Abstract
Background
A close connection between inflammation and cancer has now been firmly established. While tumor initiation is typically independent of inflammatory events, immune cells infiltrating the tumor microenvironment secrete inflammatory cytokines that enhance the aberrant growth of tumor cells and thus facilitate tumor progression. Therefore, inflammation and tumor growth are usually interpreted as closely related on a systemic level but as distinct, independently regulated processes at a molecular level.
Highlight
Recently, we reported that a sub-class of small GTPases, namely κB-Ras1 and κB-Ras2, regulate both inflammation and tumor growth, thereby providing a unique molecular bridge between the two biological processes.
Conclusion
Here, we briefly summarize the known contact points between inflammation and cancer, including oral cancers, and put into context the identification of κB-Ras proteins as molecular link between two independent pathways important for tumor growth.
Keywords: cancer, inflammation, κB-Ras, NF-κB, Ras, Ral
1) Introduction
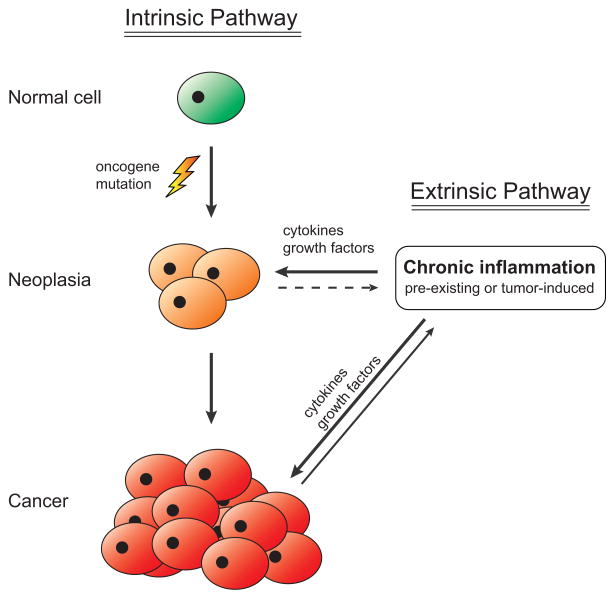
In the middle of the 19th century, German pathologist Rudolf Virchow noticed that neoplasms often develop at sites of inflammation [1]. Based on this observation, he hypothesized that chronic inflammation enhances tumor growth [2], but his idea was largely ignored for over a century. During the past two decades, however, the notion that inflammatory events can promote cancer growth has experienced a vigorous renaissance, fuelled by the development of new animal models of cancer, and rapid advances in the fields of immunology and inflammation [3–5]. These discoveries have led to a model which posits that inflammation acts as a facilitator of cancer growth (Figure 1). According to this model, oncogenic mutations in tumor precursor cells arise independently of any inflammatory processes. However, chronic inflammation may already be present at the site of tumor initiation from unrelated causes, e.g. Crohn’s disease preceding colon cancer or ulcers preceding gastric tumors. Alternatively, inflammation may be actively induced by the incipient tumor itself. Recruitment of immune cells infiltrating the tumor microenvironment then secrete cytokines that stimulate pro-proliferative and anti-apoptotic responses in the tumor cells, providing a growth advantage that facilitates tumor progression in the absence of additional oncogenic mutations (although these continue to accrue in the tumor cells) [5,6]. This enhancement of tumor growth by cytokines produced by non-tumor cells is referred to as the extrinsic pathway of cancer development, in contrast to the intrinsic pathway of oncogenic mutations. While there are a large number of signaling molecules that have been linked to various cancers, two classes of cellular regulators have emerged as particularly dominant players: the Ras family of small guanosine triphosphatases (GTPases), members of which are mutated in human solid cancers more frequently than any other type of gene; and the NF-κB family of transcription factors, which are central regulators of inflammation and growth.
Figure 1. Current model of the interplay between inflammation and cancer.
A healthy cell sustains DNA damage resulting in an oncogenic mutation (intrinsic pathway). Growth of the incipient tumor is stimulated by the secretion of cytokines and growth factors from nearby immune cells (extrinsic pathway). These immune cells may have been activated due to unrelated chronic inflammation or due to chemokines secreted by the neoplastic cells.
2) Ras proteins light the fire
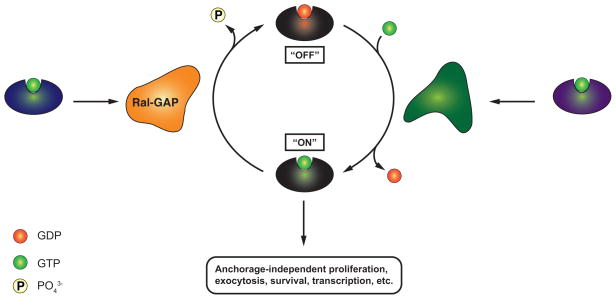
The rat sarcoma (Ras) proteins K-Ras, H-Ras and N-Ras are among the most-studied human oncogenes. They are the eponymous members of the Ras superfamily of small GTPases, which includes over 150 human proteins [7]. Proteins of the Ras superfamily cycle between two conformations: a biologically inactive, GDP-bound conformation (“off”) and a GTP-bound effector conformation (“on”). Transition between these two conformations is tightly regulated by guanine-nucleotide-exchange factors (GEFs), which catalyze replacement of GDP with GTP (“off” -> “on”); and by GTPase-activating proteins (GAPs), which stimulate the intrinsic catalytic activity of the Ras superfamily protein to hydrolyze GTP to GDP (“on” -> “off”) (cf. Figure 2). For simplicity, we will hereafter refer to the closely related, classical Ras proteins K-Ras, H-Ras and N-Ras collectively as RAS, unless noted otherwise. RAS functions as a molecular switch that can be activated by growth factors, e.g. the epidermal growth factor (EGF). GTP-bound RAS (“on”) activates a series of down-stream effectors, most notably rapidly accelerated fibrosarcoma (RAF), phosphoinositide 3-kinase (PI3K) and Ras-like (Ral), which in turn regulate a broad range of cellular processes, including cell survival, proliferation and cytoskeletal reorganization [8,9]. Given their central role in the regulation of cellular growth, it is not surprising that gain-of-function mutations in the RAS gene are highly oncogenic. Indeed, over 30% of human cancers carry mutations that lock RAS in a GTP-bound (“on”) conformation. This number is markedly higher in certain particular types of cancer: RAS gain-of-function mutations are estimated to be present in 50% of colorectal cancers and in 90% of pancreatic ductal adenocarcinomata [9], making oncogenic RAS proteins a key contributor to human carcinogenesis.
Figure 2. Regulation of the Ral GTPase.
GTP-bound Ras activates Ral-GEF, which catalyzes binding of Ral to GTP (“on”). GTP-bound Ral regulates a series of cellular processes, including anchorage-independent proliferation. Ral-GAP catalyzes the hydrolysis of GTP to GDP by Ral (“off”). κB-Ras enhances this Ral-GAP activity.
Mutations of RAS are also frequently observed in oral squamous cell carcinoma (OSCC), the sixth-most-common cancer worldwide [10,11]. A report synthesizing the results of 40 separate studies found a wide range in the prevalence of RAS mutations in OSCC tissue, varying from 0% to over 30%. Interestingly, HRAS was more commonly mutated than KRAS or NRAS in these tumors [11]. In 2004, Caulin et al. developed a mouse model that inducibly expressed mutant K-Ras only in stratified epithelia. Mice expressing the mutant K-Ras spontaneously developed prominent squamous papillomata in the oral cavity within sixteen weeks of induction, thus establishing the first genetically inducible mouse model of oral cancer [12].
3) NF-κB adds the fuel
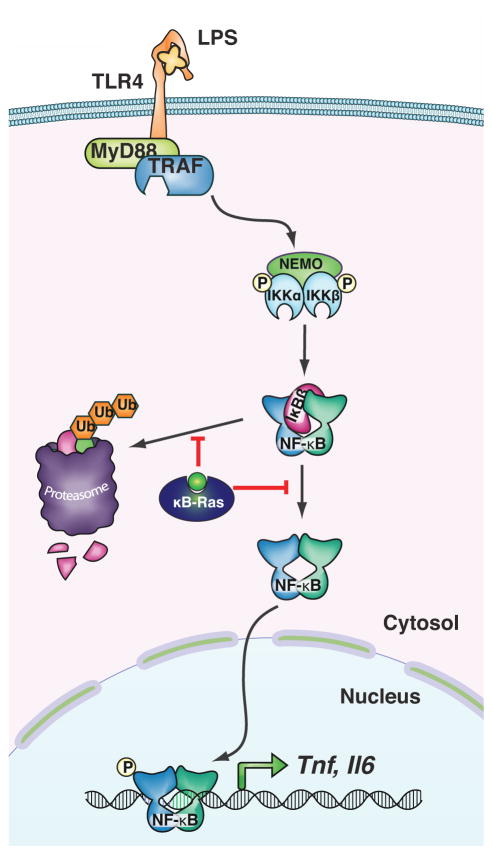
Members of the NF-κB family of transcription factors are intricately involved in the regulation of a diverse range of biological processes, including cellular proliferation, differentiation and survival, as well as innate and adaptive immunity. In the absence of activating stimuli, NF-κB dimers are retained in the cytoplasm by an interaction with inhibitor of κB (IκB) proteins, which bind to the N-terminal rel homology domain (RHD) of NF-κB subunits through a series of ankyrin repeat motifs and thus mask the nuclear localization signal (NLS) of bound NF-κB dimers. Upon binding of an activating ligand to its cognate receptor, a signaling cascade is initiated which culminates in the phosphorylation-dependent activation of the IκB kinase (IKK) complex. The IKK complex then phosphorylates IκB proteins, triggering their degradation by the proteasome. This frees the NF-κB dimers to translocate into the nucleus and induce transcription of target genes (Figure 3) [13,14]. The NF-κB pathway is of particular importance for cells of the innate and adaptive immune response, but it also regulates expression of pro-proliferative and anti-apoptotic genes in epithelial cells and other tissues. An early indication that NF-κB may be involved in cancer development came from the observation that v-Rel, a retroviral homolog of the NF-κB subunit c-Rel, exhibits oncogenic properties [15]. However, mutations in the NF-κB pathway are found only rarely in human solid tumors (but are common in lymphomas, consistent with the dominant role of NF-κB signaling in lymphocytes [16]). Nonetheless, levels of NF-κB are frequently elevated in carcinoma cells as a direct result of stimulation by immune cells in the tumor’s microenvironment [5,6]. Thus, activation of the NF-κB pathway is crucial to the development of solid tumors in two separate ways: (i) activation of tumor-infiltrating immune cells and the ensuing secretion of cytokines is dependent on NF-κB; (ii) and these cytokines induce NF-κB-dependent signaling in the tumor cells. This two-fold importance of NF-κB signaling was demonstrated elegantly in a landmark publication by Greten et al. in 2004 [17]. The group used a conditional knock-out system to first delete IKK-β, which is required for effective NF-κB activation, only in intestinal epithelial cells. When they chemically induced colitis-associated colorectal cancer with a combination of azoxymethane and dextran sulfate sodium salt in these mice, they observed that the number of tumors was significantly smaller than in control mice, even though production of pro-inflammatory cytokines by myeloid cells was increased. They subsequently deleted IKK-β only in myeloid cells, thus effectively reducing levels of pro-inflammatory cytokines secreted into the tumor microenvironment, which also reduced tumor burden in mice compared to controls. This study therefore clearly demonstrated the dual role of NF-κB in cancer development.
Figure 3. Basic schematic of NF-κB signaling through toll-like receptor 4 (TLR4).
LPS binds to TLR4 and induces a signaling cascade leading to activation of the IKK complex consisting of IKK-α, IKK-β and NEMO. Active IKK can phosphorylate IκB, which is subsequently degraded and releases NF-κB dimers to translocate into the nucleus, where they regulate transcription. κB-Ras inhibits stimulus-dependent degradation of IκB-β and thus reduces NF-κB activation.
Not surprisingly, NF-κB signaling has been implicated in oral carcinogenesis as well. The NF-κB-dependent cytokine interleukin (IL-) 6 has been associated with a range of oral diseases, including OSCC [18]. IL-1β, which is regulated by NF-κB-dependent transcription and in turn induces NF-κB activation in target cells, was recently demonstrated to be elevated in a mouse model of chemically induced OSCC [19]. The same study found that IL-1β is markedly up-regulated in human tongue cancer tissue and in OSCC tissue, compared to healthy control tissue [19]. Direct evidence for the involvement of NF-κB signaling in oral cancer was provided by a study that used a transgenic mouse model in which IKK-β was overexpressed only in stratified epithelia, causing increased and consistent activation of NF-κB in these tissues [20]. The mice consistently developed inflammation and spontaneous tumors in the oral cavity. When Ras-driven oral tumorigenesis was chemically induced in the same mouse model, tumors exhibited a higher degree of malignancy in the presence of IKK-β overexpression.
4) κB-Ras proteins form molecular connectors between cancer and inflammation
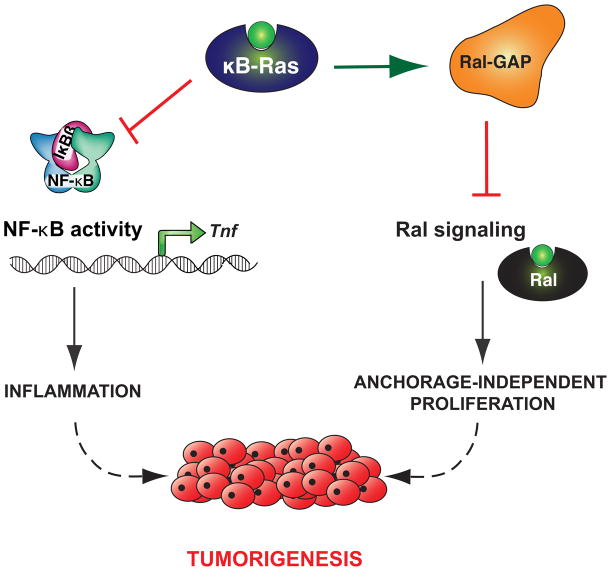
While there is evidence that activation of RAS can lead to activation of NF-κB [21,22], the intrinsic pathway (e.g. through RAS signaling) and the extrinsic pathway (through NF-κB signaling) are generally considered to be independent of each other. Recently, however, we reported that the NF-κB inhibitor-interacting Ras-like (κB-Ras) proteins actively regulate both NF-κB signaling and RAS signaling [23]. The κB-Ras proteins, κB-Ras 1 and κB-Ras 2, are small GTPases in the Ras family that appear to be locked constitutively in a GTP-bound state [24,25]. They were originally identified, as the full name indicates, as interaction partners of IκB-β (but not IκB-α), and it was demonstrated that their overexpression stabilizes IκB-β and thus inhibits NF-κB activation (Figure 3) [24,26,27]. Of note, it has also been suggested that κB-Ras proteins may interfere with phosphorylation of the NF-κB subunit RelA and inhibit transcriptional activation by this route [25]. We created a κB-Ras knock-out mouse to further investigate the role of κB-Ras in inflammation and found that in response to NF-κB activation by lipopolysaccharide (LPS), macrophages lacking κB-Ras expressed higher levels of the pro-inflammatory cytokine tumor necrosis factor α (TNF-α) than wild-type macrophages. Consistent with this observation, κB-Ras-deficient mice were hypersensitive to LPS-induced shock and perished significantly faster than wild-type controls [23]. Surprisingly, we found in the same study that κB-Ras proteins have another, completely unrelated cellular interaction partner: Ral-GAP, the complex negatively regulating the Ral GTPase. Ral is a downstream effector of RAS and regulates several different biological functions, including organization of the cytoskeleton, cell proliferation and vesicular transport [28]. Importantly, Ral has also been reported to be centrally involved in promoting anchorage-independent proliferation (AIP) of cancer cells, a crucial requirement for tumor growth and spread [29–31]. We found that cells lacking both isoforms of κB-Ras had increased levels of GTP-bound Ral (“on”), indicating that κB-Ras enhances Ral-GAP activity and serves as a negative regulator of Ral signaling (Figure 2). Consistent with this observation, immortalized fibroblasts deficient for κB-Ras exhibited a strong increase in AIP as well as enhanced tumor growth when implanted into immunodeficient mice [23]. Taken together, these data demonstrate that κB-Ras is an active regulator of NF-κB signaling and Ras/Ral signaling, making it the only known molecular bridge between these two cancer-relevant pathways (Figure 4). The biological relevance of this central position is underscored by the observation that expression levels of κB-Ras are reduced in a wide variety of human cancers, including oral cancer [23,32]. Intriguingly, human cancer cell lines carrying an oncogenic RAS mutation had consistently lower protein levels of κB-Ras than similar cell lines encoding wild-type RAS. Restoring κB-Ras protein levels in two of these cell lines by transfection reduced their ability for anchorage-independent growth [23].
Figure 4. κB-Ras forms a molecular connector between inflammation and cancer.
κB-Ras inhibits NF-κB signaling, which contributes to the extrinsic pathway of cancer development, and separately inhibits Ral signaling, which contributes to the intrinsic pathway of cancer development.
5) Conclusions
After more than a century, Virchow’s hypothesis of a functional relationship between cancer and inflammation appears to have been vindicated. What used to be seen as unrelated events are now generally considered to be deeply intertwined processes that feed on both the intrinsic and the extrinsic pathway of cancer development. The recent discovery that inflammation and tumor growth are not only parallel processes enhancing each other, but that κB-Ras proteins form a physical, molecular connector between them, further emphasizes the importance of investigating the interface of both pathways. Ras and NF-κB signaling are involved in such a breadth of biological processes that any broad-acting inhibition of either pathway – or both – is likely to have severe systemic side effects and thus be unsuitable as chronic treatment for long-term illnesses, such as cancer or chronic inflammation. Therefore, targeted intervention at the level of more specific modulators of signaling offers a more appealing strategy than shutting down the entire pathway. For instance, overexpression of κB-Ras proteins does not abrogate NF-κB signaling completely but modifies expression of a subset of IκB-β-dependent target genes [23,33,34]. Similarly, overexpression of κB-Ras does not eliminate Ras signaling but acts specifically on the Ral pathway, which is essential for anchorage-independent proliferation, while leaving PI3K-dependent signaling intact [23,29–31]. Nonetheless, expression of κB-Ras proteins is sufficient to significantly reduce the growth of tumor cells [23]. Thus, therapeutic intervention focusing on specific modulators of signaling may open up effective but safe new approaches to chemotherapy.
Acknowledgments
The work described in this review that was carried out in the author’s laboratory was funded by NIH (R01 AI093985-03 to SG) and the Deutsche Forschungsgemeinschaft (PO 1946/1-1 to TSP).
Footnotes
ETHICAL APPROVAL
No ethical approval is required for this article.
CONFLICTS OF INTEREST
There are no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Virchow R. Reizung und Reizbarkeit. Archiv F Pathol Anat. 1858;14:1–63. [Google Scholar]
- 2.Virchow R. Die krankhaften Geschwülste. Verlag Von August Hirschwald; 1863. [Google Scholar]
- 3.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? The Lancet. 2001 doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 7.Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–6. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 8.Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–31. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox AD, Der CJ. Ras history: The saga continues. Small GTPases. 2010;1:2–27. doi: 10.4161/sgtp.1.1.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasahira T, Kirita T, Kuniyasu H. Update of molecular pathobiology in oral cancer: a review. Int J Clin Oncol. 2014;19:431–6. doi: 10.1007/s10147-014-0684-4. [DOI] [PubMed] [Google Scholar]
- 11.Murugan AK, Munirajan AK, Tsuchida N. Ras oncogenes in oral cancer: the past 20 years. Oral Oncol. 2012;48:383–92. doi: 10.1016/j.oraloncology.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Caulin C, Nguyen T, Longley MA, Zhou Z, Wang X-J, Roop DR. Inducible activation of oncogenic K-ras results in tumor formation in the oral cavity. Cancer Res. 2004;64:5054–8. doi: 10.1158/0008-5472.CAN-04-1488. [DOI] [PubMed] [Google Scholar]
- 13.Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wertz IE, Dixit VM. Signaling to NF-kappaB: regulation by ubiquitination. Cold Spring Harb Perspect Biol. 2010;2:a003350. doi: 10.1101/cshperspect.a003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilmore TD. Multiple mutations contribute to the oncogenicity of the retroviral oncoprotein v-Rel. Oncogene. 1999;18:6925–37. doi: 10.1038/sj.onc.1203222. [DOI] [PubMed] [Google Scholar]
- 16.Gasparini C, Celeghini C, Monasta L, Zauli G. NF-κB pathways in hematological malignancies. Cell Mol Life Sci. 2014;71:2083–102. doi: 10.1007/s00018-013-1545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greten FR, Eckmann L, Greten TF, Park JM, Li Z-W, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Nibali L, Fedele S, D’Aiuto F, Donos N. Interleukin-6 in oral diseases: a review. Oral Dis. 2012;18:236–43. doi: 10.1111/j.1601-0825.2011.01867.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee C-H, Chang JS-M, Syu S-H, Wong T-S, Chan JY-W, Tang Y-C, et al. IL-1β promotes malignant transformation and tumor aggressiveness in oral cancer. J Cell Physiol. 2015;230:875–84. doi: 10.1002/jcp.24816. [DOI] [PubMed] [Google Scholar]
- 20.Page A, Cascallana JL, Casanova ML, Navarro M, Alameda JP, Pérez P, et al. IKKβ overexpression leads to pathologic lesions in stratified epithelia and exocrine glands and to tumoral transformation of oral epithelia. Mol Cancer Res. 2011;9:1329–38. doi: 10.1158/1541-7786.MCR-11-0168. [DOI] [PubMed] [Google Scholar]
- 21.Henry DO, Moskalenko SA, Kaur KJ, Fu M, Pestell RG, Camonis JH, et al. Ral GTPases contribute to regulation of cyclin D1 through activation of NF-kappaB. Mol Cell Biol. 2000;20:8084–92. doi: 10.1128/mcb.20.21.8084-8092.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clément J-F, Meloche S, Servant MJ. The IKK-related kinases: from innate immunity to oncogenesis. Cell Res. 2008;18:889–99. doi: 10.1038/cr.2008.273. [DOI] [PubMed] [Google Scholar]
- 23.Oeckinghaus A, Postler TS, Rao P, Schmitt H, Schmitt V, Grinberg-Bleyer Y, et al. κB-Ras Proteins Regulate Both NF-κB-Dependent Inflammation and Ral-Dependent Proliferation. Cell Rep. 2014 doi: 10.1016/j.celrep.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fenwick C, Na SY, Voll RE, Zhong H, Im SY, Lee JW, et al. A subclass of Ras proteins that regulate the degradation of IkappaB. Science. 2000;287:869–73. doi: 10.1126/science.287.5454.869. [DOI] [PubMed] [Google Scholar]
- 25.Tago K, Funakoshi-Tago M, Sakinawa M, Mizuno N, Itoh H. KappaB-Ras is a nuclear-cytoplasmic small GTPase that inhibits NF-kappaB activation through the suppression of transcriptional activation of p65/RelA. J Biol Chem. 2010;285:30622–33. doi: 10.1074/jbc.M110.117028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Vallee S, Wu J, Vu D, Sondek J, Ghosh G. Inhibition of NF-kappaB activity by IkappaBbeta in association with kappaB-Ras. Mol Cell Biol. 2004;24:3048–56. doi: 10.1128/MCB.24.7.3048-3056.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Wu J, Ghosh G. KappaB-Ras binds to the unique insert within the ankyrin repeat domain of IkappaBbeta and regulates cytoplasmic retention of IkappaBbeta x NF-kappaB complexes. J Biol Chem. 2003;278:23101–6. doi: 10.1074/jbc.M301021200. [DOI] [PubMed] [Google Scholar]
- 28.Feig LA. Ral-GTPases: approaching their 15 minutes of fame. Trends Cell Biol. 2003;13:419–25. doi: 10.1016/s0962-8924(03)00152-1. [DOI] [PubMed] [Google Scholar]
- 29.Bodemann BO, White MA. Ral GTPases and cancer: linchpin support of the tumorigenic platform. Nat Rev Cancer. 2008;8:133–40. doi: 10.1038/nrc2296. [DOI] [PubMed] [Google Scholar]
- 30.Kashatus DF. Ral GTPases in tumorigenesis: emerging from the shadows. Experimental Cell Research. 2013;319:2337–42. doi: 10.1016/j.yexcr.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirakawa R, Horiuchi H. Ral GTPases: Crucial Mediators of Exocytosis and Tumorigenesis. J Biochem. 2015 doi: 10.1093/jb/mvv029. [DOI] [PubMed] [Google Scholar]
- 32.Jou Y-J, Lin C-D, Lai C-H, Chen C-H, Kao J-Y, Chen S-Y, et al. Proteomic identification of salivary transferrin as a biomarker for early detection of oral cancer. Anal Chim Acta. 2010;681:41–8. doi: 10.1016/j.aca.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 33.Rao P, Hayden MS, Long M, Scott ML, West AP, Zhang D, et al. IkappaBbeta acts to inhibit and activate gene expression during the inflammatory response. Nature. 2010;466:1115–9. doi: 10.1038/nature09283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheibel M, Klein B, Merkle H, Schulz M, Fritsch R, Greten FR, et al. IkappaBbeta is an essential co-activator for LPS-induced IL-1beta transcription in vivo. J Exp Med. 2010;207:2621–30. doi: 10.1084/jem.20100864. [DOI] [PMC free article] [PubMed] [Google Scholar]