Abstract
microRNAs (miRNAs) are a family of small, non-coding RNA molecules that negatively regulate protein expression by either inhibiting the initiation of the translation of mRNA, or by inducing the degradation of mRNA molecules. Accumulating evidence suggests that miRNA-mediated repression of protein expression is of paramount importance in a broad range of physiologic and pathologic conditions. In particular, miRNA-induced dysregulation of molecular processes involved in inflammatory pathways has been shown to contribute to the development of chronic inflammatory diseases. n this review, we first provide an overview of miRNA biogenesis, main mechanisms of action, and currently available miRNA profiling tools. Next, we summarize the available evidence supporting a specific role for miRNAs in the pathobiology of periodontitis. Based on a review of available data on the differential expression of miRNAs in gingival tissues in states of periodontal health and disease, we address specific roles for miRNAs in molecular and cellular pathways causally linked to periodontitis. Our review points to several lines of evidence suggesting the involvement of miRNAs in periodontal tissue homeostasis and pathology. Although the intricate regulatory networks affected by miRNA function are still incompletely mapped, further utilization of systems biology tools is expected to enhance our understanding of the pathobiology of periodontitis.
Keywords: small RNAs, epigenetics, expression regulators, inflammation
The notion that biological information is transferred from DNA to messenger RNA (mRNA) to protein in a linear and sequential manner, hailed as the ‘central dogma of molecular biology’, was introduced by Crick in 1958 (33). However, a little more than a decade later, Crick himself admitted that his concept was likely a major over-simplification (32). Today, it is clear that mRNA transcription does not inevitably lead to protein translation, and the processes involved in post-transcriptional regulation are a fertile field of research. A prominent role in this context is attributed to microRNAs (miRNAs), a relatively recently discovered family of small (~ 22 base-long) non-coding RNA molecules that can either inhibit the initiation of the translation of mRNA into proteins, or induce the degradation of mRNA molecules. Either way, miRNA expression leads to significant repression of their target genes and results in profound effects on protein translation. It is assumed that ~ 60% of all protein-coding genes are targets of miRNAs (49).
The first described miRNA (lin-4) and its regulatory function were both reported in 1993, based on work carried out in the Caenorhabditis elegans nematode by the groups of Victor Ambros and Gary Ruvkun (94, 187). Since then, additional miRNAs have been discovered at an exponential pace: at the time of authorship of this manuscript, the latest version of the miRNA database miRBase (version 21, released June 26, 2014) listed a total of 2,588 mature miRNAs in humans, and 1,915 miRNAs in mice (56).
Regulation of protein expression by miRNAs is critically important in homeostasis and pathology alike. Aberrant miRNA expression triggers the dysregulation of multiple cellular processes involved in both the innate and adaptive immune responses, leading to either ineffective countering of microbial challenges, or excessive catabolic responses. In both instances, this miRNA-induced dysregulation facilitates the development of chronic inflammatory diseases. In this review, we provide an overview of current knowledge in basic miRNA biology as it relates to pathways associated with inflammatory periodontal disease.
microRNA fundamentals
Biogenesis
The Biogenesis of mammalian miRNAs is a multistep process (Fig. 1). It involves the generation of genome-encoded miRNA precursors in the nucleus and their transportation and further processing in the cytoplasm (63). Genes encoding for miRNAs can either be found (i) between protein-coding genes, (ii) as polycistronic transcripts under their own promoters, or (iii) in intronic regions of protein-coding genes that often encode multiple end-product miRNAs (107).
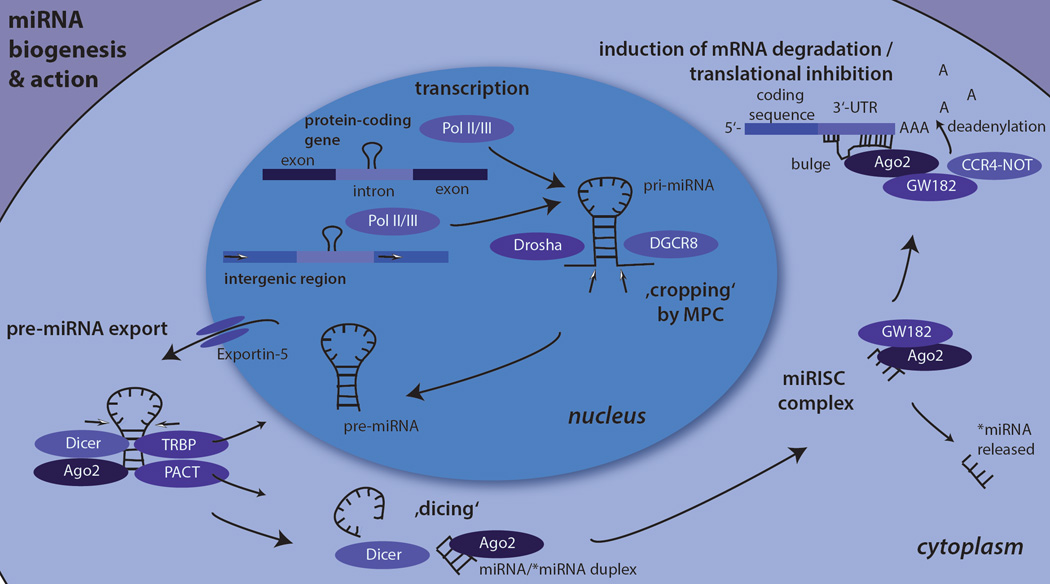
Fig. 1. miRNA biogenesis and action.
The figure shows the principal events of the canonical miRNA biogenesis pathway and their actions in human cells. First, primary-miRNA (pri-miRNA) is transcribed by RNA polymerases II or III from either intronic regions within protein-coding genes or intergenic regions. Second, pri-miRNA is cropped by the Microprocessor complex (MPC) unit comprising of Drosha and DGCR8 (Pasha) into precursor-miRNA (pre-miRNA). Third, pre-miRNAs are exported from the nucleus to the cytoplasm by Exportin-5, where pre-miRNA is further cleaved by Dicer into a short guide/passenger strand (miRNA/*miRNA) duplex. One of the strands is incorporated in the miRNA-induced silencing complex (miRISC) with GW182 and an Argonaute protein, most frequently Argonaute −2. The miRISC-attached miRNA binds to the 3’- untranslated region of a target mRNA, the CCR4-Not complex recruited by GW182 leads to translational inhibition of the mRNA and/or to deadenylation of the mRNA´s poly(A) tail and its subsequent degradation.
miRNA genes are most often transcribed by RNA polymerase II (97), or less frequently by RNA polymerase III (15). This transcription results in the production of long primary miRNAs (pri-miRNAs) that contain a characteristic stem-loop structure. Similarly to miRNAs, these long pri-miRNAs are co-transcriptionally processed (115). They are then ‘cropped’ by the RNase III Drosha into small hairpin-like precursor miRNAs (pre-miRNAs) that are approximately 60 nucleotides long (95). This ‘cropping’ process is mediated by the microprocessor complex (55). The microprocessor complex is a large protein complex comprising of Drosha and DiGeorge syndrome critical region gene 8 (DGCR8, Pasha) (138). Importantly, the processing of pri-miRNAs can be blocked post-transcriptionally (175) by RNA binding-proteins, allowing for additional regulation of these molecules.
Next, the pre-miRNAs are actively transported to the cytoplasm by the Exportin-5-Ran-GTP complex (196). There, they encounter another RNase III termed Dicer. Dicer acts together with Argonaute-2 and either or both (87, 92) of the two double-stranded RNA-binding proteins HIV-1 transactivation response RNA-binding protein (25) and protein activator of PKR kinase (96). It cleaves pre-miRNAs into approximately 22-nucleotide long “guide-strand”/”passenger strand” duplexes (miRNA/miRNA*). This process is referred to as ‘dicing’.
In addition to the classical (canonical) pathway described above, alternative processing pathways of pre-miRNA can be independent of either Drosha or Dicer. For example, in cases where introns encode pre-miRNA-like genes (‘mirtrons’) that are transcribed independently of their original genomic environment (145) and are excised by the spliceosome, there is no need for cropping by Drosha and the transcripts are direct Dicer substrates (34, 91). A variant of mirtrons that do not depend on spliceosome excision of the intronic information are termed ‘simtrons’ (67). Conversely, in the biogenesis of Dicer-independent miRNAs such as miR-451, pri-miRNAs are processed by Drosha into pre-miRNA that is directly loaded by Argonaute and cleaved by the Argonaute catalytic center (22).
Drosha and Dicer cleavage of miRNA precursors is an imprecise process that can lead to length variants or shifted sequences (59) resulting in miRNAs termed ‘isomiRs’ (58, 121). Most isomiRs vary in size from the canonical miRNA by one or more bases at the 3’ and/or 5’ end. The 5’ isomiR variants are of greater functional importance, likely because the 5’ seed regions mediate most of the miRNA-mRNA interactions (162). These isomiRs can either regulate the same targets as the canonical miRNAs (108), or may vary in their repression of gene expression because of changes to their target specificity (60, 162).
Mechanisms of action
The duplex of the guide/passenger strand (miRNA/miRNA*) is loaded on the Argonaute 2 protein during the assembly of a ribonucleoprotein complex termed the miRNA-induced silencing complex (miRISC). The miRISC is constructed from one of the four paralogs of the Argonaute protein subfamily, most frequently Argonaute 2 (110), and the trinucleotide repeat containing protein 6 (TNRC6, also known as glycine-tryptophan 182-kDa protein, GW182). The passenger strand is subsequently released and degraded. The bound guide strand then directs the miRISC to interact with partially complementary sequences in target mRNAs most often located in the 3’-untranslated region. Binding to the target mRNAs either results in the deadenylation and subsequent degradation of transcripts or, less frequently, in inhibition of the translation of the mRNA. In addition, several other less prevalent mechanisms for the regulation of gene expression by miRNAs have been described (for review see (158)).
At the molecular level, the two aforementioned major mechanisms for expression regulation by miRNAs are today thought to involve the following steps: the 5’ end of the miRISC-attached miRNA binds the target mRNA. This binding is mediated by a perfect 6 to 8 nucleotide-long complementary sequence between the mRNA and a specific, short ‘seed’ sequence on the miRNA. Adjacent to the complementary sequence, a non-complementary ‘bulge’ sequence is found (17, 18). Subsequently, the glycine-tryptophan repeats of GW182 recruit a multicomponent nuclease, the CCR4-NOT complex. This complex deadenylates the target mRNA and inhibits its translation. Mechanistically, the recruitment of CCR4-NOT triggers the release of poly(A)-binding protein from the transcript´s poly(A) tail (199). This loss leads to disrupted mRNA circularization that facilitates repression of translation and deadenylation. Based on studies in Drosophila cells, it was first assumed that both effects, i.e., translational inhibition and deadenylation/decay of target mRNAs, commence sequentially (40). However, the most frequently employed mechanism of action in mammalian cells is the destabilization of target mRNAs (57). Post-transcriptional regulation of miRNA precursors is common. The expression level of mature miRNAs can be further altered by the action of RNA binding proteins (173) or of circular RNAs acting as endogenous miRNA ‘sponges’ (65, 111). Given the limited complementarity between miRNA and the target mRNA required for miRNA action, a single miRNA usually targets many different mRNAs; conversely, a single mRNA is also targeted by several different miRNAs. Multiple mechanisms control the expression and function of miRNAs on target mRNAs, and their significance remains to be fully understood.
microRNA profiling
Assessment of expression of a single, multiple, or all possible miRNAs in a sample is achieved using available technical platforms, each of which has its own characteristics, advantages and shortcomings (for detailed review, see (133), for annotated protocols see (79)). In general, isolation of miRNAs in a cell or tissue sample is very similar to that for total RNA, but specific precautions need to be taken to preserve the small RNA fraction. Depending on the application, an enrichment or isolation of the small RNA fraction may be necessary (1). Very frequently, total RNA including the small RNA fraction is isolated from homogenized cells or tissues using modifications of the Chomczynski & Sacchi method (27) that utilizes concentrated chaotropic salts, such as guanidine thiocyanate, followed by a solid-phase extraction step using silica columns. Enrichment of the miRNA fraction can be done by size fractionation and subsequent cleanup of the excised relevant portion of the sample from a gel. Alternatively, a less accurate but faster way to enrich small RNAs is the use of specific spin columns.
The assessment of the quality and quantity of the isolated miRNA is of critical importance. In most cases where total RNA including the small RNA fraction is desired, the quality control procedures are very similar to those for mRNA profiling studies. miRNA quantity can be assessed using a spectrophotometer, while both quantity and quality can be simultaneously assessed using automated capillary electrophoresis instruments and specific chips designed to analyze total RNA integrity and to estimate miRNA abundance. Accurate estimation of miRNA abundance is possible only when the quality and integrity of the total RNA preparation is high, since small degradation products cannot be distinguished from miRNAs (11). In cases where high efficiency miRNA extraction is essential, e.g. when profiling extracellular miRNAs in plasma or serum, synthetic miRNA ‘spike-in’ controls can be used to monitor the extraction process and normalize across different samples.
Several challenges are inherent in the accurate identification of miRNAs. First, the limited length of only ~ 22 nucleotides and the absence of a poly-A tail in mature miRNAs render the annealing of ‘traditional’ primers both for reverse transcription and detection difficult or impossible (89). Second, many miRNAs within a certain family differ only marginally in sequence, often only in a single nucleotide, necessitating high accuracy of the profiling method. Third, isomiRs which often have significantly different biological effects (58) are particularly difficult to distinguish due to minimal discrepancies in length and common, but shifted, sequences (144). Lastly, the short sequence length of miRNAs results in a wide range of melting temperatures that may lead to bias in favor of certain miRNAs in subsequent annealing reactions.
The commonly used miRNA-profiling platforms today utilize (i) quantitative reverse transcription polymerase chain reaction (RT-PCR/qPCR), (ii) microarray hybridizations, or (iii) next generation RNA-sequencing. It is important to realize that data generated by different platforms are not interchangeable, as recently demonstrated in a microRNA quality control study (112). In qPCR-based miRNA expression assays, miRNA is reverse transcribed into cDNA using either stem-loop primers specific to the 3’ end of the miRNA that generate a reverse transcription binding site (174), or by enzymatic addition of a poly-A tail, so that traditional oligo-dT primers can be used (50). The cDNA obtained can then be quantified using qPCR technology, either using SYBR green-based protocols (150), or by employing hydrolysis probes (23). qPCR-based detection of miRNAs was shown to be sensitive and specific, with a high dynamic range and a possibility for absolute quantification of copy numbers (112). On the downside, the aforementioned strong variation of melting temperatures renders highly parallel qPCR for many miRNAs difficult, since the optimal annealing temperatures vary significantly. This issue is currently addressed by the use of locked nucleic acids-modified primers that allow the optimization of melting temperatures by the introduction of modified nucleotides (6). Another limitation of qPCR-based miRNA profiling is that only known miRNAs can be detected, and that isomiR detection is reduced in comparison to sequencing (157). Lastly, the platform, often in 96-well or microfluidic card format, makes high-throughput processing of large numbers of samples logistically difficult and costly, especially at a genome-wide level.
Microarray hybridization was one of the first methods developed for high-throughput analysis with respect to large numbers of miRNAs. In this platform, miRNAs in a sample are fluorometrically-labeled and hybridized to DNA-based probes on an array or beads. Commonly, this labeling is done by the T4 ligase-mediated ligation of a fluorochrome-linked nucleotide to the 3’-OH group of the miRNA (35, 178). Here, it is important to counter the risk of circularization that exists in presence of a T4 ligase, since the 5’ phosphate group of the miRNA produced during Dicer cleavage is dephosphorylated at an initial step in this protocol (178). Several other methods for labeling exist that are meant to reduce substrate sequence bias in the labeling process. It must also be kept in mind that the variable melting temperature of miRNAs is an issue that applies to microarray hybridizations as well; therefore, locked nucleic acid modifications of probes are used in some products (20). The major advantage of microarray-based miRNA profiling platforms is that they facilitate high-throughput detection of a large number of miRNAs at relatively low cost. Downsides include reduced specificity and a lower dynamic range when comparing to qPCR-based assays (112), and imprecision in the assessment of absolute copy numbers (13). As is the case in qPCR detection, the microarray hybridization assays can only detect miRNAs that have already been described and are included on the array.
Lastly, next generation RNA-sequencing platforms offer the possibility to detect both already described and new miRNAs and their sequence variants (93, 161). Several different sequencing platforms exist, but they all share common features (85). First, a small RNA library is prepared by reverse transcription into cDNA and subsequent ligation of adapters. Then, all molecules in the library are read in a ‘massively parallel’ way, i.e. millions to billions of short sequencing reads are processed per instrument run. The obtained millions of ‘reads’ are subsequently aligned to the genome and relatively quantified (10, 46). This technology is currently quite expensive, requires specialized computational infrastructure and skills for data analysis (29), and may also be subject to bias introduced during library preparation (172). Nevertheless, introduction of new, more efficient sequencing devices, as well as the development of DNA barcoding, which allows for multiplexing of several samples in a single sequencing lane but may also increase bias (4), is expected to result in more widespread utilization and reduced costs (46).
As mentioned earlier, the recent microRNA quality control study (112) revealed substantial differences in data generated across miRNA expression platforms. Therefore, it is widely recommended to independently confirm the findings of a single profiling study using another, independent method (2, 112). For genome-wide profiling in large numbers of samples, especially when comparing relative abundance of miRNAs in relation to a given diagnosis (such as in ‘health’ and ‘disease’), microarray hybridizations are still considered a reasonable option. Nevertheless, the inherent shortcomings of this platform discussed above and the ever decreasing cost of next generation sequencing make it likely that future large scale studies will employ the latter methodology. qPCR assays are ideally suited for independent confirmation of findings, due to their demonstrated superior sensitivity and specificity.
microRNAs in periodontitis and periodontitis-related mechanisms
microRNAs in states of periodontal health and disease
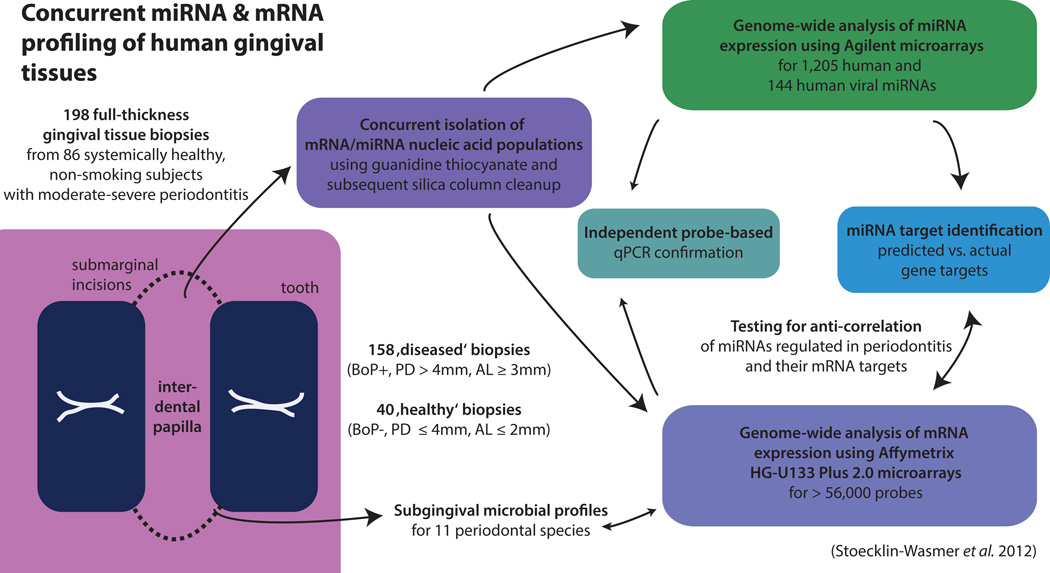
By the time of authorship of this review, only four studies have examined the expression of miRNAs in healthy and diseased gingival tissues (98, 131, 156, 191) (Table 1). Of these, two studies (98, 191) compared miRNA expression data between a single pair of pooled samples from healthy and diseased gingival tissues. Thus, no meaningful inferences related to the differential expression of specific miRNAs between states of gingival health and disease can be derived from these studies. The third available study (131) is a pilot investigation that employed a focused qPCR-based array to compare healthy and diseased gingival tissue biopsies from 20 obese or non-obese individuals with respect to the expression of 88 miRNAs with putative or known roles in inflammation. The largest study available to date (156) reported data from 86 systemically healthy, non-smoking subjects with moderate to severe periodontitis. These investigators carried out genome-wide miRNA profiling in 198 gingival tissue samples, 40 of which were harvested from periodontally ‘healthy’ sites and 158 from ‘diseased’ sites. Notably, mRNA profiles (38) and microbial colonization patterns in the proximal periodontal pockets (129) were also available from these tissue biopsies (Fig. 2).
Table 1.
Studies reporting miRNA expression in diseased vs. healthy gingival tissues
| Study | N (samples/subjects) | Method | Confirmation | Correction for multiple testing |
Findings | Comments |
|---|---|---|---|---|---|---|
| Stoecklin- Wasmer et al. (156) |
198 gingival tissue samples (158 ‘diseased’, 40 ‘healthy’) from 86 subjects |
Hybridization (Agilent microarrays) |
qPCR (probe-based) | Yes (FDR) | 159 differentially expressed miRNAs at FDR<0.05; 4 miRNAs were upregulated by >2- fold (hsa-miR-451, hsa-miR-223, hsa- miR-486-5p, hsa- miR-3917), and 7 miRNAs were downregulated by > 2-fold (hsa-miR- 1246, hsa-miR- 1260, hsa-miR-141, hsa-miR-1260b, hsa- miR-203, hsa-miR- 210, hsa-miR-205*) in disease vs. health |
Concurrent analysis of miRNA and mRNA expression; functional annotation by miRNA target Enrichment Analysis; gingival tissue samples obtained from untreated periodontitis |
| Perri et al. (131) |
40 gingival tissue samples (20 ‘diseased’, 20 ‘healthy’ gingival tissues) from 20 individuals (10 obese and 10 non-obese), with or without periodontitis |
qPCR array (SABiosciences); SYBR green- based assessment of 88 miRNAs |
No | No | Using miRNA expression at healthy gingiva from non-obese subjects as the reference, two miRNAs were upregulated in non- obese individuals with periodontitis (miR 30e, miR- 106b), nine in obese individuals with periodontitis (miR- 15a, miR-18a, miR- 22, miR-30d, miR- 30e, miR-103, miR- 106b, miR-130a, miR-142-3p, miR- 185, and miR-210), and two in obese, periodontally healthy individuals (miR-18a, miR-30e) |
|
| Xie et al. (191) | 20 gingival tissue samples samples (10 ‘diseased’, 10 ‘healthy’) from 20 subjects, half of whom had periodontitis; all samples from the same condition were pooled (two samples in total were analyzed) |
Hybridization (Exiqon microarray) |
qPCR (SYBR green based) |
No | 91 miRNAs upregulated and 34 down-regulated by at least twofold in disease vs. health |
No statistics possible due to pooling of the samples; gingival tissue samples obtained after completed initial therapy |
| Lee et al. (98) | No sample size reported | qPCR array (SABiosciences; 88 miRNAs, SYBR green- based) |
qPCR (probe-based) | No | 6 miRNAs differentially expressed > 8-fold, 22 miRNAs > 4-fold in disease vs. health |
No statistics reported |
Fig. 2. Concurrent miRNA and mRNA profiling of human gingival tissues.
The largest study of miRNA expression in human periodontitis lesions available to date (Stoecklin-Wasmer et al. [156]) reported data from 86 systemically healthy, non-smoking subjects with moderate to severe periodontitis. Genome-wide miRNA and mRNA profiling was concurrently carried out in 198 gingival tissue samples, 40 of which were harvested from periodontally ‘healthy’ sites and 158 from ‘diseased’ sites. miRNA and mRNA expression data where analyzed to distinguish between predicted and actual gene targets.
An important caveat with all available studies to date is that they have assessed miRNA expression in whole tissue gingival biopsies that contain a mixture of cellular components the proportions of which may vary significantly between health and disease. Thus, it is impossible to ascertain whether the increased expression of a specific miRNA recorded in one condition over the other is in fact the result of a true increased transcription of the particular sequence, is due to the proliferation of a specific cell type that harbors the transcript at an unchanged level, or a combination of both. For example, a miRNA that is expressed at high levels in mononuclear cells, like miR-155 (45), could thus appear as differentially expressed in ‘diseased’ versus ‘healthy’ tissue samples irrespective of an actual up-regulation of this miRNA in the particular cell type, since ‘diseased’ tissues are characterized by a large mononuclear cell infiltrate. Ideally, cell type-specific expression profiles can be generated after isolation of homogeneous cell populations using laser capture microdissection, or by fitting models that correct mathematically for the different proportions of cell populations in the individual samples (90), as recently performed in the context of natural killer cell biology in periodontitis (88).
In the following paragraphs, we review the available evidence suggesting a role for particular miRNAs in molecular and cellular pathways involved in periodontal tissue inflammation. We primarily focus on those miRNAs that were shown to be differentially expressed between healthy and periodontitis-affected gingival tissues in the above studies (Table 2), as well as on a number of miRNAs that have been studied in cell culture or animal models of periodontal infection. The observed direction of the differential regulation (i.e., overexpression in periodontal health or disease) is also described in Table 2.
Table 2.
Specific miRNAs with reported roles in periodontal inflammation
| miRNA | Differential regulation in ‘diseased’ vs. ‘healthy’ gingival tissues |
Reported function | Comment |
|---|---|---|---|
| Let-7i | Upregulated | Targets TLR4 | |
| miR-16 | Upregulated | Activated by p53, facilitates cell cycle arrest and apoptosis |
|
| miR-29a | Upregulated | Downregulates IL-23 by targeting IL12p40 and p19; inhibits both intrinsic and extrinsic apoptosis; hypoxamir, induces angiogenesis in hypoxia; mediates osteoblast differentiation by targeting Wnt signaling inhibitors |
|
| miR-30e | Downregulated | Inhibits NK cell cytotoxicity | |
| miR-31 | Downregulated | Targets a negative regulator of NF-kB; mediates osteoclastogenesis and represses osteogenesis by targeting Osterix |
|
| miR-125-3p | Upregulated | Activates p53, pro-apoptotic | |
| miR-141 | Downregulated | Targets MAP4K4 (a direct inducer of MAP kinase signaling) and SDF- 1/CXCL12 (mediator of leukocyte migration) |
|
| miR-146a | Upregulated | NF-kB dependent miRNA that targets TLR4, IRAK1/TRAF4; increases DC survival by reducing their cytokine production; regulates B cell development, ‘brake’ on autoimmunity; activated by periodontitis-associated TF Bob1 |
Critical for steering TLR sensitivity |
| miR-148a | Upregulated | Impairs innate response and antigen presentation of DCs; targets anti- apoptotic Bcl2; mediates osteoclastogenesis |
|
| miR-155 | Upregulated | miRNA that targets NF-kB signaling; activated by NF-kB, fos/jun, NOD2 signaling, mediates type 1 interferon response to infection; activates PI3K/Akt pathway; induces activation of NK cells; critical for DC maturation and function; essential for Th17 response and T cell development; inhibits DNA damage-induced apoptosis; hypoxamir that triggers cell cycle arrest, induces autophagy and targets HIF-1α in hypoxia (pro- survival); induced by adiponectin stimulation |
Critical for steering TLR sensitivity, directly affects expression level of other miRNAs; miR-155 targeting improves chronic inflammatory diseases |
| miR-185 | Upregulated | Enhances DNA damage-induced apoptosis |
|
| miR-200a | Downregulated | Negatively regulates IL-12 signaling in NK cells |
|
| miR-200c | Downregulated | Targets TLR4; targets pro-apoptotic Noxa and anti-apoptotic FAP-1; inhibits endothelial cell differentiation and angiogenesis; induces osteoclastogenesis |
|
| miR-203 | Downregulated | Targets VEGFA, inhibiting angiogenesis | |
| miR-210 | Downregulated | Impairs immune-suppressive functions of Tregs by targeting FOXP3; hypoxamir, controls host responses in hypoxia by induction of autophagy and targeting of HIF-1α (pro-survival) |
|
| miR-451 | Upregulated | Suppresses neutrophil chemotaxis via p38 MAPK; reduces cytokine secretion in DCs |
Dicer-independent miRNA |
| miR-486 | Upregulated | Disrupts NF-kB negative feedback loops, leading to stronger NF-kB activation |
|
| miR-497 | Upregulated | Facilitates intrinsic apoptosis | |
| miR-650 | Upregulated | Regulates B cell proliferation by targeting CDK1 |
microRNAs and the innate immune response
The host’s first line of immune response against pathogens, i.e., the innate immune response, involves a number of pattern-recognition receptors, most notably the family of Toll-like receptors. Upon ligation, these receptors initiate intracellular signaling cascades that trigger direct cellular responses, including the secretion of cytokines (52). Toll-like receptor-2 and Toll-like receptor-4 are by far the most studied pattern recognition receptors in the context of periodontal diseases (142), given their ability to interact with specific pathogens and their components in the periodontal biofilm, including lipopolysaccharide from Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans and other gram-negative periodontal species (12).
A crucial pathway activated downstream of Toll-like receptor binding is the one involving the NF-ΚB family, a rapid responder transcription factor that is able to mediate prompt changes in the expression of target genes. NF-ΚB signaling is targeted by several miRNAs, notably miR-146a, that is itself regulated by NF-ΚB activation in response to bacterial stimuli via Toll-like receptor-2, Toll-like receptor-4, Toll-like receptor-5 or Toll-like receptor-9, and was shown to trigger a negative feedback loop resulting in down-regulation of the two key NF-ΚB/tumor necrosis factor signaling adapter molecules interleukin-1 receptor-associated kinase-1 and tumor necrosis factor receptor-associated factor-6 (160). Importantly, this immunosuppressive miRNA was found to be significantly up-regulated in inflamed gingival tissues, as compared to clinically ‘healthy’ gingiva in humans as well as in mice (118), suggesting a role in regulation of immune responses in periodontitis. Furthermore, Toll-like receptor-2-mediated inflammatory responses were suppressed by miR-146a in keratinocytes (109) and macrophages (134), suggesting that this miRNA has a role in the regulation of Toll-like receptor-mediated sensitivity by preventing the development of excessive inflammation (155). On the other hand, stimulation with P. gingivalis lipopolysaccharide was found to induce miR-146a in macrophages without resulting in lower cytokine production (70), suggesting counteracting effects by other positive regulators. For example, the gingival health-associated miR-200c targets Toll-like receptor-4 and down-stream NF-ΚB signaling (186), translating into a Toll-like receptor sensitizing pro-inflammatory effect in diseased tissues (136).
Another miRNA with a well-established role in innate immunity that also acts via the NF-ΚB pathway is miR-155 (45, 164). This miRNA is indirectly induced via NF-ΚB and fos/jun signaling in response to Toll-like receptor ligation by both bacterial and viral stimuli, as well as after stimulation by cytokines (30, 31). In periodontal ligament cells, the induction of miR-155 was found to lead to a down-regulation of NF-ΚB signaling and promotion of cell differentiation (74). Alternatively, miR-155 was found to be activated by the sensing of bacterial peptidoglycan through the cytoplasmic pattern recognition receptor nucleotide-binding oligomerization domain containing 2 (NOD2) (147), a pathway that was most recently linked with periodontitis (198). This particular miRNA was shown to mediate a type I interferon response to infection (183), a possible feature of aggressive periodontitis (124). Furthermore, it is capable of suppressing the expression of the inositol phosphatase SHIP, leading to activation of the PI3K/Akt pathway which has been shown to be critical for the survival of primary gingival epithelial cells infected with P. gingivalis (197).
Furthermore, miR-155 was shown to induce expansion and activation of natural killer cells (169), and to increase the production of interferon-γ (168). Two additional miRNAs found to be down-regulated in inflamed gingival tissues that also have natural killer cell regulatory roles are miR-30e and miR-200a. Specifically, miR-30e inhibits natural killer cell cytotoxicity (182), whereas miR-200e negatively regulates interleukin-12 signaling in natural killer cells (72). The observed reduced expression of these miRNAs in periodontitis is compatible with the increased activation of natural killer cells and higher interferon-γ production associated with periodontal tissue destruction (21, 88).
In macrophages, both miR-146b and miR-155 were shown to mediate the increase in Toll-like receptor responsiveness triggered by leukotriene B4, a lipid mediator formed from arachidonic acid and one of the most potent stimulants of mononuclear cells. This effect was due to a decrease of suppressor of cytokine signaling-1 mRNA stability, leading to a positive regulation of MyD88 (185). Leukotriene B4 was found to be over-expressed in inflamed gingival tissues (83), and its levels in gingival crevicular fluid correlate with periodontitis severity (132). Dysregulation of the expression of miR-146 and miR-155 in primary oral epithelial cells infected with P. gingivalis resulted in increased sensitivity to activation via Toll-like receptor signaling (71, 140). Interestingly, the anti-inflammatory agent triclosan could reconstitute ‘normal’ Toll-like receptor signaling by modulation of miR-146 (176) and miR-155 expression (122).
A member of the highly conserved lethal-7 miRNA family (139), lethal-7i, was found to be overexpressed in periodontitis-affected gingival tissues. Since lethal-7i directly targets the Toll-like receptor-4 receptor (24), this observation is in line with the immuno-suppressive capacity of several miRNAs in response to infection described above. Similarly, the observed down-regulation of miR-31 in periodontitis, a miRNA that targets serine/threonine kinase 40, a negative regulator of NF-ΚB (192), may also result in containment of excessive NF-ΚB-mediated inflammation. Acting towards the opposite direction, miR-486 that was found to be strongly overexpressed in periodontitis lesions was reported to disrupt multiple NF-ΚB negative feedback loops, thereby sustaining NF-ΚB signaling (153).
The family of mitogen activating protein (MAP) kinases have been shown to be triggered in response to Toll-like receptor engagement during the course of periodontal infections (167). One of the top miRNAs associated with gingival health, miR-141, was shown to target mitogen-activated protein kinase kinase kinase kinase 4 (MAP4K4), the direct inducer of MAPK8/c-Jun N-terminal kinase-1. The down-regulation of this miRNA in periodontal disease therefore supports MAP kinase signaling and its pro-inflammatory down-stream events (204).
A key feature of the periodontal lesion is a chemotactic gradient of interleukin-8 (166) and granulocyte chemotactic protein-2 (GCP2/CXCL6) (82) mediating neutrophil migration from the gingival micro-vasculature into the periodontal pocket. A Dicer-independent miRNA that was strongly induced in periodontitis, miR-451 (194), was found to suppress neutrophil chemotaxis in an air-pouch model via the p38 MAPK pathway (117). Another Toll-like receptor-induced mediator of leukocyte migration, the chemokine stromal-cell derived factor-1 (SDF-1/CXCL12), which is strongly up-regulated in human periodontitis along with its receptor CXCR4 (66, 83), is a target of the periodontal health-associated miR-141, increasing the protein´s expression in disease (73).
Dendritic cell signaling bridges innate and adaptive immunity (for review, see (44) in this volume). They detect pathogens and their components using their surface receptors, and produce cytokines that mediate the cellular response. In periodontal infections, dendritic cell signaling is considered a critical step that steers immune responses. The pathways controlling dendritic cell functions are tightly regulated by miRNAs (152). Silencing of c-Fos expression in dendritic cells by miR-155 was shown to be critical for dendritic cell maturation and function, including their ability to trigger a cellular inflammatory response (43). Conversely, the miR-146a was demonstrated to impair Toll-like receptor signaling in dendritic cells, effectively reducing the amount of cytokines produced by the cells, as well as their survival (81). miR-451, strongly over-expressed in inflamed gingival tissues, was shown to reduce cytokine secretion by infected dendritic cells using a negative feedback loop (137). Likewise, miR-148a was found to impair innate response and antigen presentation of Toll-like receptor-triggered dendritic cells by interfering with intracellular calcium homeostasis (104). As discussed above, NOD2 signaling, a pathway recently implicated in the pathogenesis of periodontal disease (198), was demonstrated to induce miR-29a, another miRNA with elevated expression in ‘diseased’ gingival tissues. This particular miRNA was shown to down-regulate interleukin-23 by directly targeting IL-12p40 and indirectly reducing expression of IL-12p19 (16).
In conclusion, the regulation of the innate immune responses involves several miRNAs that can either inhibit or trigger inflammatory pathways, most notably via NF-ΚB signaling (135). Although multiple miRNAs can be induced in the same molecular pathway and may lead to similar functional consequences, their induction and regulatory capacity shows specificity with respect to stimulus or cellular environment (135, 147).
microRNAs and the adaptive immune response
The adaptive immune system involves expansion of antigen-specific clones of T- and B-cells and provides the host with an immunological memory. Adaptive immunity plays a significant role in periodontitis, and established periodontal lesions are characterized by high proportions of antibody-producing plasma cells (151). Below, we summarize findings related to a number of miRNAs that have emerged as potent regulators of adaptive immunity that also have known or suspected roles in periodontal disease.
Several miRNAs shown to regulate B cells (36) were found to be differentially expressed between healthy and inflamed gingival tissues. miR-146a plays a significant role in B cell development and constitutes a brake on autoimmunity, as evidenced by loss-of-function models in mice that showed massive immune defects after miR-146a deletion (14). Conversely, over-expression of miR-146a resulted in an autoimmune lympho-proliferative condition that was primarily driven by dysregulated B cells with strongly reduced expression of the apoptosis molecule Fas (61). B cell activation was shown to be mediated by the lymphocyte transcription factor Bob1, which is strongly up-regulated in periodontitis tissues (38) and activates transcription for miR-146a. The immune restriction exerted by this miRNA is critical for an adequate B cell response involving the generation of germinal centers and a robust immunoglobulin response (103). miR-650, which was also found to be up-regulated in periodontitis lesions, influences the proliferation capacity of B cells by targeting cyclin-dependent kinase 1 (CDK1) and other proteins mediating cell proliferation and survival (116).
In addition to its involvement in innate immunity discussed above, miR-155 plays a role in the regulation of the adaptive immune response (148), in particular in the regulation of T-cells (102). This miRNA was shown to control CD8 T-cell responses by regulating type I interferon signaling (54). Moreover, it activates cytokine gene expression in Th17 and T regulatory cells by exerting effects on the DNA-binding protein Jarid2, which in turn mediates repression of transcription by recruiting the Polycomb repressive Complex 2 to chromatin (47). miR155 was also found to be essential for the indirect activation of a T-helper cell-17 response by dendritic cell signaling (125) (for further details on the role of dendritic cells in the pathogenesis of periodontitis see the review by El-Awady and co-workers in this volume (44)). Induction of miR-155 primarily induced Th1 differentiation of CD4+ cells by inhibition of interferon-gamma signaling (9). In contrast, miR-210, which was found to be under-expressed in periodontitis lesions, was recently demonstrated to target FOXP3 and thereby impair the immuno-suppressive functions of regulatory T-cells (205). Thus, down-regulation of miR-210 promotes regulatory T-cell signaling that was recently shown to be involved in the pathogenesis of periodontitis (130). In addition, the biological effects of miR-155 are not only mediated by direct effects on target genes, but also through a hierarchy of miRNAs that are directly or indirectly regulated by miR-155. For example, miR-155 deficiency results in lower levels of miR-455 in murine dendritic cells, possibly due to a repression of the transcription factor C/EBPbeta by miR-155 (42). Interestingly, miR-155 deficiency or therapeutic targeting was shown to be associated with several chronic inflammatory diseases in vivo, including atherosclerosis (41), experimental myasthenia gravis (184), experimental lupus-like disease (163), and cardiac hypertrophy and failure (69). Helicobacter pylori-infected mice deficient for miR-155 were unable to control infection by the pathogen, but also exhibited less pronounced infection-induced immunopathologies such as gastritis and intestinal metaplasia (126). However, complete miR-155 deficiency, although associated with lower severity of chronic inflammatory or autoimmune diseases, is incompatible with normal adaptive immune responses based on T- or B-cells (155).
Taken together, emerging data support a role of miRNA signaling in the regulation of adaptive immunity in periodontal disease. Given that adaptive immunity is a feature exclusively found in vertebrates, and that the expansion of miRNAs is a vertebrate-only phenomenon, it is conceivable that additional miRNAs play as yet unidentified roles in adaptive immunity (135).
microRNAs and the stress response
During the course of periodontal infections, the cellular components of the periodontium are subject to the effects of several stressors including bacterial products and components, the hypoxic microenvironment, and inflammatory mediators.
Periodontal tissue cells and mononuclear cells of the inflammatory infiltrate frequently undergo programmed cell death in response to pathogen contact. Apoptotic events are particularly pronounced in cell interactions with A. actinomycetemcomitans (83), a key pathogen in aggressive periodontitis. The cytolethal distending toxin of A. actinomycetemcomitans triggers both the DNA damage pathway and the intrinsic apoptosis pathway via the ataxia telangiectasia mutated checkpoint kinase (3). DNA damage-induced apoptosis is regulated by several miRNAs found to be overexpressed in periodontitis, including the inhibitory miR-155 (86), and the enhancing miR-185 (181). Furthermore, miR-148a (202) and miR-16 (5) promote the intrinsic apoptosis pathway by targeting the key anti-apoptotic molecule C-cell lymphoma-2 (Bcl2). miR-16, which is up-regulated by stress-induced activation of the p53 tumor suppressor, also targets the checkpoint kinases Chk1 and Wee1 (100) as well as another critical inhibitor of the DNA damage response, the Wip1 phosphatase (203), leading to cell cycle arrest and facilitating apoptosis. Similarly, miR-497 targets Chk1 (190), Wee1 (28) and Bcl2 (193, 206), also facilitating apoptosis. miR-125a-3p, also over-expressed in periodontitis, was shown to activate p53 and exert pro-apoptotic effects (78). On the other hand, PUMA, a gene that is member of the BH3-only pro-apoptotic BCL2 family is targeted by miR-29a, resulting in impairment of the intrinsic apoptosis pathway (127). In fact, the same miRNA was also implicated in the inhibition of the extrinsic apoptosis pathway (149, 165) which was shown to be involved in periodontitis pathogenesis (105, 114). Likewise, miR-200c that was found to be overexpressed in periodontally healthy gingiva targets the pro-apoptotic BH3-only protein Noxa (99) and the apoptosis inhibitor FAP-1 (146).
Hypoxia, a prevalent feature of deep, inflamed periodontal pockets (123) was shown to augment cytokine secretion induced by Toll-like receptor ligation in periodontal ligament cells (77) and to result in the induction of a specific set of miRNAs termed ‘hypoxamirs’ (120). Among those, miR-155 triggers cell cycle arrest, attenuates cell proliferation, and induces autophagy by targeting the MTOR pathway (177). Autophagy in response to hypoxia has been shown to occur in periodontal ligament cells and gingival epithelial cells (154, 170), and is considered a critical process for facilitating cellular survival under stress (201). Alternatively, in a negative feedback loop, hypoxia-induced miR-155 also targets hypoxia inducible factor (HIF-) 1 α, which is the prime transcription factor that is regulated by the redox state of the cell and mediates its hypoxia response. This way, miR-155 promotes the resolution of HIF-1 α activity in hypoxia (19). A similar phenomenon was most recently described for miR-210 (75, 179) which controls host responses to hypoxia by promoting the induction of autophagic pathways, but also by targeting HIF-1 α, thereby limiting the duration of the hypoxic reaction (37).
Furthermore, hypoxia induces angiogenesis in human endothelial cells through up-regulation of miR-29a (195). Likewise, the decreased expression of miR-203 in pathologic gingival tissues is compatible with increased angiogenesis in periodontitis, as this miRNA targets vascular endothelial growth factor-alpha (207). Similarly, the observed down-regulation of miR-200c in periodontitis also translates in pro-angiogenic effects, as this miRNA was shown to inhibit endothelial cell differentiation and vasculogenesis (106).
Obesity, a pro-inflammatory condition associated with high secretion of inflammatory mediators termed adipokines (48) has been positively associated with periodontitis (53). It was recently shown that adiponectin stimulation of macrophages induces expression of miR-155 (159), similarly to the effect of other inflammatory cytokines. In the pilot investigation by Perri et al. (131), miRNAs associated with periodontal inflammation in obesity had predicted target mRNAs that regulate glucose and lipid metabolism, as well as the inflammatory response. In addition, miR-185, an enhancer of DNA damage-induced apoptosis, was found to be more strongly induced in obese vs. non-obese subjects with periodontitis (Table 1). These data suggest involvement of miRNA signaling in the aggravated periodontal destruction found in obese individuals.
microRNAs and bone biology
Several miRNAs that were differentially regulated in healthy or periodontitis-affected gingival tissues have specific functions in bone homeostasis. For example, miR-148a was found to mediate osteoclastogenesis in CD14+ peripheral blood mononuclear cells induced by receptor activator of NF-ΚB ligand (RANKL), since an synthetic antisense microRNA (antagomir) used to counter its activity resulted in increased bone mass in vivo (26). Conversely, miR-29a modulates Wnt signaling in human osteoblasts through a positive feedback loop that directly targets three negative regulators of Wnt signaling, Dkk1, Kremen2, and secreted frizzled related protein 2 (sFRP2), leading to osteoblast differentiation (80). miR-31, a miRNA found to be downregulated in periodontitis-affected gingival tissues, mediates osteoclastogenesis and represses osteogenesis by targeting Osterix, leading to impaired osteoclast formation and reduced bone resorption (8, 39, 113, 189). Likewise, the observed down-regulation of miR-200c in periodontitis-affected gingiva, a miRNA that targets Notch signaling by inhibiting the Notch-1 ligand Jagged1 (171) and thereby inducing odonto- and osteoclastogenesis (119), likely also constitutes a regulatory mechanism that contains excessive bone loss in periodontal inflammation.
Extracellular messaging by microRNAs
miRNAs are also located extracellularly in bodily fluids such as plasma and saliva. They cofractionate with Argonaute 2 in plasma (7), which offers them protection from degradation (101). These miRNA-protein complexes can also be found encapsulated in cell-secreted exosomes, from where the miRNA sequences can be recovered (143). The miRandola database provides a listing of extracellular miRNAs (141).
Importantly, vesicle-bound miRNAs can enter other cells where they can also exert biological functions, essentially acting as biological messengers. In the cardiovascular field, endothelial microparticles shedded from endothelial cells in response to tissue damage were shown to contain miR-126 (76, 200) which, upon uptake by other cells at distant sites, activated vascular protection pathways via a miR-126 dependent mechanism. Similarly, thrombopoietin-stimulated platelets released miR-223-containing microvesicles that were subsequently uptaken by endothelial cells; in turn, these cells became more susceptible to advanced glycation end product-mediated apoptosis, because of down-regulation of the insulin-like growth factor 1 receptor (128). Interestingly, the level of serum miR-223 was found to be predictive of sepsis (180), indicating a possible role of this miRNA in inflammatory circuits. In addition, circulating miRNAs were shown to activate natural killer cells, but not T-cells, via Toll-like receptor-1 irrespective of their particular sequence (68). This could be of importance in the context of periodontitis since activated natural killer cells are involved in rapid periodontal bone loss (21, 88). Due to their extraordinary stability, extracellular miRNAs can potentially serve as biomarkers for a variety of pathologic conditions, as shown in a pilot study that used a sequencing approach to identify circulating miRNAs in the bloodstream (84). Similarly, miRNAs in saliva (51, 188) hold the potential to be used as markers for periodontitis and other diseases in the future.
Conclusions
As discussed above, miRNAs play a key role in regulating gene expression in mammalian cells affecting several molecular and cellular pathways, ranging from responses to pathogen-derived signals, to mediation of stress responses. Their involvement in periodontal tissue homeostasis and pathology has only begun to be appreciated. Additional research is required to replicate and expand the early findings suggesting differential expression of several miRNAs between ‘healthy’ and ‘diseased’ gingival tissues, to refine the emerging expression patterns with respect to specific cell populations, and to further examine their roles in mechanistic studies. Ultimately, integrated bioinformatics approaches incorporating miRNA expression to reconstruct regulatory networks (62) will further our understanding of the pathobiology of periodontal diseases. Future miRNA-based applications may also include the use of extracellular miRNAs as biomarkers in bodily fluids, as well as their use as exosomal transport vehicles with potential therapeutic functions (64).
Acknowledgements
Work in Dr. Kebschull´s laboratory was supported by Deutsche Forschungsgemeinschaft (DFG KFO208, TP6), the German Society for Periodontology (DGParo) and the German Society for Oral and Maxillofacial Sciences (DGZMK). Work in Dr. Papapanou´s laboratory was supported by NIH grants DE-015649, DE-021820 and UL1-TR000040, and Colgate-Palmolive, NJ, USA.
Contributor Information
Moritz Kebschull, Associate Professor of Dental Medicine, Consultant, Department of Periodontology, Operative and Preventive Dentistry, University of Bonn, Welschnonnenstr. 17, 53111 Bonn, Germany, Tel: +49-228-28722-007, moritz.kebschull@uni-bonn.de
Panos N. Papapanou, Professor of Dental Medicine, Director, Division of Periodontics, Chair, Section of Oral and Diagnostic Sciences, Columbia University College of Dental Medicine, 630 West 168th Street, PH-7E-110, New York, NY 10032, USA, Tel: +1-212-342-3008, Fax: +1-212-305-9313, pp192@cumc.columbia.edu.
References
- 1.Accerbi M, Schmidt SA, De Paoli E, Park S, Jeong DH, Green PJ. Methods for isolation of total RNA to recover miRNAs and other small RNAs from diverse species. Methods Mol Biol. 2010;592:31–50. doi: 10.1007/978-1-60327-005-2_3. [DOI] [PubMed] [Google Scholar]
- 2.Ach RA, Wang H, Curry B. Measuring microRNAs: Comparisons of microarray and quantitative pcr measurements, and of different total RNA prep methods. BMC Biotechnol. 2008;8:69. doi: 10.1186/1472-6750-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alaoui-El-Azher M, Mans JJ, Baker HV, Chen C, Progulske-Fox A, Lamont RJ, Handfield M. Role of the ATM-checkpoint kinase 2 pathway in CDT-mediated apoptosis of gingival epithelial cells. PLoS One. 2010;5:e11714. doi: 10.1371/journal.pone.0011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alon S, Vigneault F, Eminaga S, Christodoulou DC, Seidman JG, Church GM, Eisenberg E. Barcoding bias in high-throughput multiplex sequencing of miRNA. Genome Res. 2011;21:1506–1511. doi: 10.1101/gr.121715.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An F, Gong B, Wang H, Yu D, Zhao G, Lin L, Tang W, Yu H, Bao S, Xie Q. miR-15b and miR-16 regulate tnf mediated hepatocyte apoptosis via BCL2 in acute liver failure. Apoptosis. 2012;17:702–716. doi: 10.1007/s10495-012-0704-7. [DOI] [PubMed] [Google Scholar]
- 6.Andreasen D, Fog JU, Biggs W, Salomon J, Dahslveen IK, Baker A, Mouritzen P. Improved microRNA quantification in total RNA from clinical samples. Methods. 2010;50:S6–S9. doi: 10.1016/j.ymeth.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baglio SR, Devescovi V, Granchi D, Baldini N. MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals osterix regulation by mir-31. Gene. 2013;527:321–331. doi: 10.1016/j.gene.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee A, Schambach F, DeJong CS, Hammond SM, Reiner SL. Micro-RNA-155 inhibits IFN-gamma signaling in CD4+ T cells. Eur J Immunol. 2010;40:225–231. doi: 10.1002/eji.200939381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao R, Huang L, Andrade J, Tan W, Kibbe WA, Jiang H, Feng G. Review of current methods, applications, and data management for the bioinformatics analysis of whole exome sequencing. Cancer Inform. 2014;13:67–82. doi: 10.4137/CIN.S13779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker C, Hammerle-Fickinger A, Riedmaier I, Pfaffl MW. MRNA and microRNA quality control for RT-qPCR analysis. Methods. 2010;50:237–243. doi: 10.1016/j.ymeth.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Benakanakere M, Kinane DF. Innate cellular responses to the periodontal biofilm. Front Oral Biol. 2012;15:41–55. doi: 10.1159/000329670. [DOI] [PubMed] [Google Scholar]
- 13.Bissels U, Wild S, Tomiuk S, Holste A, Hafner M, Tuschl T, Bosio A. Absolute quantification of microRNAs by using a universal reference. RNA. 2009;15:2375–2384. doi: 10.1261/rna.1754109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M, Garcia-Flores Y, Luong M, Devrekanli A, Xu J, Sun G, Tay J, Linsley PS, Baltimore D. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. 2011;208:1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borchert GM, Lanier W, Davidson BL. Rna polymerase iii transcribes human microRNAs. Nat Struct Mol Biol. 2006;13:1097–1101. doi: 10.1038/nsmb1167. [DOI] [PubMed] [Google Scholar]
- 16.Brain O, Owens BM, Pichulik T, Allan P, Khatamzas E, Leslie A, Steevels T, Sharma S, Mayer A, Catuneanu AM, Morton V, Sun MY, Jewell D, Coccia M, Harrison O, Maloy K, Schonefeldt S, Bornschein S, Liston A, Simmons A. The intracellular sensor NOD2 induces microRNA-29 expression in human dendritic cells to limit IL-23 release. Immunity. 2013;39:521–536. doi: 10.1016/j.immuni.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 17.Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS Biol. 2005;3:e85. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brodersen P, Voinnet O. Revisiting the principles of microRNA target recognition and mode of action. Nat Rev Mol Cell Biol. 2009;10:141–148. doi: 10.1038/nrm2619. [DOI] [PubMed] [Google Scholar]
- 19.Bruning U, Cerone L, Neufeld Z, Fitzpatrick SF, Cheong A, Scholz CC, Simpson DA, Leonard MO, Tambuwala MM, Cummins EP, Taylor CT. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. Mol Cell Biol. 2011;31:4087–4096. doi: 10.1128/MCB.01276-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castoldi M, Schmidt S, Benes V, Hentze MW, Muckenthaler MU. Michip: An array-based method for microRNA expression profiling using locked nucleic acid capture probes. Nat Protoc. 2008;3:321–329. doi: 10.1038/nprot.2008.4. [DOI] [PubMed] [Google Scholar]
- 21.Chaushu S, Wilensky A, Gur C, Shapira L, Elboim M, Halftek G, Polak D, Achdout H, Bachrach G, Mandelboim O. Direct recognition of Fusobacterium nucleatum by the NK cell natural cytotoxicity receptor nkp46 aggravates periodontal disease. PLoS Pathog. 2012;8:e1002601. doi: 10.1371/journal.ppat.1002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheloufi S, Dos Santos CO, Chong MMW, Hannon GJ. A dicer-independent miRNA biogenesis pathway that requires ago catalysis. Nature. 2010;465:584–589. doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, Lao KQ, Livak KJ, Guegler KJ. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen XM, Splinter PL, O'Hara SP, LaRusso NF. A cellular micro-RNA, LET-7i, regulates toll-like receptor 4 expression and contributes to cholangiocyte immune responses against cryptosporidium parvum infection. J Biol Chem. 2007;282:28929–28938. doi: 10.1074/jbc.M702633200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the dicer complex to ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng P, Chen C, He HB, Hu R, Zhou HD, Xie H, Zhu W, Dai RC, Wu XP, Liao EY, Luo XH. miR-148a regulates osteoclastogenesis by targeting v-maf musculoaponeurotic fibrosarcoma oncogene homolog b. J Bone Miner Res. 2013;28:1180–1190. doi: 10.1002/jbmr.1845. [DOI] [PubMed] [Google Scholar]
- 27.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 28.Creevey L, Ryan J, Harvey H, Bray IM, Meehan M, Khan AR, Stallings RL. MicroRNA-497 increases apoptosis in mycn amplified neuroblastoma cells by targeting the key cell cycle regulator WEE1. Mol Cancer. 2013;12:23. doi: 10.1186/1476-4598-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Creighton CJ, Reid JG, Gunaratne PH. Expression profiling of microRNAs by deep sequencing. Brief Bioinform. 2009;10:490–497. doi: 10.1093/bib/bbp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cremer TJ, Fatehchand K, Shah P, Gillette D, Patel H, Marsh RL, Besecker BY, Rajaram MV, Cormet-Boyaka E, Kanneganti TD, Schlesinger LS, Butchar JP, Tridandapani S. miR-155 induction by microbes/microbial ligands requires NF-kappaB-dependent de novo protein synthesis. Front Cell Infect Microbiol. 2012;2:73. doi: 10.3389/fcimb.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cremer TJ, Ravneberg DH, Clay CD, Piper-Hunter MG, Marsh CB, Elton TS, Gunn JS, Amer A, Kanneganti TD, Schlesinger LS, Butchar JP, Tridandapani S. miR-155 induction by F. novicida but not the virulent F. tularensis results in ship down-regulation and enhanced pro-inflammatory cytokine response. PLoS One. 2009;4:e8508. doi: 10.1371/journal.pone.0008508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crick F. Central dogma of molecular biology. Nature. 1970;227:561–563. doi: 10.1038/227561a0. [DOI] [PubMed] [Google Scholar]
- 33.Crick FH. The biological replication of macromolecules. Symp. Soc. Exp. Biol. 1958:138–163. [PubMed] [Google Scholar]
- 34.Curtis HJ, Sibley CR, Wood MJ. Mirtrons, an emerging class of atypical miRNA. Wiley Interdiscip Rev RNA. 2012;3:617–632. doi: 10.1002/wrna.1122. [DOI] [PubMed] [Google Scholar]
- 35.D'Andrade PN, Fulmer-Smentek S. Agilent microRNA microarray profiling system. Methods Mol Biol. 2012;822:85–102. doi: 10.1007/978-1-61779-427-8_6. [DOI] [PubMed] [Google Scholar]
- 36.Danger R, Braza F, Giral M, Soulillou JP, Brouard S. MicroRNAs, major players in B cells homeostasis and function. Front Immunol. 2014;5:98. doi: 10.3389/fimmu.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Lella Ezcurra AL, Bertolin AP, Melani M, Wappner P. Robustness of the hypoxic response: Another job for miRNAs? Dev Dyn. 2012;241:1842–1848. doi: 10.1002/dvdy.23865. [DOI] [PubMed] [Google Scholar]
- 38.Demmer RT, Behle JH, Wolf DL, Handfield M, Kebschull M, Celenti R, Pavlidis P, Papapanou PN. Transcriptomes in healthy and diseased gingival tissues. J Periodontol. 2008;79:2112–2124. doi: 10.1902/jop.2008.080139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng Y, Wu S, Zhou H, Bi X, Wang Y, Hu Y, Gu P, Fan X. Effects of a miR-31, RUNX2, and SATB2 regulatory loop on the osteogenic differentiation of bone mesenchymal stem cells. Stem Cells Dev. 2013;22:2278–2286. doi: 10.1089/scd.2012.0686. [DOI] [PubMed] [Google Scholar]
- 40.Djuranovic S, Nahvi A, Green R. MiRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science. 2012;336:237–240. doi: 10.1126/science.1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du F, Yu F, Wang Y, Hui Y, Carnevale K, Fu M, Lu H, Fan D. MicroRNA-155 deficiency results in decreased macrophage inflammation and attenuated atherogenesis in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2014;34:759–767. doi: 10.1161/ATVBAHA.113.302701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dueck A, Eichner A, Sixt M, Meister G. A miR-155-dependent microRNA hierarchy in dendritic cell maturation and macrophage activation. FEBS Lett. 2014;588:632–640. doi: 10.1016/j.febslet.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Dunand-Sauthier I, Santiago-Raber ML, Capponi L, Vejnar CE, Schaad O, Irla M, Seguin-Estevez Q, Descombes P, Zdobnov EM, Acha-Orbea H, Reith W. Silencing of c-FOS expression by microRNA-155 is critical for dendritic cell maturation and function. Blood. 2011;117:4490–4500. doi: 10.1182/blood-2010-09-308064. [DOI] [PubMed] [Google Scholar]
- 44.El-Awady A, Arce R, Cutler C. Dendritic cells: Microbial clearance via autophagy and potential immunobiological consequences for periodontal disease. Periodontol 2000 2015. 2015 doi: 10.1111/prd.12096. (this volume) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elton TS, Selemon H, Elton SM, Parinandi NL. Regulation of the miR155 host gene in physiological and pathological processes. Gene. 2013;532:1–12. doi: 10.1016/j.gene.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 46.Eminaga S, Christodoulou DC, Vigneault F, Church GM, Seidman JG. Quantification of microRNA expression with next-generation sequencing. Curr Protoc Mol Biol. 2013 doi: 10.1002/0471142727.mb0417s103. Chapter 4 Unit 4 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Escobar TM, Kanellopoulou C, Kugler DG, Kilaru G, Nguyen CK, Nagarajan V, Bhairavabhotla RK, Northrup D, Zahr R, Burr P, Liu X, Zhao K, Sher A, Jankovic D, Zhu J, Muljo SA. miR-155 activates cytokine gene expression in TH17 cells by regulating the DNA-binding protein JARID2 to relieve polycomb-mediated repression. Immunity. 2014;40:865–879. doi: 10.1016/j.immuni.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fantuzzi G. Adiponectin in inflammatory and immune-mediated diseases. Cytokine. 2013;64:1–10. doi: 10.1016/j.cyto.2013.06.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu HJ, Zhu J, Yang M, Zhang ZY, Tie Y, Jiang H, Sun ZX, Zheng XF. A novel method to monitor the expression of microRNAs. Mol Biotechnol. 2006;32:197–204. doi: 10.1385/MB:32:3:197. [DOI] [PubMed] [Google Scholar]
- 51.Gallo A, Alevizos I. Isolation of circulating microRNA in saliva. Methods Mol Biol. 2013;1024:183–190. doi: 10.1007/978-1-62703-453-1_14. [DOI] [PubMed] [Google Scholar]
- 52.Garlet GP. Destructive and protective roles of cytokines in periodontitis: A re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89:1349–1363. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]
- 53.Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2000. 2013;62:59–94. doi: 10.1111/j.1600-0757.2012.00457.x. [DOI] [PubMed] [Google Scholar]
- 54.Gracias DT, Stelekati E, Hope JL, Boesteanu AC, Doering TA, Norton J, Mueller YM, Fraietta JA, Wherry EJ, Turner M, Katsikis PD. The microRNA miR-155 controls CD8(+) T cell responses by regulating interferon signaling. Nat Immunol. 2013;14:593–602. doi: 10.1038/ni.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 56.Griffiths-Jones S. The microRNA registry. Nucleic Acids Res. 2004;32:D109–D111. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo L, Chen F. A challenge for miRNA: Multiple isomirs in miRNAomics. Gene. 2014;544:1–7. doi: 10.1016/j.gene.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 59.Guo L, Zhao Y, Yang S, Zhang H, Chen F. A genome-wide screen for non-template nucleotides and isomir repertoires in miRNAs indicates dynamic and versatile microRNAome. Mol Biol Rep. 2014;41:6649–6658. doi: 10.1007/s11033-014-3548-0. [DOI] [PubMed] [Google Scholar]
- 60.Guo L, Zhao Y, Yang S, Zhang H, Chen F. An integrated analysis of miRNA, lncRNA, and mRNA expression profiles. Biomed Res Int. 2014;2014:345605. doi: 10.1155/2014/345605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo Q, Zhang J, Li J, Zou L, Zhang J, Xie Z, Fu X, Jiang S, Chen G, Jia Q, Li F, Wan Y, Wu Y. Forced miR-146a expression causes autoimmune lymphoproliferative syndrome in mice via downregulation of fas in germinal center B cells. Blood. 2013;121:4875–4883. doi: 10.1182/blood-2012-08-452425. [DOI] [PubMed] [Google Scholar]
- 62.Gurtan AM, Sharp PA. The role of miRNAs in regulating gene expression networks. J Mol Biol. 2013;425:3582–3600. doi: 10.1016/j.jmb.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 64.Hagiwara K, Ochiya T, Kosaka N. A paradigm shift for extracellular vesicles as small RNA carriers: From cellular waste elimination to therapeutic applications. Drug Deliv Transl Res. 2014;4:31–37. doi: 10.1007/s13346-013-0180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 66.Havens AM, Chiu E, Taba M, Wang J, Shiozawa Y, Jung Y, Taichman LS, D'Silva NJ, Gopalakrishnan R, Wang C, Giannobile WV, Taichman RS. Stromal-derived factor-1alpha (CXCL12) levels increase in periodontal disease. J Periodontol. 2008;79:845–853. doi: 10.1902/jop.2008.070514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Havens MA, Reich AA, Duelli DM, Hastings ML. Biogenesis of mammalian microRNAs by a non-canonical processing pathway. Nucleic Acids Res. 2012;40:4626–4640. doi: 10.1093/nar/gks026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He S, Chu J, Wu LC, Mao H, Peng Y, Alvarez-Breckenridge CA, Hughes T, Wei M, Zhang J, Yuan S, Sandhu S, Vasu S, Benson DM, Jr, Hofmeister CC, He X, Ghoshal K, Devine SM, Caligiuri MA, Yu J. MicroRNAs activate natural killer cells through toll-like receptor signaling. Blood. 2013;121:4663–4671. doi: 10.1182/blood-2012-07-441360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heymans S, Corsten MF, Verhesen W, Carai P, van Leeuwen RE, Custers K, Peters T, Hazebroek M, Stoger L, Wijnands E, Janssen BJ, Creemers EE, Pinto YM, Grimm D, Schurmann N, Vigorito E, Thum T, Stassen F, Yin X, Mayr M, de Windt LJ, Lutgens E, Wouters K, de Winther MP, Zacchigna S, Giacca M, van Bilsen M, Papageorgiou AP, Schroen B. Macrophage microRNA-155 promotes cardiac hypertrophy and failure. Circulation. 2013;128:1420–1432. doi: 10.1161/CIRCULATIONAHA.112.001357. [DOI] [PubMed] [Google Scholar]
- 70.Honda T, Takahashi N, Miyauchi S, Yamazaki K. Porphyromonas gingivalis lipopolysaccharide induces mir-146a without altering the production of inflammatory cytokines. Biochem Biophys Res Commun. 2012;420:918–925. doi: 10.1016/j.bbrc.2012.03.102. [DOI] [PubMed] [Google Scholar]
- 71.Hou J, Wang P, Lin L, Liu X, Ma F, An H, Wang Z, Cao X. MicroRNA-146a feedback inhibits RIG-i-dependent type i IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol. 2009;183:2150–2158. doi: 10.4049/jimmunol.0900707. [DOI] [PubMed] [Google Scholar]
- 72.Huang Y, Lei Y, Zhang H, Hou L, Zhang M, Dayton AI. MicroRNA regulation of stat4 protein expression: Rapid and sensitive modulation of IL-12 signaling in human natural killer cells. Blood. 2011;118:6793–6802. doi: 10.1182/blood-2011-05-356162. [DOI] [PubMed] [Google Scholar]
- 73.Huang Z, Shi T, Zhou Q, Shi S, Zhao R, Shi H, Dong L, Zhang C, Zeng K, Chen J, Zhang J. miR-141 regulates colonic leukocytic trafficking by targeting CXCL12beta during murine colitis and human crohn's disease. Gut. 2014;63:1247–1257. doi: 10.1136/gutjnl-2012-304213. [DOI] [PubMed] [Google Scholar]
- 74.Hung PS, Chen FC, Kuang SH, Kao SY, Lin SC, Chang KW. miR-146a induces differentiation of periodontal ligament cells. J Dent Res. 2010;89:252–257. doi: 10.1177/0022034509357411. [DOI] [PubMed] [Google Scholar]
- 75.Ivan M, Huang X. miR-210: Fine-tuning the hypoxic response. Adv Exp Med Biol. 2014;772:205–227. doi: 10.1007/978-1-4614-5915-6_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jansen F, Yang X, Hoyer FF, Paul K, Heiermann N, Becher MU, Abu Hussein N, Kebschull M, Bedorf J, Franklin BS, Latz E, Nickenig G, Werner N. Endothelial microparticle uptake in target cells is annexin i/phosphatidylserine receptor dependent and prevents apoptosis. Arterioscler Thromb Vasc Biol. 2012;32:1925–1935. doi: 10.1161/ATVBAHA.112.253229. [DOI] [PubMed] [Google Scholar]
- 77.Jian C, Li C, Ren Y, He Y, Li Y, Feng X, Zhang G, Tan Y. Hypoxia augments lipopolysaccharide-induced cytokine expression in periodontal ligament cells. Inflammation. 2014;37:1413–1423. doi: 10.1007/s10753-014-9865-6. [DOI] [PubMed] [Google Scholar]
- 78.Jiang L, Chang J, Zhang Q, Sun L, Qiu X. MicroRNA HSA-miR-125a-3p activates p53 and induces apoptosis in lung cancer cells. Cancer Invest. 2013;31:538–544. doi: 10.3109/07357907.2013.820314. [DOI] [PubMed] [Google Scholar]
- 79.Kaeuferle T, Bartel S, Dehmel S, Krauss-Etschmann S. MicroRNA methodology: Advances in miRNA technologies. Methods Mol Biol. 2014;1169:121–130. doi: 10.1007/978-1-4939-0882-0_12. [DOI] [PubMed] [Google Scholar]
- 80.Kapinas K, Kessler C, Ricks T, Gronowicz G, Delany AM. miR-29 modulates wnt signaling in human osteoblasts through a positive feedback loop. J Biol Chem. 2010;285:25221–25231. doi: 10.1074/jbc.M110.116137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karrich JJ, Jachimowski LC, Libouban M, Iyer A, Brandwijk K, Taanman-Kueter EW, Nagasawa M, de Jong EC, Uittenbogaart CH, Blom B. MicroRNA-146a regulates survival and maturation of human plasmacytoid dendritic cells. Blood. 2013;122:3001–3009. doi: 10.1182/blood-2012-12-475087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kebschull M, Demmer R, Behle JH, Pollreisz A, Heidemann J, Belusko PB, Celenti R, Pavlidis P, Papapanou PN. Granulocyte chemotactic protein 2 (GCP-2/CXCL6) complements interleukin-8 in periodontal disease. J Periodontal Res. 2009;44:465–471. doi: 10.1111/j.1600-0765.2008.01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kebschull M, Guarnieri P, Demmer RT, Boulesteix AL, Pavlidis P, Papapanou PN. Molecular differences between chronic and aggressive periodontitis. J Dent Res. 2013;92:1081–1088. doi: 10.1177/0022034513506011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keller A, Leidinger P, Bauer A, Elsharawy A, Haas J, Backes C, Wendschlag A, Giese N, Tjaden C, Ott K, Werner J, Hackert T, Ruprecht K, Huwer H, Huebers J, Jacobs G, Rosenstiel P, Dommisch H, Schaefer A, Muller-Quernheim J, Wullich B, Keck B, Graf N, Reichrath J, Vogel B, Nebel A, Jager SU, Staehler P, Amarantos I, Boisguerin V, Staehler C, Beier M, Scheffler M, Buchler MW, Wischhusen J, Haeusler SF, Dietl J, Hofmann S, Lenhof HP, Schreiber S, Katus HA, Rottbauer W, Meder B, Hoheisel JD, Franke A, Meese E. Toward the blood-borne mirnome of human diseases. Nat Methods. 2011;8:841–843. doi: 10.1038/nmeth.1682. [DOI] [PubMed] [Google Scholar]
- 85.Koboldt DC, Steinberg KM, Larson DE, Wilson RK, Mardis ER. The next-generation sequencing revolution and its impact on genomics. Cell. 2013;155:27–38. doi: 10.1016/j.cell.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koch M, Mollenkopf HJ, Klemm U, Meyer TF. Induction of microRNA-155 is tlr- and type iv secretion system-dependent in macrophages and inhibits DNA-damage induced apoptosis. Proc Natl Acad Sci U S A. 2012;109:E1153–E1162. doi: 10.1073/pnas.1116125109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koscianska E, Starega-Roslan J, Krzyzosiak WJ. The role of dicer protein partners in the processing of microRNA precursors. PLoS One. 2011;6:e28548. doi: 10.1371/journal.pone.0028548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kramer B, Kebschull M, Nowak M, Demmer RT, Haupt M, Korner C, Perner S, Jepsen S, Nattermann J, Papapanou PN. Role of the NK cell-activating receptor CRACC in periodontitis. Infect Immun. 2013;81:690–696. doi: 10.1128/IAI.00895-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kramer MF. Stem-loop rt-qpcr for miRNAs. Curr Protoc Mol Biol. 2011 doi: 10.1002/0471142727.mb1510s95. Chapter 15 Unit 15 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuhn A, Thu D, Waldvogel HJ, Faull RL, Luthi-Carter R. Population-specific expression analysis (psea) reveals molecular changes in diseased brain. Nature methods. 2011;8:945–947. doi: 10.1038/nmeth.1710. [DOI] [PubMed] [Google Scholar]
- 91.Ladewig E, Okamura K, Flynt AS, Westholm JO, Lai EC. Discovery of hundreds of mirtrons in mouse and human small RNA data. Genome Res. 2012;22:1634–1645. doi: 10.1101/gr.133553.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee HY, Zhou K, Smith AM, Noland CL, Doudna JA. Differential roles of human dicer-binding proteins TRBP and PACT in small RNA processing. Nucleic Acids Res. 2013;41:6568–6576. doi: 10.1093/nar/gkt361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee LW, Zhang S, Etheridge A, Ma L, Martin D, Galas D, Wang K. Complexity of the microRNA repertoire revealed by next-generation sequencing. RNA. 2010;16:2170–2180. doi: 10.1261/rna.2225110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee RC, Feinbaum RL, Ambros V. The c. Elegans heterochronic gene LIN-4 encodes small RNAs with antisense complementarity to LIN-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 95.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN. The nuclear RNAse iii drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 96.Lee Y, Hur I, Park SY, Kim YK, Suh MR, Kim VN. The role of PACT in the RNA silencing pathway. EMBO J. 2006;25:522–532. doi: 10.1038/sj.emboj.7600942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee YH, Na HS, Jeong SY, Jeong SH, Park HR, Chung J. Comparison of inflammatory microRNA expression in healthy and periodontitis tissues. Biocell. 2011;35:43–49. [PubMed] [Google Scholar]
- 99.Lerner M, Haneklaus M, Harada M, Grander D. miR-200c regulates NOXA expression and sensitivity to proteasomal inhibitors. PLoS One. 2012;7:e36490. doi: 10.1371/journal.pone.0036490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lezina L, Purmessur N, Antonov AV, Ivanova T, Karpova E, Krishan K, Ivan M, Aksenova V, Tentler D, Garabadgiu AV, Melino G, Barlev NA. miR-16 and miR-26a target checkpoint kinases WEE1 and CHK1 in response to p53 activation by genotoxic stress. Cell Death Dis. 2013;4:e953. doi: 10.1038/cddis.2013.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li L, Zhu D, Huang L, Zhang J, Bian Z, Chen X, Liu Y, Zhang CY, Zen K. Argonaute 2 complexes selectively protect the circulating microRNAs in cell-secreted microvesicles. PLoS One. 2012;7:e46957. doi: 10.1371/journal.pone.0046957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lind EF, Ohashi PS. miR-155, a central modulator of T-cell responses. Eur J Immunol. 2014;44:11–15. doi: 10.1002/eji.201343962. [DOI] [PubMed] [Google Scholar]
- 103.Lindner JM, Kayo H, Hedlund S, Fukuda Y, Fukao T, Nielsen PJ. Cutting edge: The transcription factor BOB1 counteracts B cell activation and regulates miR-146a in B cells. J Immunol. 2014;192:4483–4486. doi: 10.4049/jimmunol.1303022. [DOI] [PubMed] [Google Scholar]
- 104.Liu X, Zhan Z, Xu L, Ma F, Li D, Guo Z, Li N, Cao X. MicroRNA-148/152 impair innate response and antigen presentation of TLR-triggered dendritic cells by targeting camkiialpha. J Immunol. 2010;185:7244–7251. doi: 10.4049/jimmunol.1001573. [DOI] [PubMed] [Google Scholar]
- 105.Lucas H, Bartold PM, Dharmapatni AA, Holding CA, Haynes DR. Inhibition of apoptosis in periodontitis. J Dent Res. 2010;89:29–33. doi: 10.1177/0022034509350708. [DOI] [PubMed] [Google Scholar]
- 106.Luo Z, Wen G, Wang G, Pu X, Ye S, Xu Q, Wang W, Xiao Q. MicroRNA-200c and −150 play an important role in endothelial cell differentiation and vasculogenesis by targeting transcription repressor ZEB1. Stem Cells. 2013;31:1749–1762. doi: 10.1002/stem.1448. [DOI] [PubMed] [Google Scholar]
- 107.Marco A, Ninova M, Griffiths-Jones S. Multiple products from microRNA transcripts. Biochem Soc Trans. 2013;41:850–854. doi: 10.1042/BST20130035. [DOI] [PubMed] [Google Scholar]
- 108.Martin HC, Wani S, Steptoe AL, Krishnan K, Nones K, Nourbakhsh E, Vlassov A, Grimmond SM, Cloonan N. Imperfect centered miRNA binding sites are common and can mediate repression of target mRNAs. Genome Biol. 2014;15:R51. doi: 10.1186/gb-2014-15-3-r51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meisgen F, Xu Landen N, Wang A, Rethi B, Bouez C, Zuccolo M, Gueniche A, Stahle M, Sonkoly E, Breton L, Pivarcsi A. miR-146a negatively regulates TLR2-induced inflammatory responses in keratinocytes. J Invest Dermatol. 2014;134:1931–1940. doi: 10.1038/jid.2014.89. [DOI] [PubMed] [Google Scholar]
- 110.Meister G. Argonaute proteins: Functional insights and emerging roles. Nat Rev Genet. 2013;14:447–459. doi: 10.1038/nrg3462. [DOI] [PubMed] [Google Scholar]
- 111.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 112.Mestdagh P, Hartmann N, Baeriswyl L, Andreasen D, Bernard N, Chen C, Cheo D, D'Andrade P, DeMayo M, Dennis L, Derveaux S, Feng Y, Fulmer-Smentek S, Gerstmayer B, Gouffon J, Grimley C, Lader E, Lee KY, Luo S, Mouritzen P, Narayanan A, Patel S, Peiffer S, Ruberg S, Schroth G, Schuster D, Shaffer JM, Shelton EJ, Silveria S, Ulmanella U, Veeramachaneni V, Staedtler F, Peters T, Guettouche T, Wong L, Vandesompele J. Evaluation of quantitative miRNA expression platforms in the microRNA quality control (MIRQC) study. Nat Meth. 2014;11:809–815. doi: 10.1038/nmeth.3014. [DOI] [PubMed] [Google Scholar]
- 113.Mizoguchi F, Murakami Y, Saito T, Miyasaka N, Kohsaka H. miR-31 controls osteoclast formation and bone resorption by targeting rhoa. Arthritis Res Ther. 2013;15:R102. doi: 10.1186/ar4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mori G, Brunetti G, Colucci S, Oranger A, Ciccolella F, Sardone F, Pignataro P, Mori C, Karapanou V, Ballini A, Mastrangelo F, Tete S, Grassi FR, Grano M. Osteoblast apoptosis in periodontal disease: Role of TNF-related apoptosis-inducing ligand. Int J Immunopathol Pharmacol. 2009;22:95–103. doi: 10.1177/039463200902200111. [DOI] [PubMed] [Google Scholar]
- 115.Morlando M, Ballarino M, Gromak N, Pagano F, Bozzoni I, Proudfoot NJ. Primary microRNA transcripts are processed co-transcriptionally. Nat Struct Mol Biol. 2008;15:902–909. doi: 10.1038/nsmb.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mraz M, Dolezalova D, Plevova K, Stano Kozubik K, Mayerova V, Cerna K, Musilova K, Tichy B, Pavlova S, Borsky M, Verner J, Doubek M, Brychtova Y, Trbusek M, Hampl A, Mayer J, Pospisilova S. MicroRNA-650 expression is influenced by immunoglobulin gene rearrangement and affects the biology of chronic lymphocytic leukemia. Blood. 2012;119:2110–2113. doi: 10.1182/blood-2011-11-394874. [DOI] [PubMed] [Google Scholar]
- 117.Murata K, Yoshitomi H, Furu M, Ishikawa M, Shibuya H, Ito H, Matsuda S. MicroRNA-451 down-regulates neutrophil chemotaxis via p38 MAPK. Arthritis Rheumatol. 2014;66:549–559. doi: 10.1002/art.38269. [DOI] [PubMed] [Google Scholar]
- 118.Nahid MA, Rivera M, Lucas A, Chan EK, Kesavalu L. Polymicrobial infection with periodontal pathogens specifically enhances microRNA miR-146a in APOE−/− mice during experimental periodontal disease. Infect Immun. 2011;79:1597–1605. doi: 10.1128/IAI.01062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nakao A, Kajiya H, Fukushima H, Fukushima A, Anan H, Ozeki S, Okabe K. PTHRP induces notch signaling in periodontal ligament cells. J Dent Res. 2009;88:551–556. doi: 10.1177/0022034509337899. [DOI] [PubMed] [Google Scholar]
- 120.Nallamshetty S, Chan SY, Loscalzo J. Hypoxia: A master regulator of microRNA biogenesis and activity. Free Radic Biol Med. 2013;64:20–30. doi: 10.1016/j.freeradbiomed.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Neilsen CT, Goodall GJ, Bracken CP. IsomiRs--the overlooked repertoire in the dynamic microRNAome. Trends Genet. 2012;28:544–549. doi: 10.1016/j.tig.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 122.Neiva KG, Calderon NL, Alonso TR, Panagakos F, Wallet SM. Type 1 diabetes-associated TLR responsiveness of oral epithelial cells. J Dent Res. 2014;93:169–174. doi: 10.1177/0022034513516345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ng KT, Li JP, Ng KM, Tipoe GL, Leung WK, Fung ML. Expression of hypoxia-inducible factor-1alpha in human periodontal tissue. J Periodontol. 2011;82:136–141. doi: 10.1902/jop.2010.100100. [DOI] [PubMed] [Google Scholar]
- 124.Nowak M, Kramer B, Haupt M, Papapanou PN, Kebschull J, Hoffmann P, Schmidt-Wolf IG, Jepsen S, Brossart P, Perner S, Kebschull M. Activation of invariant NK T cells in periodontitis lesions. J Immunol. 2013;190:2282–2291. doi: 10.4049/jimmunol.1201215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.O'Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, Kahn ME, Rao DS, Baltimore D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33:607–619. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Oertli M, Engler DB, Kohler E, Koch M, Meyer TF, Muller A. MicroRNA-155 is essential for the T cell-mediated control of Helicobacter pylori infection and for the induction of chronic gastritis and colitis. J Immunol. 2011;187:3578–3586. doi: 10.4049/jimmunol.1101772. [DOI] [PubMed] [Google Scholar]
- 127.Ouyang YB, Xu L, Lu Y, Sun X, Yue S, Xiong XX, Giffard RG. Astrocyte-enriched miR-29a targets puma and reduces neuronal vulnerability to forebrain ischemia. Glia. 2013;61:1784–1794. doi: 10.1002/glia.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pan Y, Liang H, Liu H, Li D, Chen X, Li L, Zhang CY, Zen K. Platelet-secreted microRNA-223 promotes endothelial cell apoptosis induced by advanced glycation end products via targeting the insulin-like growth factor 1 receptor. J Immunol. 2014;192:437–446. doi: 10.4049/jimmunol.1301790. [DOI] [PubMed] [Google Scholar]
- 129.Papapanou PN, Behle JH, Kebschull M, Celenti R, Wolf DL, Handfield M, Pavlidis P, Demmer RT. Subgingival bacterial colonization profiles correlate with gingival tissue gene expression. BMC Microbiol. 2009;9:221. doi: 10.1186/1471-2180-9-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Parachuru VP, Coates DE, Milne TJ, Hussaini HM, Rich AM, Seymour GJ. Forkhead box p3-positive regulatory T-cells and interleukin 17-positive T-helper 17 cells in chronic inflammatory periodontal disease. J Periodontal Res. 2014 doi: 10.1111/jre.12169. [DOI] [PubMed] [Google Scholar]
- 131.Perri R, Nares S, Zhang S, Barros SP, Offenbacher S. MicroRNA modulation in obesity and periodontitis. J Dent Res. 2012;91:33–38. doi: 10.1177/0022034511425045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pradeep AR, Manjunath SG, Swati PP, Shikha C, Sujatha PB. Gingival crevicular fluid levels of leukotriene B4 in periodontal health and disease. J Periodontol. 2007;78:2325–2330. doi: 10.1902/jop.2007.070135. [DOI] [PubMed] [Google Scholar]
- 133.Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: Approaches and considerations. Nat Rev Genet. 2012;13:358–369. doi: 10.1038/nrg3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Quinn EM, Wang JH, O'Callaghan G, Redmond HP. MicroRNA-146a is upregulated by and negatively regulates TLR2 signaling. PLoS One. 2013;8:e62232. doi: 10.1371/journal.pone.0062232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rebane A, Akdis CA. MicroRNAs: Essential players in the regulation of inflammation. J Allergy Clin Immunol. 2013;132:15–26. doi: 10.1016/j.jaci.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 136.Rokavec M, Wu W, Luo JL. IL6-mediated suppression of miR-200c directs constitutive activation of inflammatory signaling circuit driving transformation and tumorigenesis. Mol Cell. 2012;45:777–789. doi: 10.1016/j.molcel.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rosenberger CM, Podyminogin RL, Navarro G, Zhao GW, Askovich PS, Weiss MJ, Aderem A. miR-451 regulates dendritic cell cytokine responses to influenza infection. J Immunol. 2012;189:5965–5975. doi: 10.4049/jimmunol.1201437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roth BM, Ishimaru D, Hennig M. The core microprocessor component diGeorge syndrome critical region 8 (DGCR8) is a nonspecific RNA-binding protein. J Biol Chem. 2013;288:26785–26799. doi: 10.1074/jbc.M112.446880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Roush S, Slack FJ. The LET-7 family of microRNAs. Trends Cell Biol. 2008;18:505–516. doi: 10.1016/j.tcb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 140.Ruggiero T, Trabucchi M, De Santa F, Zupo S, Harfe BD, McManus MT, Rosenfeld MG, Briata P, Gherzi R. LPS induces kh-type splicing regulatory protein-dependent processing of microRNA-155 precursors in macrophages. FASEB J. 2009;23:2898–2908. doi: 10.1096/fj.09-131342. [DOI] [PubMed] [Google Scholar]
- 141.Russo F, Di Bella S, Nigita G, Macca V, Lagana A, Giugno R, Pulvirenti A, Ferro A. Mirandola: Extracellular circulating microRNAs database. PLoS One. 2012;7:e47786. doi: 10.1371/journal.pone.0047786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sanz M, van Winkelhoff AJ. Periodontal infections: Understanding the complexity--consensus of the seventh european workshop on periodontology. J Clin Periodontol. 2011;11(38 Suppl):3–6. doi: 10.1111/j.1600-051X.2010.01681.x. [DOI] [PubMed] [Google Scholar]
- 143.Schageman J, Zeringer E, Li M, Barta T, Lea K, Gu J, Magdaleno S, Setterquist R, Vlassov AV. The complete exosome workflow solution: From isolation to characterization of RNA cargo. Biomed Res Int. 2013;2013:253957. doi: 10.1155/2013/253957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schamberger A, Orban TI. 3' isomir species and DNA contamination influence reliable quantification of microRNAs by stem-loop quantitative pcr. PLoS One. 2014;9:e106315. doi: 10.1371/journal.pone.0106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schamberger A, Sarkadi B, Orban TI. Human mirtrons can express functional microRNAs simultaneously from both arms in a flanking exon-independent manner. RNA Biol. 2012;9:1177–1185. doi: 10.4161/rna.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schickel R, Park SM, Murmann AE, Peter ME. miR-200c regulates induction of apoptosis through CD95 by targeting FAP-1. Mol Cell. 2010;38:908–915. doi: 10.1016/j.molcel.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schulte LN, Westermann AJ, Vogel J. Differential activation and functional specialization of miR-146 and miR-155 in innate immune sensing. Nucleic Acids Res. 2013;41:542–553. doi: 10.1093/nar/gks1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Seddiki N, Brezar V, Ruffin N, Levy Y, Swaminathan S. Role of miR-155 in the regulation of lymphocyte immune function and disease. Immunology. 2014;142:32–38. doi: 10.1111/imm.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sharbati J, Lewin A, Kutz-Lohroff B, Kamal E, Einspanier R, Sharbati S. Integrated microRNA-mRNA-analysis of human monocyte derived macrophages upon Mycobacterium avium subsp. hominissuis infection. PLoS One. 2011;6:e20258. doi: 10.1371/journal.pone.0020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shi R, Chiang VL. Facile means for quantifying microRNA expression by real-time PCR. Biotechniques. 2005;39:519–525. doi: 10.2144/000112010. [DOI] [PubMed] [Google Scholar]
- 151.Smith M, Seymour GJ, Cullinan MP. Histopathological features of chronic and aggressive periodontitis. Periodontol 2000. 2010;53:45–54. doi: 10.1111/j.1600-0757.2010.00354.x. [DOI] [PubMed] [Google Scholar]
- 152.Smyth LA, Boardman D, Tung S, Lechler R, Lombardi G. MicroRNAs affect dendritic cells function and phenotype. Immunology. 2014 doi: 10.1111/imm.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Song L, Lin C, Gong H, Wang C, Liu L, Wu J, Tao S, Hu B, Cheng SY, Li M, Li J. miR-486 sustains NF-kappaB activity by disrupting multiple NF-kappaB-negative feedback loops. Cell Res. 2013;23:274–289. doi: 10.1038/cr.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Song ZC, Zhou W, Shu R, Ni J. Hypoxia induces apoptosis and autophagic cell death in human periodontal ligament cells through HIF-1alpha pathway. Cell Prolif. 2012;45:239–248. doi: 10.1111/j.1365-2184.2012.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Staedel C, Darfeuille F. MicroRNAs and bacterial infection. Cell Microbiol. 2013;15:1496–1507. doi: 10.1111/cmi.12159. [DOI] [PubMed] [Google Scholar]
- 156.Stoecklin-Wasmer C, Guarnieri P, Celenti R, Demmer RT, Kebschull M, Papapanou PN. MicroRNAs and their target genes in gingival tissues. J Dent Res. 2012;91:934–940. doi: 10.1177/0022034512456551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stokowy T, Eszlinger M, Swierniak M, Fujarewicz K, Jarzab B, Paschke R, Krohn K. Analysis options for high-throughput sequencing in miRNA expression profiling. BMC Res Notes. 2014;7:144. doi: 10.1186/1756-0500-7-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stroynowska-Czerwinska A, Fiszer A, Krzyzosiak WJ. The panorama of miRNA-mediated mechanisms in mammalian cells. Cell Mol Life Sci. 2014;71:2253–2270. doi: 10.1007/s00018-013-1551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Subedi A, Park PH. Autocrine and paracrine modulation of microRNA-155 expression by globular adiponectin in raw 264.7 macrophages: Involvement of MAPK/NF-kappaB pathway. Cytokine. 2013;64:638–641. doi: 10.1016/j.cyto.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 160.Taganov KD, Boldin MP, Chang KJ, Baltimore D. Nf-kappaB-dependent induction of microRNA mir-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tam S, de Borja R, Tsao MS, McPherson JD. Robust global microRNA expression profiling using next-generation sequencing technologies. Lab Invest. 2014;94:350–358. doi: 10.1038/labinvest.2013.157. [DOI] [PubMed] [Google Scholar]
- 162.Tan GC, Chan E, Molnar A, Sarkar R, Alexieva D, Isa IM, Robinson S, Zhang S, Ellis P, Langford CF, Guillot PV, Chandrashekran A, Fisk NM, Castellano L, Meister G, Winston RM, Cui W, Baulcombe D, Dibb NJ. 5' isomir variation is of functional and evolutionary importance. Nucleic Acids Res. 2014;42:9424–9435. doi: 10.1093/nar/gku656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Thai TH, Patterson HC, Pham DH, Kis-Toth K, Kaminski DA, Tsokos GC. Deletion of microRNA-155 reduces autoantibody responses and alleviates lupus-like disease in the FAS(LPR) mouse. Proc Natl Acad Sci U S A. 2013;110:20194–20199. doi: 10.1073/pnas.1317632110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thompson RC, Vardinogiannis I, Gilmore TD. Identification of an NF-kappaB p50/p65-responsive site in the human miR155hg promoter. BMC Mol Biol. 2013;14:24. doi: 10.1186/1471-2199-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tiao MM, Wang FS, Huang LT, Chuang JH, Kuo HC, Yang YL, Huang YH. MicroRNA-29a protects against acute liver injury in a mouse model of obstructive jaundice via inhibition of the extrinsic apoptosis pathway. Apoptosis. 2014;19:30–41. doi: 10.1007/s10495-013-0909-4. [DOI] [PubMed] [Google Scholar]
- 166.Tonetti MS, Imboden MA, Lang NP. Neutrophil migration into the gingival sulcus is associated with transepithelial gradients of interleukin-8 and ICAM-1. J Periodontol. 1998;69:1139–1147. doi: 10.1902/jop.1998.69.10.1139. [DOI] [PubMed] [Google Scholar]
- 167.Travan S, Li F, D'Silva NJ, Slate EH, Kirkwood KL. Differential expression of mitogen activating protein kinases in periodontitis. J Clin Periodontol. 2013;40:757–764. doi: 10.1111/jcpe.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Trotta R, Chen L, Ciarlariello D, Josyula S, Mao C, Costinean S, Yu L, Butchar JP, Tridandapani S, Croce CM, Caligiuri MA. miR-155 regulates IFN-gamma production in natural killer cells. Blood. 2012;119:3478–3485. doi: 10.1182/blood-2011-12-398099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Trotta R, Chen L, Costinean S, Josyula S, Mundy-Bosse BL, Ciarlariello D, Mao C, Briercheck EL, McConnell KK, Mishra A, Yu L, Croce CM, Caligiuri MA. Overexpression of miR-155 causes expansion, arrest in terminal differentiation and functional activation of mouse natural killer cells. Blood. 2013;121:3126–3134. doi: 10.1182/blood-2012-12-467597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tsuda H, Ning Z, Yamaguchi Y, Suzuki N. Programmed cell death and its possible relationship with periodontal disease. J Oral Sci. 2012;54:137–149. doi: 10.2334/josnusd.54.137. [DOI] [PubMed] [Google Scholar]
- 171.Vallejo DM, Caparros E, Dominguez M. Targeting notch signalling by the conserved miR-8/200 microRNA family in development and cancer cells. EMBO J. 2011;30:756–769. doi: 10.1038/emboj.2010.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.van Dijk EL, Jaszczyszyn Y, Thermes C. Library preparation methods for next-generation sequencing: Tone down the bias. Exp Cell Res. 2014;322:12–20. doi: 10.1016/j.yexcr.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 173.van Kouwenhove M, Kedde M, Agami R. MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat Rev Cancer. 2011;11:644–656. doi: 10.1038/nrc3107. [DOI] [PubMed] [Google Scholar]
- 174.Varkonyi-Gasic E, Hellens RP. Quantitative stem-loop RT-PCR for detection of microRNAs. Methods Mol Biol. 2011;744:145–157. doi: 10.1007/978-1-61779-123-9_10. [DOI] [PubMed] [Google Scholar]
- 175.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by LIN28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wallet MA, Calderon N, Alonso TR, Choe CS, Catalfamo D, Lalane CJ, Neiva KG, Panagakos F, Wallet SM. Triclosan alters antimicrobial and inflammatory responses of epithelial cells. Oral Dis. 2013;19:296–302. doi: 10.1111/odi.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wan G, Xie W, Liu Z, Xu W, Lao Y, Huang N, Cui K, Liao M, He J, Jiang Y, Yang BB, Xu H, Xu N, Zhang Y. Hypoxia-induced miR155 is a potent autophagy inducer by targeting multiple players in the mtor pathway. Autophagy. 2014;10:70–79. doi: 10.4161/auto.26534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang H, Ach RA, Curry B. Direct and sensitive miRNA profiling from low-input total RNA. RNA. 2007;13:151–159. doi: 10.1261/rna.234507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang H, Flach H, Onizawa M, Wei L, McManus MT, Weiss A. Negative regulation of HIF1a expression and TH17 differentiation by the hypoxia-regulated microRNA mir-210. Nat Immunol. 2014;15:393–401. doi: 10.1038/ni.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang HJ, Zhang PJ, Chen WJ, Feng D, Jia YH, Xie LX. Four serum microRNAs identified as diagnostic biomarkers of sepsis. J Trauma Acute Care Surg. 2012;73:850–854. doi: 10.1097/TA.0b013e31825a7560. [DOI] [PubMed] [Google Scholar]
- 181.Wang J, He J, Su F, Ding N, Hu W, Yao B, Wang W, Zhou G. Repression of atr pathway by miR-185 enhances radiation-induced apoptosis and proliferation inhibition. Cell Death Dis. 2013;4:e699. doi: 10.1038/cddis.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang P, Gu Y, Zhang Q, Han Y, Hou J, Lin L, Wu C, Bao Y, Su X, Jiang M, Wang Q, Li N, Cao X. Identification of resting and type I IFN-activated human NK cell mirnomes reveals microRNA-378 and microRNA-30e as negative regulators of NK cell cytotoxicity. J Immunol. 2012;189:211–221. doi: 10.4049/jimmunol.1200609. [DOI] [PubMed] [Google Scholar]
- 183.Wang P, Hou J, Lin L, Wang C, Liu X, Li D, Ma F, Wang Z, Cao X. Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J Immunol. 2010;185:6226–6233. doi: 10.4049/jimmunol.1000491. [DOI] [PubMed] [Google Scholar]
- 184.Wang YZ, Tian FF, Yan M, Zhang JM, Liu Q, Lu JY, Zhou WB, Yang H, Li J. Delivery of an miR155 inhibitor by anti-cd20 single-chain antibody into B cells reduces the acetylcholine receptor-specific autoantibodies and ameliorates experimental autoimmune myasthenia gravis. Clin Exp Immunol. 2014;176:207–221. doi: 10.1111/cei.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang Z, Filgueiras LR, Wang S, Serezani AP, Peters-Golden M, Jancar S, Serezani CH. Leukotriene B4 enhances the generation of proinflammatory microRNAs to promote myd88-dependent macrophage activation. J Immunol. 2014;192:2349–2356. doi: 10.4049/jimmunol.1302982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wendlandt EB, Graff JW, Gioannini TL, McCaffrey AP, Wilson ME. The role of microRNAs miR-200b and miR-200c in TLR4 signaling and NF-kappaB activation. Innate Immun. 2012;18:846–855. doi: 10.1177/1753425912443903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene LIN-14 by LIN-4 mediates temporal pattern formation in c. Elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 188.Wong DT. Salivaomics. J Am Dent Assoc. 2012;143:19S–24S. doi: 10.14219/jada.archive.2012.0339. [DOI] [PubMed] [Google Scholar]
- 189.Xie Q, Wang Z, Bi X, Zhou H, Wang Y, Gu P, Fan X. Effects of miR-31 on the osteogenesis of human mesenchymal stem cells. Biochem Biophys Res Commun. 2014;446:98–104. doi: 10.1016/j.bbrc.2014.02.058. [DOI] [PubMed] [Google Scholar]
- 190.Xie Y, Wei RR, Huang GL, Zhang MY, Yuan YF, Wang HY. Checkpoint kinase 1 is negatively regulated by miR-497 in hepatocellular carcinoma. Med Oncol. 2014;31:844. doi: 10.1007/s12032-014-0844-4. [DOI] [PubMed] [Google Scholar]
- 191.Xie YF, Shu R, Jiang SY, Liu DL, Zhang XL. Comparison of microRNA profiles of human periodontal diseased and healthy gingival tissues. Int J Oral Sci. 2011;3:125–134. doi: 10.4248/IJOS11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xu N, Meisgen F, Butler LM, Han G, Wang XJ, Soderberg-Naucler C, Stahle M, Pivarcsi A, Sonkoly E. MicroRNA-31 is overexpressed in psoriasis and modulates inflammatory cytokine and chemokine production in keratinocytes via targeting serine/threonine kinase 40. J Immunol. 2013;190:678–688. doi: 10.4049/jimmunol.1202695. [DOI] [PubMed] [Google Scholar]
- 193.Yadav S, Pandey A, Shukla A, Talwelkar SS, Kumar A, Pant AB, Parmar D. miR-497 and miR-302b regulate ethanol-induced neuronal cell death through BCL2 protein and cyclin D2. J Biol Chem. 2011;286:37347–37357. doi: 10.1074/jbc.M111.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yang JS, Maurin T, Robine N, Rasmussen KD, Jeffrey KL, Chandwani R, Papapetrou EP, Sadelain M, O'Carroll D, Lai EC. Conserved vertebrate miR-451 provides a platform for Dicer-independent, AGO2-mediated microRNA biogenesis. Proc Natl Acad Sci U S A. 2010;107:15163–15168. doi: 10.1073/pnas.1006432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang Z, Wu L, Zhu X, Xu J, Jin R, Li G, Wu F. miR-29a modulates the angiogenic properties of human endothelial cells. Biochem Biophys Res Commun. 2013;434:143–149. doi: 10.1016/j.bbrc.2013.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yilmaz O, Jungas T, Verbeke P, Ojcius DM. Activation of the phosphatidylinositol 3-kinase/akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis . Infect Immun. 2004;72:3743–3751. doi: 10.1128/IAI.72.7.3743-3751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yuan H, Zelka S, Burkatovskaya M, Gupte R, Leeman SE, Amar S. Pivotal role of NOD2 in inflammatory processes affecting atherosclerosis and periodontal bone loss. Proc Natl Acad Sci U S A. 2013;110:E5059–E5068. doi: 10.1073/pnas.1320862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zekri L, Kuzuoglu-Ozturk D, Izaurralde E. Gw182 proteins cause pabp dissociation from silenced miRNA targets in the absence of deadenylation. EMBO J. 2013;32:1052–1065. doi: 10.1038/emboj.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Koppel T, Jahantigh MN, Lutgens E, Wang S, Olson EN, Schober A, Weber C. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 201.Zhai H, Fesler A, Ju J. MicroRNA: A third dimension in autophagy. Cell Cycle. 2013;12:246–250. doi: 10.4161/cc.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang H, Li Y, Huang Q, Ren X, Hu H, Sheng H, Lai M. Mir-148a promotes apoptosis by targeting BCL-2 in colorectal cancer. Cell Death Differ. 2011;18:1702–1710. doi: 10.1038/cdd.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang X, Wan G, Mlotshwa S, Vance V, Berger FG, Chen H, Lu X. Oncogenic WIP1 phosphatase is inhibited by miR-16 in the DNA damage signaling pathway. Cancer Res. 2010;70:7176–7186. doi: 10.1158/0008-5472.CAN-10-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhao G, Wang B, Liu Y, Zhang JG, Deng SC, Qin Q, Tian K, Li X, Zhu S, Niu Y, Gong Q, Wang CY. MiRNA-141, downregulated in pancreatic cancer, inhibits cell proliferation and invasion by directly targeting MAP4K4. Mol Cancer Ther. 2013;12:2569–2580. doi: 10.1158/1535-7163.MCT-13-0296. [DOI] [PubMed] [Google Scholar]
- 205.Zhao M, Wang LT, Liang GP, Zhang P, Deng XJ, Tang Q, Zhai HY, Chang CC, Su YW, Lu QJ. Up-regulation of microRNA-210 induces immune dysfunction via targeting FOXP3 in CD4(+) T cells of psoriasis vulgaris. Clin Immunol. 2014;150:22–30. doi: 10.1016/j.clim.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 206.Zhu W, Zhu D, Lu S, Wang T, Wang J, Jiang B, Shu Y, Liu P. miR-497 modulates multidrug resistance of human cancer cell lines by targeting BCL2. Med Oncol. 2012;29:384–391. doi: 10.1007/s12032-010-9797-4. [DOI] [PubMed] [Google Scholar]
- 207.Zhu X, Er K, Mao C, Yan Q, Xu H, Zhang Y, Zhu J, Cui F, Zhao W, Shi H. miR-203 suppresses tumor growth and angiogenesis by targeting VEGFA in cervical cancer. Cell Physiol Biochem. 2013;32:64–73. doi: 10.1159/000350125. [DOI] [PubMed] [Google Scholar]