Abstract
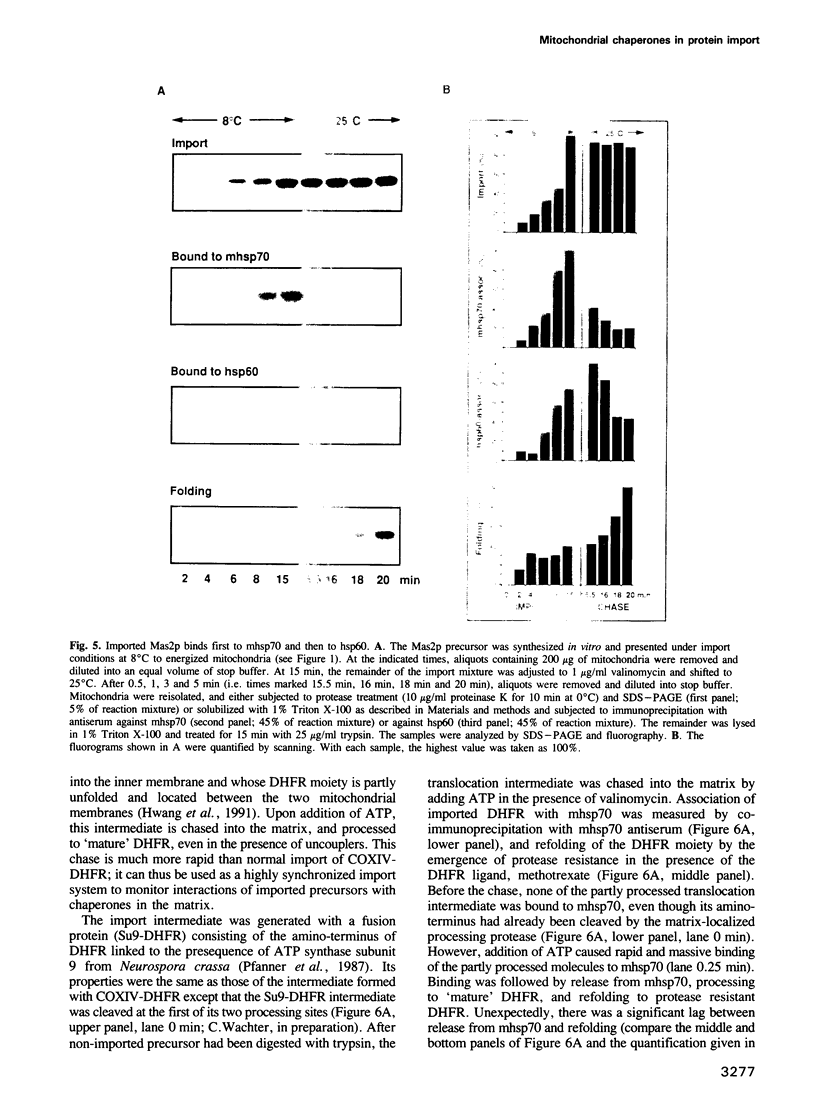
Translocation and folding of proteins imported into mitochondria are mediated by two matrix-localized chaperones, mhsp70 and hsp60. In order to investigate whether these chaperones act sequentially or in parallel, we studied their interaction with newly imported precursor proteins in isolated yeast mitochondria by coimmunoprecipitation. All precursors bound transiently to mhsp70. Release from mhsp70 required hydrolysis of ATP and did not immediately generate a tightly folded protein. For example, after imported mouse dihydrofolate reductase (a soluble monomeric enzyme) had been released from mhsp70, folding to a protease resistant conformation occurred only after a lag and was much slower than the release. Under standard import conditions, no significant association of DHFR with hsp60 could be detected. Similarly, newly imported hsp60 subunit was released from mhsp70 as an incompletely folded, unassembled intermediate which accumulated at low temperature and assembled to hsp60 14-mer at higher temperature in an ATP-dependent manner. Mas2p (the larger subunit of the MAS-encoded processing protease) first bound to mhsp70, then to hsp60, and only then assembled with its partner subunit, Mas1p. We propose that ATP-dependent release from mhsp70 is insufficient to cause folding of imported proteins and that assembly of hsp60 and Mas2p requires sequential, ATP-dependent interactions with mhsp70 and hsp60.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker K. P., Schatz G. Mitochondrial proteins essential for viability mediate protein import into yeast mitochondria. Nature. 1991 Jan 17;349(6306):205–208. doi: 10.1038/349205a0. [DOI] [PubMed] [Google Scholar]
- Blumberg H., Silver P. A. A homologue of the bacterial heat-shock gene DnaJ that alters protein sorting in yeast. Nature. 1991 Feb 14;349(6310):627–630. doi: 10.1038/349627a0. [DOI] [PubMed] [Google Scholar]
- Cheng M. Y., Hartl F. U., Horwich A. L. The mitochondrial chaperonin hsp60 is required for its own assembly. Nature. 1990 Nov 29;348(6300):455–458. doi: 10.1038/348455a0. [DOI] [PubMed] [Google Scholar]
- Cheng M. Y., Hartl F. U., Martin J., Pollock R. A., Kalousek F., Neupert W., Hallberg E. M., Hallberg R. L., Horwich A. L. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 1989 Feb 16;337(6208):620–625. doi: 10.1038/337620a0. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Kramer J., Kosic-Smithers J. SSC1, a member of the 70-kDa heat shock protein multigene family of Saccharomyces cerevisiae, is essential for growth. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4156–4160. doi: 10.1073/pnas.84.12.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Kramer J., Shilling J., Werner-Washburne M., Holmes S., Kosic-Smithers J., Nicolet C. M. SSC1, an essential member of the yeast HSP70 multigene family, encodes a mitochondrial protein. Mol Cell Biol. 1989 Jul;9(7):3000–3008. doi: 10.1128/mcb.9.7.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Engman D. M., Kirchhoff L. V., Donelson J. E. Molecular cloning of mtp70, a mitochondrial member of the hsp70 family. Mol Cell Biol. 1989 Nov;9(11):5163–5168. doi: 10.1128/mcb.9.11.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M. K., Hajra A. K. A rapid method for the isolation of peroxisomes from rat liver. Anal Biochem. 1986 Nov 15;159(1):169–174. doi: 10.1016/0003-2697(86)90323-4. [DOI] [PubMed] [Google Scholar]
- Gray R. E., Grasso D. G., Maxwell R. J., Finnegan P. M., Nagley P., Devenish R. J. Identification of a 66 KDa protein associated with yeast mitochondrial ATP synthase as heat shock protein hsp60. FEBS Lett. 1990 Jul 30;268(1):265–268. doi: 10.1016/0014-5793(90)81024-i. [DOI] [PubMed] [Google Scholar]
- Haid A., Suissa M. Immunochemical identification of membrane proteins after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Methods Enzymol. 1983;96:192–205. doi: 10.1016/s0076-6879(83)96017-2. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Neupert W. Protein sorting to mitochondria: evolutionary conservations of folding and assembly. Science. 1990 Feb 23;247(4945):930–938. doi: 10.1126/science.2406905. [DOI] [PubMed] [Google Scholar]
- Hase T., Müller U., Riezman H., Schatz G. A 70-kd protein of the yeast mitochondrial outer membrane is targeted and anchored via its extreme amino terminus. EMBO J. 1984 Dec 20;3(13):3157–3164. doi: 10.1002/j.1460-2075.1984.tb02274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Schatz G. The amino-terminal region of an imported mitochondrial precursor polypeptide can direct cytoplasmic dihydrofolate reductase into the mitochondrial matrix. EMBO J. 1984 Dec 20;3(13):3149–3156. doi: 10.1002/j.1460-2075.1984.tb02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. T., Schatz G. Translocation of proteins across the mitochondrial inner membrane, but not into the outer membrane, requires nucleoside triphosphates in the matrix. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8432–8436. doi: 10.1073/pnas.86.21.8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. E., Yaffe M. P. Import of proteins into yeast mitochondria: the nuclear MAS2 gene encodes a component of the processing protease that is homologous to the MAS1-encoded subunit. EMBO J. 1988 Dec 1;7(12):3863–3871. doi: 10.1002/j.1460-2075.1988.tb03272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P. J., Ostermann J., Shilling J., Neupert W., Craig E. A., Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990 Nov 8;348(6297):137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Krieg U. C., Walter P., Johnson A. E. Photocrosslinking of the signal sequence of nascent preprolactin to the 54-kilodalton polypeptide of the signal recognition particle. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8604–8608. doi: 10.1073/pnas.83.22.8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leustek T., Dalie B., Amir-Shapira D., Brot N., Weissbach H. A member of the Hsp70 family is localized in mitochondria and resembles Escherichia coli DnaK. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7805–7808. doi: 10.1073/pnas.86.20.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissin N. M., Venyaminov SYu, Girshovich A. S. (Mg-ATP)-dependent self-assembly of molecular chaperone GroEL. Nature. 1990 Nov 22;348(6299):339–342. doi: 10.1038/348339a0. [DOI] [PubMed] [Google Scholar]
- Lubben T. H., Gatenby A. A., Donaldson G. K., Lorimer G. H., Viitanen P. V. Identification of a groES-like chaperonin in mitochondria that facilitates protein folding. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7683–7687. doi: 10.1073/pnas.87.19.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullin T. W., Hallberg R. L. A highly evolutionarily conserved mitochondrial protein is structurally related to the protein encoded by the Escherichia coli groEL gene. Mol Cell Biol. 1988 Jan;8(1):371–380. doi: 10.1128/mcb.8.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullin T. W., Hallberg R. L. A normal mitochondrial protein is selectively synthesized and accumulated during heat shock in Tetrahymena thermophila. Mol Cell Biol. 1987 Dec;7(12):4414–4423. doi: 10.1128/mcb.7.12.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H., Pain D., Blobel G. 70-kD heat shock-related protein is one of at least two distinct cytosolic factors stimulating protein import into mitochondria. J Cell Biol. 1988 Dec;107(6 Pt 1):2051–2057. doi: 10.1083/jcb.107.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert W., Hartl F. U., Craig E. A., Pfanner N. How do polypeptides cross the mitochondrial membranes? Cell. 1990 Nov 2;63(3):447–450. doi: 10.1016/0092-8674(90)90437-j. [DOI] [PubMed] [Google Scholar]
- Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989 Sep 14;341(6238):125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Ostermann J., Voos W., Kang P. J., Craig E. A., Neupert W., Pfanner N. Precursor proteins in transit through mitochondrial contact sites interact with hsp70 in the matrix. FEBS Lett. 1990 Dec 17;277(1-2):281–284. doi: 10.1016/0014-5793(90)80865-g. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Müller H. K., Harmey M. A., Neupert W. Mitochondrial protein import: involvement of the mature part of a cleavable precursor protein in the binding to receptor sites. EMBO J. 1987 Nov;6(11):3449–3454. doi: 10.1002/j.1460-2075.1987.tb02668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Rassow J., Guiard B., Söllner T., Hartl F. U., Neupert W. Energy requirements for unfolding and membrane translocation of precursor proteins during import into mitochondria. J Biol Chem. 1990 Sep 25;265(27):16324–16329. [PubMed] [Google Scholar]
- Prasad T. K., Hack E., Hallberg R. L. Function of the maize mitochondrial chaperonin hsp60: specific association between hsp60 and newly synthesized F1-ATPase alpha subunits. Mol Cell Biol. 1990 Aug;10(8):3979–3986. doi: 10.1128/mcb.10.8.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Scherer P. E., Krieg U. C., Hwang S. T., Vestweber D., Schatz G. A precursor protein partly translocated into yeast mitochondria is bound to a 70 kd mitochondrial stress protein. EMBO J. 1990 Dec;9(13):4315–4322. doi: 10.1002/j.1460-2075.1990.tb07880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stueber D., Ibrahimi I., Cutler D., Dobberstein B., Bujard H. A novel in vitro transcription-translation system: accurate and efficient synthesis of single proteins from cloned DNA sequences. EMBO J. 1984 Dec 20;3(13):3143–3148. doi: 10.1002/j.1460-2075.1984.tb02271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suissa M., Reid G. A. Preparation and use of antibodies against insoluble membrane proteins. Methods Enzymol. 1983;97:305–311. doi: 10.1016/0076-6879(83)97142-2. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Brunner J., Baker A., Schatz G. A 42K outer-membrane protein is a component of the yeast mitochondrial protein import site. Nature. 1989 Sep 21;341(6239):205–209. doi: 10.1038/341205a0. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Schatz G. Mitochondria can import artificial precursor proteins containing a branched polypeptide chain or a carboxy-terminal stilbene disulfonate. J Cell Biol. 1988 Dec;107(6 Pt 1):2045–2049. doi: 10.1083/jcb.107.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J. P., Misra L. M., Rose M. D. Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J Cell Biol. 1990 Jun;110(6):1885–1895. doi: 10.1083/jcb.110.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Jensen R. E., Yaffe M. P., Oppliger W., Schatz G. Import of proteins into yeast mitochondria: the purified matrix processing protease contains two subunits which are encoded by the nuclear MAS1 and MAS2 genes. EMBO J. 1988 Dec 1;7(12):3857–3862. doi: 10.1002/j.1460-2075.1988.tb03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]










