] [Google Scholar]
- 40.Lichtenberg PA, Ross T, Millis SR, et al. The relationship between depression and cognition in older adults: a cross-validation study. J Gerontol B Psychol Sci Soc Sci. 1995;50:25–32. doi: 10.1093/geronb/50b.1.p25. [DOI] [PubMed] [Google Scholar]
- 41.Klein BE, Klein R, Knudtson MD, et al. Frailty, morbidity and survival. Arch Gerontol Geriatr. 2005;41:141–149. doi: 10.1016/j.archger.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Nash DT, Fillit H. Cardiovascular disease risk factors and cognitive impairment. Am J Cardiol. 2006;97:1262–1265. doi: 10.1016/j.amjcard.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 43.Fisher CM. Hydrocephalus as a cause of disturbances of gait in the elderly. Neurology. 1982;32:1358–1363. doi: 10.1212/wnl.32.12.1358. [DOI] [PubMed] [Google Scholar]
- 44.Graff-Radford NR, Godersky JC. Normal-pressure hydrocephalus. Onset of gait abnormality before dementia predicts good surgical outcome. Arch Neurol. 1986;43:940–942. doi: 10.1001/archneur.1986.00520090068020. [DOI] [PubMed] [Google Scholar]
- 45.Gallia GL, Rigamonti D, Williams MA. The diagnosis and treatment of idiopathic normal pressure hydrocephalus. Nat Clin Pract Neurol. 2006;2:375–381. doi: 10.1038/ncpneuro0237. [DOI] [PubMed] [Google Scholar]