Abstract
Objective
Tight junctions are multicomponent structures, with claudin proteins defining paracellular permeability. Claudin 3 is a candidate for the exceptional “tightness” of human urothelium, being localised to the terminal tight junction (TJ) of superficial cells. Our aim was to determine whether claudin 3 plays an instigating and/or a functional role in the urothelial TJ.
Materials and Methods
Normal human urothelial (NHU) cells maintained as non-immortalised cell lines were retrovirally-transduced to over-express or silence claudin 3 expression. Stable sublines induced to stratify or differentiate were assessed for TJ formation by immunocytochemistry and transepithelial electrical resistance (TER). Expression of claudin 3, ZO-1 and ZO-1α+ was examined in native urothelium by immunohistochemistry.
Results
Claudin 3 expression was associated with differentiation and development of a tight barrier and along with ZO-1 and ZO-1α+ was localised to the apical tight junction in native urothelium. Knockdown of claudin 3 inhibited formation of a tight barrier in three independent cell lines, however, overexpression of claudin 3 was not sufficient to induce tight barrier development in the absence of differentiation. A differentiation-dependent induction of the ZO-1α+ isoform was found to coincide with barrier formation. Whereas claudin 3 overexpression did not induce the switch to co-expression of ZO-1α−/ZO-1α+, claudin 3 knockdown decreased localisation of ZO-1 to the TJ and resulted in compromised barrier function.
Conclusions
Urothelial cytodifferentiation is accompanied by induction of claudin 3 which is essential for the development of a terminal TJ. A coordinated switch to the ZO-1α+ isotype was also observed and for the first time may indicate that ZO-1α+ is involved in the structural assembly and function of the urothelial terminal TJ.
Keywords: claudin, differentiation, tight junction, urothelium, zonula occludens
Introduction
A common feature of all epithelia is the ability to form a selective barrier to limit the passage of solutes from the apical to basal aspect. Epithelial “tightness” is commonly assessed by measurement of transepithelial electrical resistance (TER), with epithelia displaying a TER >500 Ω.cm2 classified as “tight” [1]. The paracellular barrier is defined by tight junctions (TJs) found in the terminal junction complex, which also serve to establish cell polarity by segregating apical from baso-lateral membrane components. TJs are multicomponent complexes incorporating members of the claudin family, of which there are 24 in man, along with occludin and the PDZ-containing zonula occludens (ZO). Whereas occludin is dispensible [2], ZO-1 forms an essential link between the TJ and the peri-junctional cytoskeleton, but does not itself limit solute permeation [3,4]. The claudins show expression profiles that reflect epithelial barrier properties and are considered to govern permselectivity [5]. Although the relationship between composition and properties of the TJ is well-studied in some experimental systems [6], it is less clear how individual claudins contribute to the barrier properties of differentiated epithelial tissues.
The least permeable epithelium is the urothelium - the transitional epithelium that lines the bladder and associated urinary tract (reviewed in [7]). Urothelium provides a urinary barrier that is maintained during the filling and voiding cycles of bladder accommodation and has features that limit permeability via both trans- and para-cellular routes. The Asymmetric Unit Membrane (AUM) is unique to urothelium and covers the surface of the apical cells in hexagonal plaques [8]. The AUM prevents transcellular absorption of water and solutes from the urine, but does not itself contribute to the paracellular barrier [9]. In the rat, the TJs of superficial urothelial cells undergo structural and functional reorganisation to preserve barrier integrity during filling and voiding cycles [10]. In the mouse, germline deletion of CLDN4 resulted in a diffuse urothelial hyperplasia leading to urinary tract obstruction and progressive hydronephrosis, indicating a possible role for claudin 4 in maintaining the homeostatic integrity of the normal murine urothelium. However, the absence of claudin 4 did not perturb barrier function, which was considered to be due to molecular compensation by claudin 7 [11]. In man, disruption of urothelial barrier integrity and TJ structure has been linked to several bladder pathologies, including interstitial cystitis [12-16] and urinary tract infections [17,18].
We have previously investigated the TJ constituents of normal human urothelium in situ, where we showed differentiation-associated expression of claudins 3, 4, 5 and 7 [19]. The expression of claudin 3 was restricted with ZO-1 to the terminally-differentiated superficial cells, where it localised specifically to the terminal “kissing points” between cells. Using PPARγ activation as a means to induce the urothelial differentiation-associated gene expression programme in cultured normal human urothelial (NHU) cells, we demonstrated that the claudin 3 gene was transcriptionally-activated during late/terminal urothelial cytodifferentiation in vitro [19], suggesting that claudin 3 may play a crucial role in urothelial barrier function in man. To test the hypothesis that claudin 3 is an essential component of the urothelial TJ required for barrier function, we have here investigated the effect of claudin 3 knockdown and over-expression on functional barrier development in NHU cell cultures, which has revealed an unexpected change in expression of ZO-1 isoforms from ZO-1α− to its alternatively-spliced ZO-1α+ variant.
Materials and Methods
Tissues
National Health Service Research Ethics Committee Approvals were obtained for the research use of surgical specimens of normal human urinary tract, which were collected from patients and donors with no history of urothelial cancer. Full informed consent was obtained as required and the study was approved locally by the Department of Biology Ethics Committee under the auspices of the University of York Ethics Committee.
Immunohistochemistry
Sections (5 μM) of human ureter were dewaxed and rehydrated. For immunolabelling with ZO-1 and claudin 3 antibodies, endogenous avidin and biotin were blocked and antigen retrieval was performed by boiling sections for 10 min in 10 mM citric acid buffer, pH 6.0. After a 16 h incubation of primary antibody at 4°C, slides were washed, incubated in biotinylated secondary antibody and visualised by addition of streptavidin-biotin horseradish peroxidase complex (DAKO) and 3,3′-diaminobenzidine (Sigma Aldrich). For ZO-1α+ labelling, antigen retrieval was performed by boiling of slides in 1 mM EDTA in 10 mM Tris-HCl buffer (pH 9.0) before incubating with primary antibody for 16 h at 4°C. Antibody binding was visualised using the ImPRESS™ Excel Polymer system (Vector labs), according to the manufacturer's instructions. All slides were counterstained in Mayer's haematoxylin and mounted in DPX (Sigma).
Cell culture
Normal human urothelial (NHU) cells were isolated from human ureter and bladder biopsies and maintained as finite cell lines in vitro [20,21]. Cultures were propagated on Primaria™ plasticware (BD Biosciences) in low calcium [0.09 mM] keratinocyte serum-free medium containing recombinant epidermal growth factor and bovine pituitary extract (Life Technologies), supplemented with 30 ng/ml cholera toxin (KSFMc) and used for experiments between passages 3-5. In these conditions, NHU cells proliferate as a monolayer that becomes contact-inhibited at confluence and can be propagated by serial, but finite sub-culture. Supplementing the medium to 2 mM Ca2+ (near physiological) results in stratification accompanied by the formation of adherens and tight junctions, but without urothelial cytodifferentiation [20,21]. Urothelial cytodifferentiation with tight barrier formation was induced by subculturing the cells in KSFMc supplemented with 5% adult bovine serum and 2 mM Ca2+, as described [22]. In all cases, cultures were grown on 24 mm Transwell™ membranes (Corning) for mRNA and protein extractions and on 12 mm Snapwell™ membranes (Corning) for electrophysiological studies [23].
Quantitative real-time polymerase chain reaction
RNA was extracted from non-differentiated, stratified and differentiated NHU cell cultures and cDNA synthesis performed as previously described [15]. Gene transcript quantification assays were performed using an ABI Prism 7300 Real-Time PCR System (Applied Biosystems) following the TaqMan™ assay protocol. Primers, probes and PCR conditions for UPK2 and GADPH transcripts were as described previously [15]. A pre-validated TaqMan™ gene expression assay for claudin 3 (Hs00265816_s1) was used (Applied Biosystems). Genes of interest were normalised to endogenous GAPDH in the same sample and the comparative CT method was used for relative quantification [24].
Generation of claudin 3 shRNA
For RNA interference experiments, siRNA oligonucleotides were designed to target the CLDN3 coding sequence (ENSG00000165215), with further addition of a hairpin loop, restriction overhangs for directional cloning and a Mlu1 restriction site to verify cloned inserts, thus generating the following CLDN3 sense shRNA sequences:
CLDN3-(1): gatccAACATCATCACGTCGCAGAACTTCAAGAGAGTTCTGCGACGTGATGATGTTTTTTTTACGCGTg
CLDN3-(2): gatccAACACCATTATCCGGGACTTCTTCAAGA-GAGAAGTCCCGGATAATGGTGTTTTTTTTACGCGTg
CLDN3-(3): gatccAAGGGCATCTTTTGGGTACCTTTCAAGAGAAGGTACCCAAAAGATGCCCTTTTTTTTACGCGTg
shRNA sequences were ligated into pSIREN-RetroQ vector and a firefly luciferase negative control shRNA was included (Clontech). After bacterial transformation, successful ligation was confirmed by Mlu1 restriction digest. Plasmids were designated shRNA CLDN3-(1), shRNA CLDN3-(2), shRNA CLDN3-(3) and shRNA control.
Claudin 3 over-expression
Full-length claudin 3 cDNA was amplified by RT-PCR from freshly-isolated bladder-derived urothelial cDNA using the Expand™ High Fidelity PCR system (Roche) and the oligonucleotides 5′-ACCcggaattccgGCCACCATGTCCATGGGCCTGGAGAT-3′ (sense) and 5′-GTCcgggatcccgTTAGACGTAGTCCTTGCGGTC-3′ (antisense). The primers were designed to incorporate a Kozac sequence (under-lined), and restriction sites for EcoR1 (lowercase, sense) and BamH1 (lowercase, antisense).
The amplified product was purified using the QIAquick Gel Extraction Kit (Qiagen) and directionally-cloned into the pLXSN vector (Clontech) using EcoR1 and BamH1 restriction sites. An empty vector control reaction was included from which claudin 3 cDNA was absent. After bacterial transformation, purified plasmid DNA was sequenced to verify successful cloning of full length claudin 3.
Generation of claudin 3 knockdown and overexpressing cell lines
After transfection of pLXSN and pSIREN-RetroQ vectors into the PT67 packaging cell line (Clontech), retroviral particles were harvested from the growth medium, filtered through 0.45 μM low-binding Tuffryn® membranes (Pall Corporation) and applied to proliferating NHU cells for retroviral transduction, as previously described [25]. Transduced NHU cells were subjected to antibiotic selection and thereafter subcultured in KSFMc prior to experimental study.
Following initial shRNA studies, construct CLDN3-(1) was selected for further study as it gave the most effective knockdown of claudin 3 protein. Stable sub-lines were generated from four independent donor NHU cell lines following transduction with shRNA control and shRNA CLDN3-(1) retroviral particles.
Immunofluorescence labelling
For immunolabelling, NHU cells were seeded onto 12-well Multispot slides (CA Hendley, Essex) at 2 × 104 cells/cm2 and maintained in non-differentiated (KSFMc), stratified (KSFMc with 2 mM calcium), or differentiated (KSFMc with 5% ABS and 2 mM CaCl2) culture conditions, as described above and in [22].
Slides were fixed in methanol:acetone (v/v) and air dried before applying primary antibodies at 4°C for 16 h. Primary antibodies are detailed in Table 1. Goat anti-rabbit IgG (Alexa 488, 1/400) or goat anti-mouse IgG (Alexa 594, 1/700) secondary antibodies (Molecular Probes) were applied for 1 h at ambient temperature before washing. Hoechst 33258 (0.1 mg/ml; Sigma-Aldrich) was used to counterstain nuclei. Samples were mounted using Fluorescent Mounting Medium (Dako) and visualised on an Olympus BX60 microscope under epifluorescence illumination.
Table 1. List of antibodies used.
| Antigen | Clone/antibody | Host | Working concentration (μg/ml) | Supplier | ||
|---|---|---|---|---|---|---|
| IB | IF | IHC | ||||
| Claudin 1 | JAY.8 | Rb | 0.25 | n/a | n/a | Life Technologies |
| Claudin 3 | Z23.JM | Rb | 0.25 | 2.5 | n/a | Life Technologies |
| Claudin 3 | Ab52231 | Rb | n/a | n/a | 1 | Abcam |
| Claudin 4 | 3E2C1 | M | 0.5 | n/a | n/a | Life Technologies |
| Claudin 5 | 4C3C2 | M | 0.5 | n/a | n/a | Life Technologies |
| Claudin 7 | ZMD.241 | Rb | 0.25 | n/a | n/a | Life Technologies |
| ZO-1 | ZO-1-1A12 | Rb | 1 | 1.25 | 10 | Life Technologies |
| ZO-1α+ | Anti-ZO-1α+ | Rb | 1.3 | 2 | 0.04 | Hycult Biotech |
| β-actin | AC-15 | M | 0.004 | n/a | n/a | Sigma Aldrich |
Primary antibodies used for immunoblotting (IB), immunofluorescence (IF) and immunohistochemistry (IHC). M, mouse; Rb, Rabbit
Immunoblotting
Whole cell lysates were resolved by polyacrylamide gel electrophoresis on Nu-Page™ Novex 4-12% bis-Tris gels (for detecting claudin proteins) or Nu-Page™ Novex 3-8% Tris-acetate gels (for ZO-1 proteins) and electrotransferred onto polyvinylidene fluoride membranes (Merck Millipore). Each track was loaded with 15 μg protein, as determined by the Bradford assay (Pierce). Membranes were incubated with pre-titrated primary antibody (Table 1) for 16 h at 4°C. Bound antibody was detected with goat anti-mouse IgG IRDye™ 680 or goat anti-rabbit IgG IRDye™ 800 (Invitrogen) and visualised using an Odyssey™ Infra-red Imaging System (Li-Cor Biosciences). Following antibody stripping (Alpha Diagnostic International), blots were re-probed with anti-β-actin antibody for normalisation of protein loading.
Electrophysiological studies
NHU or transduced NHU cells were seeded at 5 × 105 cells per Snap-well™ membrane (3-6 replicates) in either undifferentiated, stratified or differentiated culture conditions for 7 days before measuring the TER using an EVOM™ Voltohmmeter (World Precision Instruments) [23]. Blank membrane (no cell) readings were subtracted from each TER value.
Statistics
Unless otherwise stated, descriptive statistics are mean ± SD. Statistical analysis was by means of analysis of variance (ANOVA) with Tukey post-test correction.
Results
Induction of barrier function
TER was used to assess barrier function of cultures grown on permeable membranes. Neither cultures maintained as undifferentiated monolayers in 0.09 mM [Ca2+] nor as stratified cultures in 2 mM [Ca2+] formed a tight epithelial barrier (>500 Ω.cm2). By contrast and as previously recorded [22], a tight barrier was obtained following differentiation of cultures in 5% ABS and 2 mM [Ca2+ ] (Fig. 1A).
Figure 1. Barrier function and tight junction analysis of NHU cultures following stratification and/or differentiation.
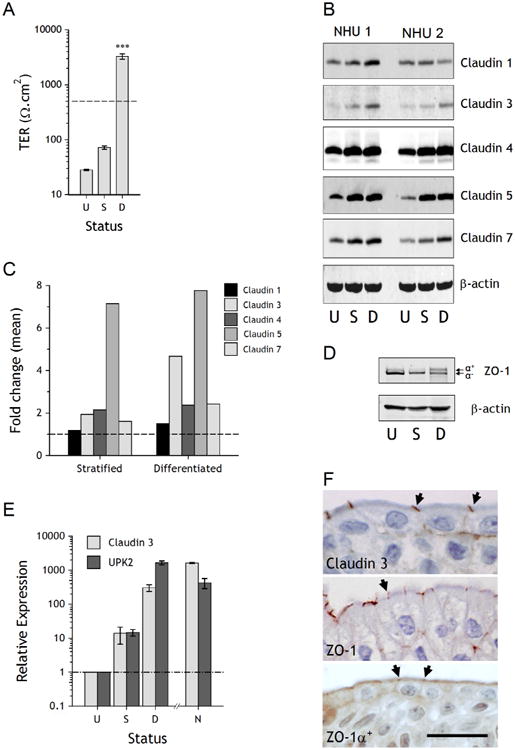
A. Barrier function was determined by measurement of trans-epithelial electrical resistance (TER) in undifferentiated cultures (U), stratified cultures grown in 2 mM calcium (S) and cultures differentiated in 5% ABS and 2 mM calcium (D). The dashed line at 500 Ω.cm2 represents the threshold for a “tight” epithelium. Bars are means (± SD) from a representative experiment, which has previously been performed on numerous independent NHU cell lines [22]. *** P < 0.001 (ANOVA with Tukey post correction) relative to undifferentiated and stratified cultures. B. Expression of claudins 1, 3, 4, 5 and 7 was assessed by immunoblotting in undifferentiated (U), stratified (S) and differentiated (D) cultures from two independent NHU cell lines. β-actin was included as a loading control. C. Densitometry analysis of Western blot bands was performed to compare expression of claudin proteins in stratified and differentiated cultures (normalised to β-actin) and plotted as mean fold change relative to undifferentiated cultures (dashed line). Data represents mean values from experiments performed with two independent cell lines. D. Expression of ZO-1 was assessed by immunoblotting in undifferentiated (U), stratified (S) and differentiated (D) cultures. The ZO-1a+ and ZO-1a− variants are arrowed. E. Transcript expression of claudin 3 and uroplakin 2 (UPK2) was determined by quantitative real-time PCR in undifferentiated (U), stratified (S) and differentiated (D) cultures (each point represents three experimental replicates). For comparison, native (N) freshly-isolated urothelium was examined from bladder and ureteric urothelium. Expression relative to undifferentiated cells is plotted after normalisation to the housekeeper gene GAP-DH. F. Immunohistological labelling of claudin 3, ZO-1 (total) and ZO-1α+ in human ureteric urothelium. Labelling at the terminal junctions is indicated by arrows. Scale bar = 25 μm.
Constitution of the urothelial tight junction
Claudin expression was examined by immunoblotting in undifferentiated, stratified and differentiated NHU cell cultures from two independent NHU cell lines grown on permeable membrane supports. The expression of claudin 3, 4, 5 and 7 proteins was enhanced in stratified and differentiated cultures compared to undifferentiated cultures, whereas there was little change in the expression of claudin 1 (Fig. 1B). Comparison of stratified and differentiated cultures showed equivalent expression of claudins 4, 5 and 7, but a marked increase in expression of claudin 3 was associated with the differentiated, barrier-forming phenotype (Fig. 1C).
The TJ anchoring protein ZO-1α− was expressed by NHU cell cultures in all states, but differentiation was accompanied by a partial switch to the alternatively-spliced ZO-1α+ isoform (Fig. 1D).
Quantitative transcript analysis revealed that claudin 3 expression was upregulated in NHU cells following differentiation in vitro, with relative expression of claudin 3 enhanced > 300 fold. The expression of claudin 3 transcript by in vitro-differentiated NHU cells mirrored that of the archetypal urothelial differentiation-restricted UPK2 transcript and was of similar magnitude to the expression found in situ (Fig. 1E).
By immunohistochemistry, antibodies against claudin 3, ZO-1 (total) and ZO-1α+ all showed localisation to the terminal junction between superficial cells of human urothelium, with some basolateral membrane localisation apparent with all three (Fig. 1F).
Claudin 3 knock-down studies
Due to the association between claudin 3 expression and NHU cell differentiation, we examined the role of claudin 3 in barrier development by generating stable shRNA knockdowns in four independent donor NHU cell lines. Following selection, the sublines expressing shRNA were induced to differentiate before analysis by immunoblotting, with the untreated parental control included for comparison purposes. Transduction with claudin 3 shRNA resulted in failure to upregulate claudin 3 protein expression following differentiation in vitro, as illustrated in one representative cell line (Fig. 2A) and collectively in all four (Fig. 2B). Knockdown of claudin 3 had no significant effect on the differentiation-induced expression of other tight junction-associated moieties, including claudin 5, which was included as a shRNA specificity control since it shares 50.3% sequence identity with claudin 3 (Fig. 2B). Qualitatively, the failure to upregulate claudin 3 led to some reduction in the extent of membrane-localised total ZO-1 and ZO-1α+ observed by immunocytochemistry (Fig. 2C).
Figure 2. Barrier function and tight junction analysis of NHU cultures following claudin 3 shRNA knock-down.
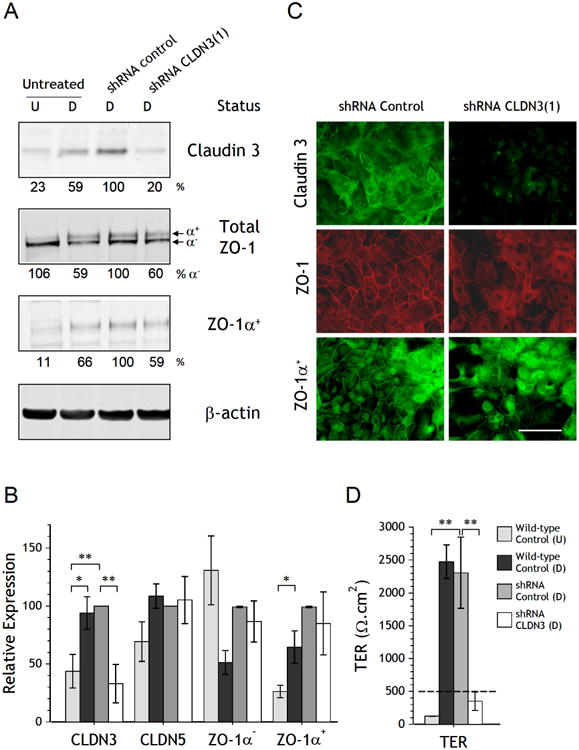
A. Expression of claudin 3, total ZO-1 (both isoforms) and ZO-1α+ proteins was assessed in undifferentiated cultures (U) and in cultures differentiated with 5% ABS and 2 mM calcium (D) by Western blotting, as well as in differentiated NHU cells transduced with control shRNA and CLDN3 shRNA (lanes 3 and 4, respectively). The densitometry analysis of Western blot bands was normalised to the loading control β-actin and the percentage expression relative to control shRNA cells is indicated. B. Compilation of results from shRNA transduction performed on four independent donor NHU cell lines. Expression of CLDN3, CLDN5, ZO-1α− and ZO-1α+ was analysed by western blotting following differentiation of NHU cells transduced with control and CLDN3 shRNA alongside wild-type undifferentiated (U) and differentiated (D) NHU cultures. Expression is plotted as relative expression (± SEM) after normalisation to β-actin. C. Immunofluorescence localisation of claudin 3, ZO-1 (total) and ZO-1α+ in control and CLDN3 shRNA transduced NHU cells following induction of differentiation. Scale bar = 50 μm. D. Barrier function of control shRNA and CLDN3 shRNA transduced cells, alongside undifferentiated (U) and differentiated (D) non-transduced NHU cells, was determined by measurement of transepithelial electrical resistance (TER) after seven days. Results compiled from three independent donor cell lines. Statistical analysis was by ANOVA with Tukey post-test correction. ** P < 0.01; * P < 0.05
Barrier function following claudin 3 shRNA knock-down was determined by measurement of the TER in three independent donor cell lines (Fig. 2D). TER measurements were low in undifferentiated cultures (<150 Ω.cm2). Following differentiation in 5% ABS and 2mM calcium, non-transduced and control shRNA cultures generated tight barriers with all TER readings exceeding 2000 Ω.cm2, however claudin 3 shRNA cells were unable to generate a tight barrier and in all cases showed a significant reduction in TER (Fig. 2D).
Overexpression of claudin 3
Claudin 3 is upregulated by differentiated NHU cells, coinciding with the development of a tight barrier, which is abrogated in differentiated cells that lack induction of claudin 3 expression. We reasoned that if development of a tight barrier was dependent on the expression of claudin 3, then the overexpression of claudin 3 by TJ-forming stratified cultures would facilitate barrier formation and result in a high TER. Claudin 3 overexpressing cells were generated by stable transduction with the full length claudin 3 coding sequence.
Overexpression was confirmed by quantitative transcript analysis of claudin 3. Claudin 3 expression was highly upregulated (>100,000-fold) in undifferentiated and stratified pLXSN-claudin 3 cultures, compared to wild-type (non-transduced) and empty vector transduced NHU cells (Fig. 3A). However, following differentiation, the amount of claudin 3 transcript found in overexpressing cells was comparable to the endogenous expression observed in differentiated wild-type and empty vector cells. These results were confirmed by immunoblotting, where overexpression of claudin 3 resulted in a 64 and a 136-fold increase in claudin 3 protein expression in undifferentiated and stratified cells, respectively (Fig. 3B). By contrast, following differentiation, the amount of claudin 3 protein detected in overexpressing cells was equivalent to that detected in differentiated wild type and empty vector sublines, again indicating that claudin 3 is regulated endogenously in differentiated cells (Fig. 3B). Overexpression of claudin 3 did not appear to influence the overall expression of either ZO-1α+ or ZO-1α− proteins in undifferentiated, stratified or differentiated NHU cultures, as observed by immunoblotting (Fig. 3C).
Figure 3. Analysis of NHU cultures following stable overexpression of claudin 3.
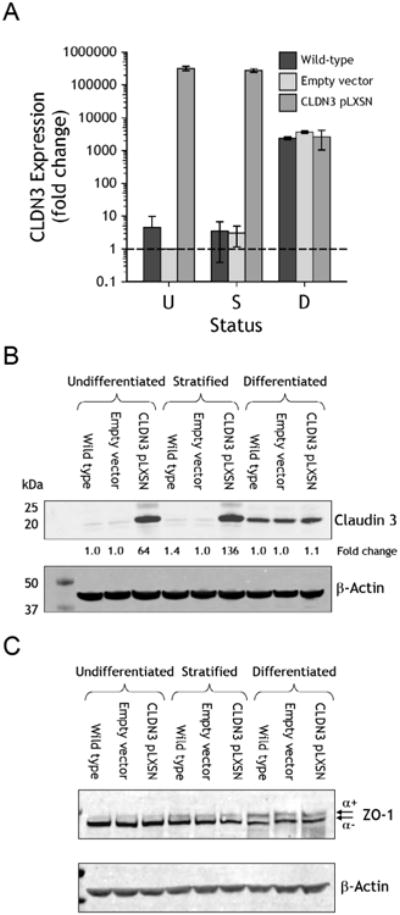
Claudin 3 overexpressing transductants were generated from isogeneic NHU cells using the pLXSN retroviral vector; controls comprised empty-vector transduced and non-transduced (wild-type) cells. Results representative of experiments performed on NHU cell lines from four independent donors and collated as functional results in Figure 4C. A. Claudin 3 transcript expression was analysed by quantitative real-time PCR in undifferentiated (U), stratified (S) and differentiated (D) cultures. Expression was plotted as fold-change relative to undifferentiated empty-vector control cells after normalisation to GAPDH. B and C. Claudin 3 overexpression was examined by immunoblotting in parallel cultures, in addition to the loading control β-actin and total ZO-1. Fold change calculated relative to empty-vector control cultures is indicated.
Overexpression of claudin 3 was evident by immunolabelling of cultures induced to stratify in 2 mM [Ca2+], where much of the overex-pressed protein was cytoplasmic, with small patches of membrane-localised protein indicative of association with TJs (Fig. 4A). Claudin 3 overexpression resulted in an increase in the intensity and extent of intercellular-localised ZO-1 in stratified cultures, but did not affect either the extent or localisation of ZO-1α+, which was detected at tight junctions in differentiated cultures, with minimal intercellular localisation in stratified cell cultures (Fig. 4A). Finally, the overexpression of claudin 3 had no effect on the expression or immunolocalisation of claudins 1, 4, 5, and 7 (data not shown).
Figure 4. Analysis of NHU cultures following stable overexpression of claudin 3.
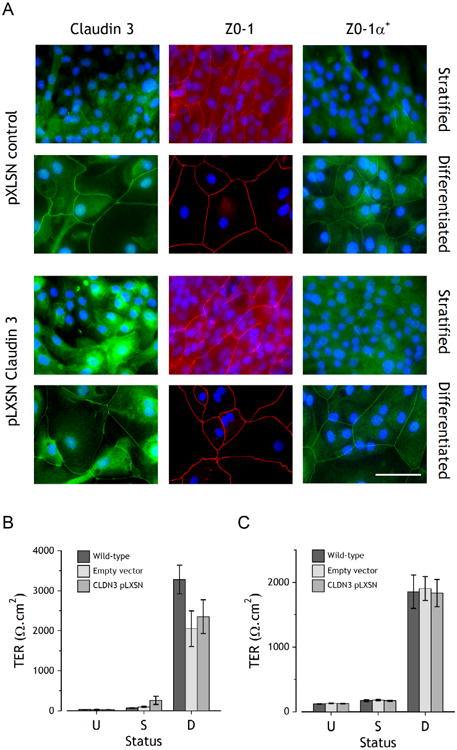
Claudin 3 overexpressing NHU cell transductants were generated as in Figure 3. A. Indirect immunofluorescent localisation of claudin 3, ZO-1 and ZO-1α+ in pLXSN control (empty vector) and pLXSN-claudin 3 overexpressing NHU cells, following stratification or differentiation. Scale bar = 50 μm. B and C. Barrier function of wild-type, empty-vector and claudin 3-pLXSN NHU cells was determined by measurement of transepithelial electrical resistance (TER) in undifferentiated (U), stratified (S) and differentiated (D) cultures. B shows a representative cell line, with n=3 replicates (mean ± SD), C shows averaged data from four independent cell lines (mean ± SEM). There were no statistically significant differences between wild-type or transduced cells and claudin 3 overexpressing cells of any status.
The effect of claudin 3 overexpression on barrier development was determined by measurement of TER in control (wild-type and pLXSN-empty vector) and claudin 3 overexpressing cultures (Fig. 4B and 4C). No significant changes were observed in barrier function resulting from claudin 3 overexpression in proliferative, stratified or differentiated NHU cells.
Discussion
This is the first study to directly link the specific protein composition of the urothelial TJ to the development and maintenance of barrier function. Here we provide evidence that claudin 3 is indispensable to urothelial tight barrier function in human urothelium, although it is not alone sufficient to instigate tight barrier formation in vitro, even under conditions of physiological calcium that are permissive for TJ formation.
Urothelial cells afford an ideal model for the study of barrier function: not only is urothelium the “tightest” of all epithelia [26,27], but urothelial cell phenotype can be manipulated in vitro by modification of the culture environment. Optimised growth conditions involve a low calcium serum-free medium originally developed for keratinocyte growth and in these conditions NHU cells adopt a basal squamous cell phenotype. The raising of extracellular [Ca2+] to near-physiological [2 mM] has been shown to induce stratification and support the development of adherens and tight junctions. However, such cultures maintain a CK14-positive squamous phenotype and do not express late/terminal urothelial markers nor form a functional tight barrier [20,22]. The capacity of NHU cells to undergo differentiation along a transitional cytokeratin 13-expressing pathway, with expression of urothelial differentiation markers and tight barrier formation can be achieved by culturing NHU cells in serum and physiological [Ca2+] [22]. Although the differentiation-inducing bioactive factor(s) in serum have yet to be identified, it is known that activation of the nuclear receptor PPARγ induces a programme of gene expression changes associated with late/terminal urothelial differentiation, including TJ components [19]. Despite there being close correspondence in the gene expression programmes entrained by the two differentiation-inducing methods, only the serum-induced method results in formation of an organised stratified epithelium with barrier function [28]. One study has reported TJ formation and expression of various claudins in the HPV16E6E7-im-mortalised TEU-2 human urothelial cell line, although neither barrier formation nor expression of claudin 3 was described [29]. In this con-text, it should be noted that immortalisation of human urothelial cells has been shown to compromise their capacity to differentiate [30,31], including their ability to form a functional barrier [31].
In situ, the superficial urothelial cell layer holds ultimate responsibility for containment of urine. In human urothelium, the superficial cell is distinguished from the other layers by the expression of claudins 3, 4 and 5 [19]. The superficial urothelial TJs in mouse comprise claudins 4, 8 and 12, with claudin 3 transcript reported as not detected in whole mouse bladder RNA [32], although this may reflect an issue of sensitivity, as sparse claudin 3 was immunolocalised to the lateral borders of superficial cells in another study [11]. We have previously proposed claudin 3 as a prime candidate for urothelial tight barrier function due to its specific localisation to the terminal TJ in human urothelium in situ. In addition, claudin 3 gene expression is induced de novo upon PPARγ-induced differentiation, unlike claudin 4, which is regulated at the protein level through stabilisation with claudin 5 [19]. It is known that claudin 4 can contribute to barrier function: forced expression in MDCK II and LLC-PK1 cells resulted in increased TER and decreased paracellular permeability [27]. However, deletion of CLDN4 did not perturb barrier function in mouse urothelium [11], suggesting it may not be the principal barrier-forming component.
Here, we have confirmed claudin 3 as a urothelial differentiation-regulated gene and provided functional evidence of abrogated barrier function following claudin 3 knockdown. Although the possibility of off-target effects from the claudin 3 shRNA sequence cannot be completely disregarded, we did show that there was no effect on the expression of claudin 5, which we included as a specificity control due to the fact that it shares 50% sequence identity. Claudin 3 is expressed by a wide variety of epithelia and is an important component of the blood-brain barrier [33]. Forced expression of human claudin 3 in MDCK II cells elevated the transepithelial electrical resistance and sealed the paracellular pathway against both charged and uncharged solutes, acting as a general barrier-forming protein [34].
One unexpected aspect of the differentiated phenotype was the lowering of claudin 3 transcript (and protein) to baseline amounts in overexpressing cells, indicating that a physiological feedback mechanism operating at the transcriptional level is acquired during differentiation to regulate the absolute amount of claudin 3. Although claudin 3 was successfully overexpressed in undifferentiated and stratified cultures, the finding that it did not result in the development of a high TER, even in conditions conducive to TJ formation, demonstrated that expression of claudin 3 alone is not sufficient for full barrier development. TJs function as large multi-protein complexes and whilst claudins are thought to be the barrier-defining components, molecular association between the cytoplasmic domain of claudins with the PDZ-domains of the ZO is recognised as critical to TJ development, with both ZO-1 and ZO-2 implicated in the timing and positioning of claudin polymerisation at TJ strands [35]. Thus, the association between claudin 3 with the scaffold structure of the ZO is likely to be essential to the correct assembly and functioning of the urothelial terminal TJ. Although experimental over-expression of claudin 3 did not affect overall ZO-1 expression, it did increase the amount of ZO-1 localising to the TJ in stratified cultures, suggesting some recruitment. This is in keeping with the hypothesis that ZO-1 is stabilised at the TJ under the influence of claudin 3. However, the increased presence of ZO-1 did not result in any significant increase in barrier function, as assessed by TER.
What did coincide with barrier development was a differentiation-dependent induction of the ZO-1α+ splice variant, which includes an 80 amino acid sequence not present in the ZO-1α− isoform, resulting in co-expression of both isoforms. This is the first report of ZO-1α+ splice variant expression by human urothelium and our results implicate ZO-1α+ in the structural assembly and function of the urothelial terminal TJ. Whereas claudin 3 overexpression did not induce the ZO-1 isoform switch, claudin 3 knockdown decreased localisation of ZO-1/ZO-1α+to the TJ and resulted in compromised barrier function. Together, this indicates coordination between claudin 3 and ZO1α+ in the development of tight barrier structure and function.
In conclusion, urothelial differentiation is accompanied by induction of claudin 3 which may act in conjunction with variant ZO-1α+ isoform to develop the terminal TJ that represents one of the tightest barriers in the human body.
Acknowledgments
This work was supported by the Urology Foundation and the Royal College of Surgeons of England (Clinical Research Fellowships to NJS), Yorkshire Kidney Research Fund (CLV) and York Against Cancer (JH & JS).
Abbreviations
- ABS
adult bovine serum
- AUM
asymmetric unit membrane
- KSFM
keratinocyte serum-free medium
- KSFMc
KSFM complete
- PPARγ
peroxisome proliferator-activated receptor gamma
- PDZ
post synaptic density protein (PSD95), Drosophila disc large tumor suppressor (Dlg1) and zonula occludens-1 protein (ZO-1), RT-qPCR, reverse transcription quantitative polymerase chain reaction
- shRNA
short hairpin RNA
- TJ
tight junction
- TER
transepithelial electrical resistance
- NHU
normal human urothelial
- ZO
zonula occludens
Footnotes
Competing interests: Invention for producing biomimetic urothelium is protected by patent WO/2004/011630 (J.S.).
References
- 1.Fromter E, Diamond J. Route of passive ion permeation in epithelia. Nat New Biol. 1972;235:9–13. doi: 10.1038/newbio235009a0. [DOI] [PubMed] [Google Scholar]
- 2.Schulzke JD, Gitter AH, Mankertz J, Spiegel S, Seidler U, et al. Epithelial transport and barrier function in occludin-deficient mice. Biochim Biophys Acta. 2005;1669:34–42. doi: 10.1016/j.bbamem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke J, et al. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010;123:1913–1921. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 4.Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20:3930–3940. doi: 10.1091/mbc.E09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Itallie CM, Anderson JM. The role of claudins in determining paracellular charge selectivity. Proc Am Thorac Soc. 2004;1:38–41. doi: 10.1513/pats.2306013. [DOI] [PubMed] [Google Scholar]
- 6.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1 doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hicks RM. The fine structure of the transitional epithelium of rat ureter. J Cell Biol. 1965;26:25–48. doi: 10.1083/jcb.26.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu P, Meyers S, Liang F, Deng F, Kachar B, et al. Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. Am J Physiol Renal Physiol. 2002;283:1200–1207. doi: 10.1152/ajprenal.00043.2002. [DOI] [PubMed] [Google Scholar]
- 10.Carattino MD, Prakasam HS, Ruiz WG, Clayton DR, McGuire M, et al. Bladder filling and voiding affect umbrella cell tight junction organization and function. Am J Physiol Renal Physiol. 2013;305:1158–1168. doi: 10.1152/ajprenal.00282.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita H, Hamazaki Y, Noda Y, Oshima M, Minato N. Claudin-4 deficiency results in urothelial hyperplasia and lethal hydronephrosis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eldrup J, Thorup J, Nielsen SL, Hald T, Hainau B. Permeability and ultrastructure of human bladder epithelium. Br J Urol. 1983;55:488–492. doi: 10.1111/j.1464-410x.1983.tb03354.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee JD, Lee MH. Decreased expression of zonula occludens-1 and occludin in the bladder urothelium of patients with interstitial cystitis/painful bladder syndrome. J Formos Med Assoc. 2014;113:17–22. doi: 10.1016/j.jfma.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Shie J, Chen S, Wang Y, Kuo H. Differences in mast cell infiltration, E-cadherin, and zonula occludens-1 expression between patients with overactive bladder and interstitial cystitis/bladder pain syndrome. Urology. 2012;80:225. doi: 10.1016/j.urology.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 15.Southgate J, Varley CL, Garthwaite MAE, Hinley J, Marsh F, et al. Differentiation potential of urothelium from patients with benign bladder dysfunction. BJU Int. 2007;99:1506–1516. doi: 10.1111/j.1464-410X.2007.06795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang CO, Wang JY, Koch KR, Keay S. Regulation of tight junction proteins and bladder epithelial paracellular permeability by an antiproliferative factor from patients with interstitial cystitis. J Urol. 2005;174:2382–2387. doi: 10.1097/01.ju.0000180417.11976.99. [DOI] [PubMed] [Google Scholar]
- 17.Mysorekar IU, Mulvey MA, Hultgren SJ, Gordon JI. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J Biol Chem. 2002;277:7412–7419. doi: 10.1074/jbc.M110560200. [DOI] [PubMed] [Google Scholar]
- 18.Wood MW, Breitschwerdt EB, Nordone SK, Linder KE, Gookin JL. Uropathogenic E. coli promote a paracellular urothelial barrier defect characterized by altered tight junction integrity, epithelial cell sloughing and cytokine release. J Comp Pathol. 2011;147:11–19. doi: 10.1016/j.jcpa.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varley CL, Garthwaite MAE, Cross W, Hinley J, Trejdosiewicz LK, et al. PPARgamma-regulated tight junction development during human urothelial cytodifferentiation. J Cell Physiol. 2006;208:407–417. doi: 10.1002/jcp.20676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Southgate J, Hutton KA, Thomas DF, Trejdosiewicz LK. Normal human urothelial cells in vitro: proliferation and induction of stratification. Lab Invest. 1994;71:583–594. [PubMed] [Google Scholar]
- 21.Southgate J, Masters JR, Trejdosiewicz LK. Culture of human urothelium. In: Freshney RI, Freshney MG, editors. Culture of Epithelial Cells. 2nd. New York: J Wiley and Sons, Inc; 2002. pp. 381–400. [Google Scholar]
- 22.Cross WR, Eardley I, Leese HJ, Southgate J. A biomimetic tissue from cultured normal human urothelial cells: analysis of physiological function. Am J Physiol Renal Physiol. 2005;289:459–468. doi: 10.1152/ajprenal.00040.2005. [DOI] [PubMed] [Google Scholar]
- 23.Rubenwolf P, Southgate J. Permeability of differentiated human urothelium in vitro. Methods Mol Biol. 2011;763:207–222. doi: 10.1007/978-1-61779-191-8_14. [DOI] [PubMed] [Google Scholar]
- 24.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 25.Shaw NJ, Georgopoulos NT, Southgate J, Trejdosiewicz LK. Effects of loss of p53 and p16 function on life span and survival of human urothelial cells. Int J Cancer. 2005;116:634–639. doi: 10.1002/ijc.21114. [DOI] [PubMed] [Google Scholar]
- 26.Lewis JR, Lewis SA. Colistin interactions with the mammalian urothelium. Am J Physiol Cell Physiol. 2004;286:913–922. doi: 10.1152/ajpcell.00437.2003. [DOI] [PubMed] [Google Scholar]
- 27.Negrete HO, Lavelle JP, Berg J, Lewis SA, Zeidel ML. Permeability properties of the intact mammalian bladder epithelium. Am J Physiol. 1996;271:886–894. doi: 10.1152/ajprenal.1996.271.4.F886. [DOI] [PubMed] [Google Scholar]
- 28.Böck M, Hinley J, Schmitt C, Wahlicht T, Kramer S, et al. Identification of ELF3 as an early transcriptional regulator of human urothelium. Dev Biol. 2013;386:321–330. doi: 10.1016/j.ydbio.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 29.Rickard A, Dorokhov N, Ryerse J, Klumpp DJ, McHowat J. Characterization of tight junction proteins in cultured human urothelial cells. In Vitro Cell Dev Biol Anim. 2008;44:261–267. doi: 10.1007/s11626-008-9116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chapman EJ, Hurst CD, Pitt E, Chambers P, Aveyard JS, et al. Expression of hTERT immortalises normal human urothelial cells without inactivation of the p16/Rb pathway. Oncogene. 2006;25:5037–5045. doi: 10.1038/sj.onc.1209513. [DOI] [PubMed] [Google Scholar]
- 31.Georgopoulos NT, Kirkwood LA, Varley CL, MacLaine NJ, Aziz N, et al. Immortalisation of normal human urothelial cells compromises differentiation capacity. Eur Urol. 2011;60:141–149. doi: 10.1016/j.eururo.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 32.Acharya P, Beckel J, Ruiz WG, Wang E, Rojas R, et al. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am J Physiol Renal Physiol. 2004;287:305–318. doi: 10.1152/ajprenal.00341.2003. [DOI] [PubMed] [Google Scholar]
- 33.Wolburg H, Wolburg-Buchholz K, Kraus J, Rascher-Eggstein G, Liebner S, et al. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003;105:586–592. doi: 10.1007/s00401-003-0688-z. [DOI] [PubMed] [Google Scholar]
- 34.Milatz S, Krug SM, Rosenthal R, Günzel D, Müller D, et al. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim Biophys Acta. 2010;1798:2048–2057. doi: 10.1016/j.bbamem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]


