Abstract
Cutaneous leishmaniasis (CL) was first detected in Sri Lanka in 1992.Local disease is caused by a genetically different variant of Leishmania donovani. Early case detection and management is the mainstay of L. donovani control. High degree of clinical suspicion is critical but a clinical diagnostic tool is not available for leishmaniasis. Current study described, for the first time, a two-staged clinical algorhythm that facilitates screening of CL in Sri Lanka by primary health care worker in stage 1 and management by medical professional in stage 2.Selected clinical markers of 400 patients suspected of CL were analysed retrospectively with laboratory confirmation of leishmaniasis. Ten clinical markers predicted CL with a over 90% accuracy. Subsets of markers showed high levels of sensitivities (60–97.2%) and/or significant association with positive laboratory results as compared to negative lesions [typical onset (acne-form, painless non-itchy), (P = 0.026), size up to 2 cm (P = 0.046), well-defined edges (P = 0.002), regular edges (P = 0.018), rounded shape (P = 0.030), and lesions at 5–8 months (P = 0.052)]. Five of them (typical onset, number up to 2, small size, rounded edges, and rounded shape) also had > 70% sensitivity levels as compared to laboratory findings. Typical onset had the highest sensitivity of 97% and a PPV of 72%. Lesions at 5–8 months duration having defined edges (P = 0.013, specificity 89.7%, PPV 83.1) or having regular edges (P = 0.006, specificity 86.2%, PPV 82.4%) were also predictive of CL. Most of early laboratory-confirmed ( < 12 months) lesions remained < 3 cm (sensitivity > 67%, PPV > 70%) and had defined edges (sensitivity of 52–71%, specificity 46.7–68.8%), (PPV 75.1–86%). Four clinical markers served as good diagnostic markers in both early ( ≤ 4) and late (>12 months) lesions, viz. typical onset (91.3–98.4%), presence of ≤ 2 lesions (sensitivity 82.6–94.7%), size ≤ 2 cm (66.9–73.7%), and regular edges (68.6–76.3%). Reliability of clinical markers generally declined in chronic lesions. However, small lesions of over 12 months were highly indicative of CL (sensitivity of 66%, specificity 66.7%). None of the single/combination markers, however, were 100% sensitive or specific, highlighting the undeniable usefulness of laboratory confirmation, in diagnosis. Decision-making algorithm used 10 basic clinical features for screening and seven specific clinical markers for clinical handling and referral for investigations.
Keywords: Leishmaniasis, Diagnostic tools, Clinical markers, Scoring system, Screening, Case detection
Introduction
Human leishmaniasis is one of the widely prevalent and emerging infections in the world and is recognized as a neglected tropical disease.1–3 Locally acquired cutaneous leishmaniasis (CL) was first described in Sri Lanka in 1992.4 Disease remained sporadic, until year 2001 in this country when a soldier working in Northern Sri Lanka was identified by us to have locally acquired CL. Following this, awareness campaigns held in Northern Sri Lanka by some of us at home institution resulted in the detection of increasing numbers of locally acquired CL.5 Globally known and most virulent usually visceralizing species Leishmania donovani results in CL in this endemic setting, where the local parasite is a genetic variant within the L. donovani complex.6,7 Though the reported cases were confined to Northern parts of the country during early parts of the outbreak, case distribution has widened during the latter part of the epidemic.8,9 More virulent forms also have recently emerged resulting in mucosal and visceral (MCL and VL) forms of disease.10–12 The need for urgent disease prevention and control has been highlighted in this country.13,14 Early case detection and management is generally considered as the mainstay of L. donovani control.2 However, under reporting is a known fact in many leishmaniasis endemic settings, and approximately only two-thirds of endemic countries notify leishmaniasis. 15
None of the clinical markers are pathognomonic for any form of leishmaniasis. A variety of other dermatological conditions including lupus vulgaris, leprosy, and psoriasis are considered by clinicians in the differential diagnosis in Sri Lanka. Some locally developed guidelines are available for management of CL.16 Cases need to be preferably laboratory confirmed prior to treatment. Antimonials (intra-lesional or systemic) and cryotherapy are used.16 Thermotherapy is being tested. There is limited literature on efficacy or side effects of these treatment modalities in Sri Lanka. Intra-lesional SSG and hypertonic saline have shown some encouraging results.17,18 Timely laboratory confirmation is useful to prevent unnecessary administration of toxic and expensive drugs and unpleasant sequelae of long-lasting untreated illness. Light microscopy is generally considered the first-line investigation in CL while in vitro culturing and PCR remain as second-line investigations.19 Accurate clinical suspicion remains critical to enhance case confirmation.
Self-referral is usually not immediate in leishmaniasis due to non-disturbing nature of lesions, lack of characteristic features and lack of awareness, making efforts for active detection critical. Furthermore, an understanding of early and late occurring clinical markers is also important since the disease may be slowly evolving with multiple clinical stages in this disease.19 Availability of a uniform set of clinical markers across an endemic area will, therefore, facilitate case screening, narrow down referrals for investigations, minimize unnecessary invasive procedures, investigation costs, and ensure a more accurate clinical diagnosis.
Clinical markers are widely used in many clinical conditions.20–23 Combination of clinical markers and laboratory markers have also been studied.24 As far as it is known, a graded scoring system is not available for CL. Furthermore, generalizability of findings of small-scale studies is hindered by regional variation which is a known barrier in leishmaniasis control.15 In Sri Lanka, general clinical profile of CL has already been well described.5,8,9,25 Current study examined the reliability and validity of clinical markers, previously described by authors as a screening and diagnostic tool for CL in Sri Lanka and a decision-making algorithm was developed.
MATERIALS AND METHODS
Four hundred CL-suspected patients from different districts within the island were recruited into a descriptive study after informed written consent. A hypothetical estimation of the least prevalent clinical marker was made as 15%. A minimum sample size of 242 patients was calculated assuming a 5% alpha error and a confidence interval ranging from 10.5 to 19.5%. Data were collected from a much larger population of 400 cases covering a period of 8 years. Clinical assessment was carried out by the medical professionals, using a previously validated data collection form25 and the diagnosis was confirmed by (one or more) microscopy/culture/PCR on lesion material.
Details regarding symptoms, socio-demographic patterns, lesion characteristics, surrounding skin, and disease spread were recorded. Forty (40) such selected criteria (all not shown) were analysed against the gold standard being the positive collective laboratory finding. Patient or lesion totals were considered as denominators depending on the feature and P values, frequencies and percentages were obtained using SPSS 20 version. Missing and doubtful information was excluded.
Working definitions
Typical onset of a lesion – non tender, non itchy, skin coloured, papule of less than 1 cm in size.
Size – maximum diameter of the observable lesion excluding induration measured to the closest centimetre.
Typical stage – common lesion types that are seen during the progression of a skin lesion in CL; a papule of less than 1 cm, a nodule of over 1 cm, an ulcerating nodule or an ulcer.
Likely place of infection acquisition (LPIA) – district from where the patient most likely acquired the infection. Determined as the place where patient lived/worked for most of the time during 12 months prior to the onset of the skin lesion.
Analysis of data
Socio-demographic and clinical markers were analysed (all variables not shown) to determine the frequencies and significance of associations with parasite positivity as compared to laboratory-negative lesions. Ten markers (age, sex, occupation, region, typical onset, number, size, site, type, and duration) among other markers were selected based on their high presence in the study population. A further subset of markers was selected based on statistically significant P values. Five parameters (P values, sensitivity, specificity, positive, and negative predictive values-PPV, NPV) were calculated for each selected marker in the total study population and sub-populations at different durations in comparison with the gold standard (laboratory finding). Different combinations of these significant markers were also tested in the total study population.
Diagnostic scoring system
The scores were analysed at two levels. First stage is based on general features and predicts the possibility of a patient/lesion to be CL, i.e. allows pre-selection of suitable candidates for medical referral by primary health care workers in the field. The second stage is based on specific clinical features and assists the clinicians in decision making towards further management.
During the first-level analysis, the probability of the presence of 10 basic clinical markers in the parasitologically confirmed group was converted to a score. If a particular marker was present in over 75% patients/lesions, score of 4 was assigned, in 50–74% = 3, 25–49% = 2, < 25% = 1. Therefore, at first-level analysis total possible score for a lesion ranged between 10 and 40 (from 1 × 10 to 4 × 10) when all 10 markers were considered. Total observed score for all 10 features was calculated as a percentage of the total possible score. A cut-off value for screening was defined for the study population based on this percentage value (detailed in results section).
At the second-level analysis, 7/10 clinical markers which were highly prevalent in the laboratory-confirmed group as compared to the laboratory-negative group (typical onset, number, size, duration, defined edge of lesion, regular edge of lesion, and shape) were further considered. Five statistical parameters were selected to examine each clinical marker in comparison with the gold standard. Each parameter value was given a score depending on the value (P value < 0.05 = 2 points; if sensitivity, specificity, PPV or NPV was over 80% = 3, 65–79% = 2 points, less than 65% = 0) with a possible maximum of 14 points for each clinical marker. Total score obtained for each feature and an average score for the panel of markers were calculated. In a given patient average score obtained by this system indicated the likelihood of obtaining a laboratory confirmed result and, therefore, would justify (or refute) the need for repeated investigations or investigation for alternative possibilities. A cut-off average score for stage 2 was defined based on the average score obtained in the study.
Selected individual clinical features were tested with same statistical parameters at different durations of lesion. Combined clinical markers were also tested in a similar manner.
Ethical aspects
Ethical clearance was obtained from the Ethics Review Committee of Faculty of Medicine, University of Colombo, Sri Lanka.
Results
Socio-demographic data and lesion pattern data from 284 laboratory positive patients were compared with those data from 116 laboratory negative patients. Age ranged from 1 to 82 years (median 33; SD = 14.354). Majority was males and soldiers working/living in Northern/Southern Sri Lanka. Most of soldiers had their residences in other parts of the country (data not shown).
Laboratory-confirmed patients
Socio-demographic patterns (age, sex, residence, and occupation) of the laboratory-confirmed patients followed that of total study population (data not shown). Most patients presented at a mean duration of 7.8 months with few late presentations (ranging up to 72 months). However, 42% of laboratory-confirmed lesions were seen within 4 months duration (n = 120). Clinical patterns of laboratory positive and negative groups were compared. Young adult males (21–40 years) who were either inhabitants or employers in these areas were more commonly affected (Northern/Southern Sri Lanka). Most lesions started in the typical manner on the exposed body areas and remained single or double in number (Table 1). All the typical clinical forms of lesions (papules, nodules, ulcerating, or completed ulcers) were seen in approximately equal proportions. Higher proportion of ulcerating nodules demonstrated parasites as compared to that of ulcers (data not shown).
Table 1. Distribution of selected features among the total study population.
| Variable | Laboratory-confirmed patients, number (%) | Clinically-confirmed patients, number (%) | P-value | ||
| Occupation | |||||
| Outdoor activities | 178 | (62.7) | 69 | (58.9) | P > 0.05 |
| Other | 106 | (37.3) | 47 | (41.1) | |
| Likely place of acquisition of infection†‡ (LPIA) | |||||
| Northern | 102 | (55.7) | 42 | (53.2) | P > 0.05 |
| Western | 3 | (1.6) | 5 | (6.3) | |
| Southern | 72 | (39.3) | 29 | (36.7) | |
| Other | 6 | (3.4) | 3 | (3.8) | |
| Number of lesions | |||||
| Up to 2 | 251 | 88.4 | 108 | 93.1 | P>0.05 |
| More than 2 | 33 | 11.6 | 8 | 6.9 | |
| Type of lesion | |||||
| Typical lesion | 53 | 18.7 | 22 | 19.0 | P > 0.05 |
| Nodule | 67 | 23.6 | 29 | 25.0 | |
| Ulcerating nodule | 66 | 23.2 | 19 | 16.4 | |
| Ulcer | 74 | 26.0 | 34 | 29.3 | |
| Plaque | 19 | 6.7 | 10 | 8.6 | |
| All other | 5 | 1.8 | 2 | 1.7 | |
| Site of lesion | |||||
| Trunk | 21 | 7.3 | 10 | 8.6 | P > 0.05 |
| Head and neck | 95 | 33.4 | 42 | 36.2 | |
| Lower limbs | 21 | 7.3 | 16 | 13.8 | |
| Upper limbs | 147 | 51.8 | 48 | 41.4 | |
| Size of lesion | |||||
| Up to 2 cm | 197 | 69.4 | 72 | 62.1 | P > 0.05 |
| Above 2 cm | 87 | 30.6 | 44 | 37.9 | |
| Duration | |||||
| Up to 4 | 120 | 42.3 | 50 | 42.3 | 0.052§ |
| >4–8 minutes | 105 | 37.0 | 30 | 25.4 | |
| >8–12 minutes | 37 | 13.0 | 18 | 15.5 | |
| >12 minutes | 22 | 7.7 | 18 | 15.5 | |
| Onset typical* | |||||
| Yes | 273 | 96.1 | 108 | 91.5 | 0.026§ |
| No | 11 | 3.9 | 10 | 8.5 | |
| Definition of the edge of the lesion†‡ | |||||
| Well defined | 145 | 57.1 | 41 | 43.2 | 0.002§ |
| Ill defined | 109 | 42.9 | 55 | 57.9 | |
| Regularity of the lesion edge†‡ | |||||
| Regular | 205 | 67.2 | 70 | 63.0 | 0.018§ |
| Irregular | 100 | 32.8 | 51 | 37.0 | |
| Shape of the lesion†‡ | |||||
| Rounded | 222 | 73.8 | 80 | 64.0 | 0.030§ |
| Oval | 23 | 7.6 | 10 | 8.0 | |
| Irregular | 55 | 18.6 | 35 | 28.0 |
*Painless acne-form non-itchy lesions of < 2 mm size. †Total number of lesions (not cases) was considered. ‡Doubtful information and missing cases have been excluded. §Statistically significant P values (P < 0.05) in comparison with laboratory negative lesions or patients.
Stage 1: scoring system for screening
Selected set of criteria included 10 markers, i.e. four socio-demographic features (age, sex, occupation, and LPIA) and six clinical markers (duration, nature of onset, site, size, number, and type of lesion) were used in this stage (Table 2). Male gender, Northern or Southern areas of LPIA, history of typical nature of onset, being single, typical lesion stage, occurrence over exposed areas of body, and being below 8 months duration each scored highest (4 points). Young adult age group (21–40 years), engagement in outdoor occupations, and small size each scored 3 points. Different combinations of these factors resulted in different total scores. Total possible score ranged from 10 to 40. Total observed highest score for the selected 10 features was 37, giving a percentage mark of 92.5 for the possibility of being CL (Table 2). Therefore, a cut-off of 80% (32/40) was decided for screening. It is assumed that atypical CL lesions will also be included by lowering the value from 92.5% to 80%. Calculation of a cut-off value for initial screening was done (Table 3).
Table 2. Stage 1 Scoring system for pre-selection of cases for investigation.
| Clinical feature | Category | % within category | Score* |
| Age group (years) | 21–40 | 64.4 | 3 |
| Other ages | 35.6 | 2 | |
| Sex | Male | 79 | 4 |
| Female | 21 | 1 | |
| Occupation | Outdoor activities | 70.8 | 3 |
| Other | 29.2 | 2 | |
| Region | North or south | 95.0 | 4 |
| Other | 5.0 | 1 | |
| Onset typical# | Yes | 96.4 | 4 |
| No | 3.6 | 1 | |
| Number of lesions | Single | 73.9 | 4 |
| Multiple | 26.1 | 1 | |
| Type of lesion | A typical stage | 91.0 | 4 |
| An atypical stage | 9.0 | 1 | |
| Site of lesion | An exposed area | 89.7 | 4 |
| Other | 10.3 | 1 | |
| Size of lesion (maximum) diameter | |||
| Up to 2 cm | 68.7 | 3 | |
| Above 2 cm | 31.3 | 2 | |
| Duration (months) | Within 8 months | 79.3 | 4 |
| Above 8 months | 20.7 | 1 |
# Painless acne-form non-itchy lesions of < 2 mm size, *If percentage within category in each clinical variable was over 75% patients or lesions, score of 4 was given, if 50–74% = 3, 25–49% = 2 and less than 25% = 1 was given.
Table 3. Calculation of a cut-off value for screening.
| Indicator | Calculation procedure | Calculation in the current project |
| Number of selected features | n | 10 |
| Lowest possible score if all features are considered* | nX1 | 1 × 10 = 10 |
| Highest possible score if all features are considered* | nX4 | 10 × 4 = 40 |
| Total observed score in the study sample | y | 37 |
| Possibility of the lesion being leishmaniasis | (y/nX4)100 | (37/40) × 100 = 92.5% |
| Cut-off value for referring (investigations/medical evaluation) | May be decided. E.g.: over 80% |
80% is suggested. Total 32/40 marks required for a patient. |
*If any selected feature was found in over 75% patients or lesions, score of 4 was given, if 50–74% = 3, 25–49% = 2 and less than 25% = 1 was given.
Stage 2: scoring system for prediction of laboratory confirmation
Single clinical markers of significance
Out of all studied patient or lesion characteristics, a subset of six clinical characteristics were significantly associated (P < 0.05) with positive laboratory results as compared to those of laboratory-negative patients (Table 1). These were typical onset (P = 0.026), small size < 2 cm (P = 0.046), defined edges (P = 0.002), regular edges (P = 0.018), and rounded shape (P = 0.030). Lesser numbers of lesions ( < 3) was a highly sensitive feature (88.4%) though this was found in association with laboratory negative lesions as well (P = 0.061), (Table 4). Likelihood of parasite isolation was higher in lesions at 5–8 months duration when compared to younger or older lesions (P = 0.050), (Table 4). Therefore, all seven features were considered in calculating stage 2 cut-off value.
Table 4. Validity of clinical markers in relation to laboratory diagnosis tested using selected parameters.
| Clinical marker | P value | Sensitivity | Specificity | PPV | NPV | Total score |
| (assigned score) | ||||||
| Typical onset* | 0.026 | 97.2 | 8.6 | 71.9 | 47.6 | 7 |
| (2) | (3) | (0 | (2) | (0) | ||
| Number up to 2 | 0.061 | 88.4 | 6.9 | 70.0 | 19.5 | |
| (0) | (3) | (0) | (2) | (0) | 5 | |
| Size up to 2 cm | 0.046 | 74.3 | 39.7 | 72.3 | 32.4 | |
| (2) | (2) | (0) | (2) | (0) | 6 | |
| Duration between 5 and 8 months | 0.050 | 37 | 73.3 | 77.2 | 32.2 | |
| (2) | (0) | (2) | (2) | (0) | 6 | |
| Definition of the ledge of lesion | 0.002 | 59.9 | 57.9 | 43.2 | 22.2 | |
| (2) | (0) | (0) | (0) | (0) | 2 | |
| Regularity of the edge of the lesion | 0.018 | 72.2 | 44.0 | 74.5 | 25.5 | |
| (2) | (2) | (0) | (2) | (0) | 6 | |
| Rounded lesion shape | 0.030 | 78.2 | 31.0 | 73.5 | 68.8 | |
| (2) | (2) | (0) | (2) | (2) | 8 | |
*Painless acne-form non-itchy lesions of < 2 mm size, PPV: positive predictive value; NPV: negative predictive value, score allocation; P value < 0.05 = 2 points, sensitivity, specificity, PPV or NPV over 80% = 3, over 65% = 2 points each less than 65% = 0.
Typical onset, small size, defined and rounded edges, and rounded shape were both highly sensitive and had good positive predictive values (Table 4). Typical onset of a skin lesion was most suggestive of CL (sensitivity of 97%, PPV of 72%), (Table 4). Total score obtained by different markers ranged from 2 to 8 in a scale of 14 while five of seven markers scored >6. Average score obtained by a single marker was 5.7. Since the difference between these 2 values was less than 1, a cut-off value of 6 was decided for stage 2. Calculation of a cut-off for stage 2 is detailed in the Table 5.
Table 5. Calculation of a cut-off for laboratory investigations, treatment, or interpretation.
| Indicator | Generalized calculation | Calculation in the current project |
| Total possible range (scale) of marks | a + b + c + d + e = Y | 0–14 (2 + 3 + 3 + 3 + 3) |
| Total possible score for all considered features | YXn* | 14 × 7 = 98 |
| Total marks obtained by the features | Y1+Y2+….+Yn | 40/98 (40.8%) |
| Average score of a second-stage clinical marker (f) | (Y1+Y2+….+Yn)/n | 40/7 = 5.7 out of 14 |
| Number obtained > 6 out of total 14 marks (g) | As observed | 5/7 markers |
| Average suggested cut-off for a single marker to be considered suitable as a marker in CL (most frequent) | As observed | 6 |
| Average total mark to be considered as the cut-off to be considered as CL | As decided | 5.7 × 7 = 39.9 ( = 40) |
*Number of features.
Combinations of clinical markers
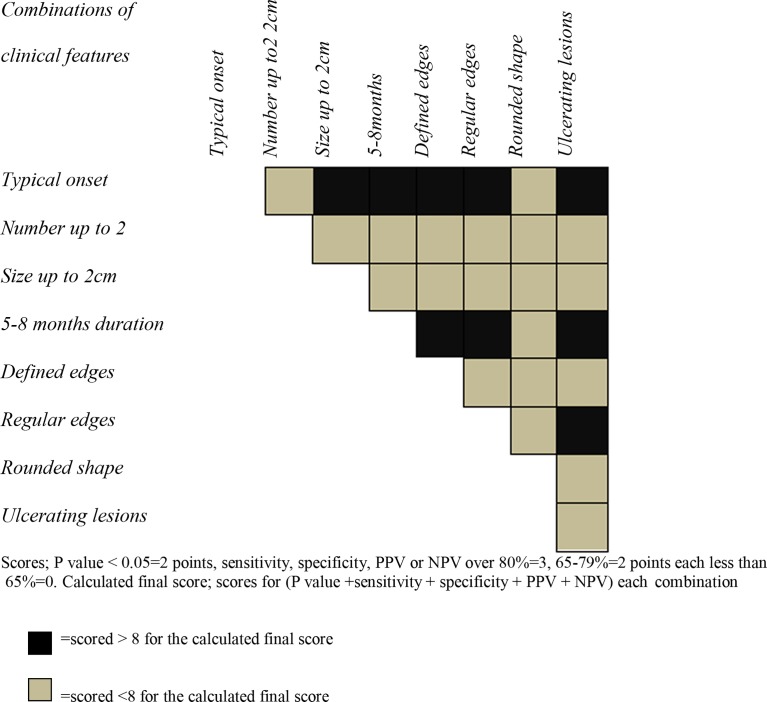
Usefulness and reliability of different combinations of clinical markers were examined. Many combinations demonstrated a high possibility for being laboratory positive as compared to lesions that are lacking the combination of features (Fig. 1). Lesions at 5–8 months duration with defined edges, with regular edges or at ulcerating stage were identified as reliable clinical markers, (Fig. 1). Specificity and PPV could be increased to 57–99% and 70–84%, respectively, in almost all the combinations (all data not shown). However, none of the combinations reached 100% sensitivity or specificity. Total observed score ranged between 4 and 8 for a given combination in a scale of 14 (calculated as described for single lesions in methodology), (Fig. 1). An average of 5.6 was obtained by combination markers. This value did not show any large increase from the total average score obtained for non-combined clinical markers.
Figure 1.
Validity demonstrated by the scores obtained by different combinations of clinical markers in the total study population.
Clinical markers at varying durations
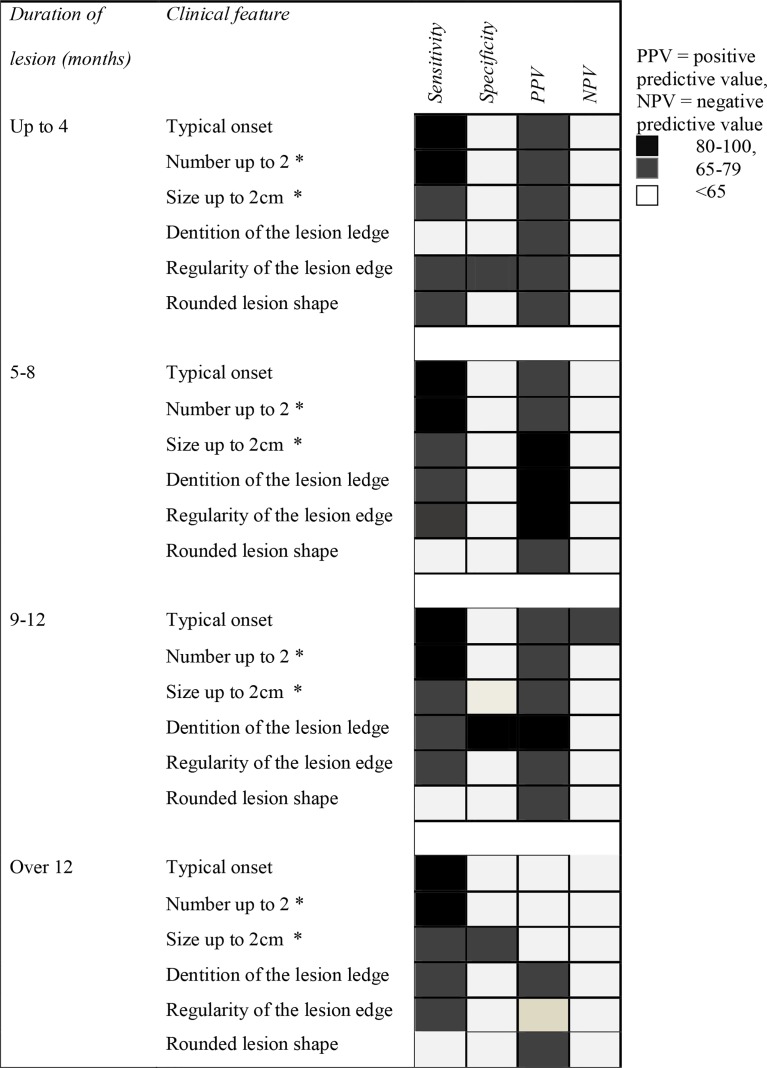
Four statistical parameters of six clinical markers (typical onset, less than 3 lesions, less than 2 cm size, defined edges, regular edges, and rounded shape) were examined for the variations related to duration of lesion development (Fig. 2). Four clinical markers remained highly sensitive in both early ( ≤ 4 months) and late (>12 months) lesions, viz. typical onset (91.3–98.4%), presence of ≤ 2 lesions sensitivity (82.6–94.7%), size ≤ 2 cm (66.9–73.7%), and regular edges (68.6–76.3%), (Fig. 2). Well-defined edges of lesion were a good indicator in over 4 months to over 1 year lesions. All the markers had a good PPV throughout the first 12 months. Size in CL lesions mostly remained less than 3 cm irrespective of the lesion progression. Small lesion size, defined edges, and regularity of lesion edge served as good markers in lesions within their first year (Fig. 2). Most of the markers were not specific for leishmaniasis at any time point. However, the PPV ranged between 60 and 86% for all the parameters of lesions within the first 12 months (Fig. 2). Lesions remaining small after 12 months were also highly indicative of CL. Generally clinical markers did not stand out in chronic lesions that had a higher possibility to start in an atypical manner.
Figure 2.
Validity demonstrated by scores obtained for selected clinical features at different durations of lesion.
Total score obtained by all six clinical features at different durations was also calculated. This was clearly low in chronic lesions (scored10) as compared to early lesions (up 4 months – 14, 5–8 months – 14, 9–12 months – 15). Therefore, cut-off values for different durations were not calculated.
Discussion
This study describes a double-staged panel of clinical markers suitable for screening of patients in the first stage and for management decision in the second stage, respectively, in CL in Sri Lanka. Study also revealed that clinical evaluation per se is inadequate for an accurate diagnosis, in spite of the high levels of sensitivities achieved by many individual markers. This fact is further highlighted in chronic lesions as reliability of clinical markers declined remarkably.
In stage 1, a panel of 10 markers was identified to be highly prevalent among patients’ populations with clinically suggestive CL and demonstrated their suitability as a screening tool. A single small lesion over an exposed area in a young adultmale presenting from Northern or Southern Sri Lanka with a history of a typical onset (painless, acne-form papules) and a known developmental stage at a duration of less than 8 months may provide over 90% prediction as CL. Young adult age group, male gender, history of typical onset, exposed sites, and typical CL developmental stages seem to be highly prevalent among the patient population of suspected CL even when considered independently. Most lesions seem to remain single and small across successive developmental stages over the first year.
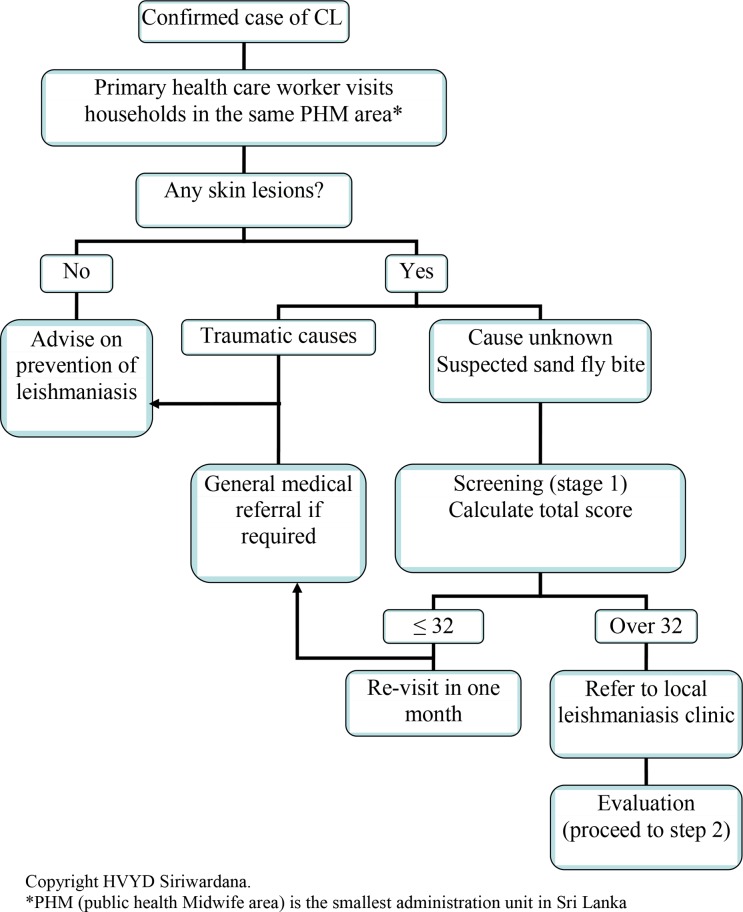
All these features are less subjective and easy to assess. Features such as nature of onset, site, duration, and number often remain constant allowing constant scores irrespective of the duration or the examiner. Therefore, this panel can be used by trained primary health care workers with minimum inter-observer errors. Once a case is reported in the hospital, field assistants may follow the a diagnostic algorithm in screening and referring further patients from immediate environment of the case (Public Health Midwife area in case of Sri Lanka), (Fig. 3). Cut-off value may be used to preselect the candidates for referral. Local leishmaniasis clinics may be conducted monthly to manage the screened cases.
Figure 3.
Step-wise management decision in cutaneous leishmaniasis (CL) due to L. donovani in Sri Lanka: stage 1 – screening.
Passive case detection may not reflect true distribution, even though CL was also very likely in cases presenting from the main two disease foci in the island (North and East). However, minimal errors are expected as Colombo served as country's main referral centre. Patients with history of outdoor occupations were also at a higher risk of acquiring CL. Most of the patients were soldiers by occupation and worked in outdoor areas for long hours and slept in bunkers and peripheral rural camps at night. However, pattern of age, sex, occupational, and spatial distribution can change with time as shown previously.5,9 Therefore, predictors may be adjusted for the time and setting and used individually or in combination. Also, these features were equally prevalent among both laboratory-confirmed and non-confirmed (already clinically justified) cases and, therefore, do not qualify as specific diagnostic markers for exclusion of non-leishmanial cases.
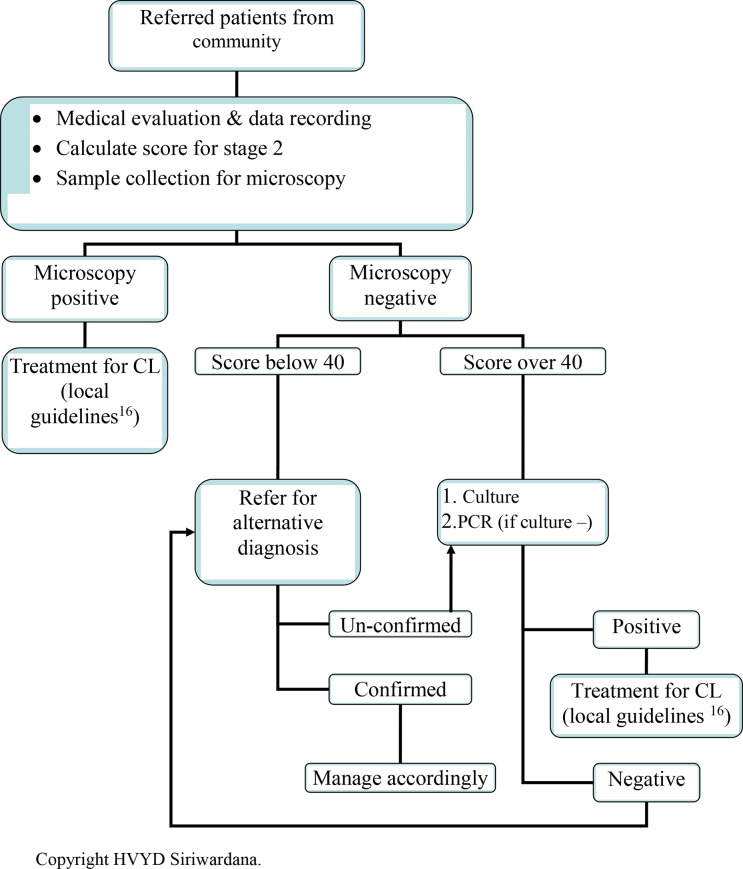
Second stage identified clinical features which were strongly associated with parasite-positive lesions as compared to laboratory-negative group, demonstrating the suitability as diagnostic markers (typical onset, size up to 2 cm, number < 2, duration in 5–8 months, regular and defined edges, rounded shape). They become valuable This sentence at the hand of an expert since some of them are measurable only in a large lesion, difficult to assess or highly subjective. Stage 2 algorithm may be used by medical personnel (Fig. 4). All the screened individuals may be investigated by inexpensive and field friendly light microscopy on lesion material. Even though a positive report always indicates necessary conservative or interventional anti-leishmanial measures, false negatives are also possible when parasite load is low in chronic lesions, treated lesions, some clinical forms (E.g. L. recidivans), and due to missed diagnoses. Stage 2 clinical scores predict the possibility of a true positive or negative laboratory report. Therefore, it is advisable to repeat microscopy and proceed with second-line investigations (culture followed by PCR if negative) in all light microscopy negative cases scoring over 40 in stage 2. If the score is less than 40, clinician may opt for investigating alternative possibilities and failing this, leishmaniasis can be revisited with repeat microscopy and second-line investigations.
Figure 4.
Step-wise management decision in cutaneous leishmaniasis (CL) due to L. donovani in Sri Lanka: stage 2 – clinical handling.
Usefulness of the tool may be further increased by combination of some features. However, considering multiple features for statistical evaluation did not increase P values (data not shown) or the average scores while combination of markers remarkably improved specificity and PPV (Fig. 1). Lesions in 5–8 months of duration retaining defined and regular edges or ulcerating nodules retaining regularity of the edges of lesion tended to give positive laboratory results (Fig. 1).
Statistical significance of different clinical profiles for different durations of lesion was not very obvious in the study group. This may be explained by individual variation in disease progression. In fact, four features, viz. presence of ≤ 3 lesions, size ≤ 2 cm, well-defined edges, and regular edges, remained highly sensitive in early ( ≤ 4) and late (24 months) lesions making them suitable for interpretation of laboratory reports. Lesions remaining single after 12 months were highly indicative of CL (sensitivity of 66%, specificity 66.7%). In chronic lesions, history of typical onset, less lesion number, and regular and defined edges of lesion were useful for prediction, though clinical reliability decreased in chronic lesions after the first year. However, these become important as leishmaniasis evolve slowly in a chronic fashion and features may vary duration wise.19 Decisions regarding treatment are important as anti-leishmanials are toxic and expensive. It is recommended that a practical guide is developed for screening, investigation, and management of CL in this country. National guidelines can be made available using prescribed tool considered together with other factors such as availability of investigation and treatment resources, beneficial versus ill effects of treatment and government policies.
Current study also proves that any individual or combination of features will not provide 100% accuracy in predicting CL, indicating the requirement of laboratory investigations as the gold standard, the value of pre-treatment investigations at all possible occasions, re-investigation of a single negative smear and thorough active case screening.
Reliable initial clinical detection carries a great importance in any new disease focus. This is particularly applicable to Sri Lanka since majority of CL infections occur in remote areas in dry zone where laboratory facilities, content expertise, professional, and public awareness are still limited.5,8,9 If a true case of CL is missed at the beginning, the possibility of CL is only revisited much later when all other diagnoses are excluded as clinical features evolve slowly in leishmaniasis. Use of a uniform clinical tool will improve case identification, enable standardization between health care centres and, therefore, will minimize errors in variations.
Conclusions
Uniform and measured clinical criteria and an algorithm are now available for detection and decision making in CL in Sri Lanka. Other endemic settings may adjust this tool as appropriate for their own use. Patient selection for investigations/treatment is better carried out based on appropriate clinical criteria in this two-staged scoring system. Interpretation of a negative result should be done with extreme care before, deciding on alternative diagnoses. However, this does not by any means undermine the importance of laboratory confirmation of diagnosis prior to treatment in all instances. It is important that these facts are considered in patient selection as well as active screening of populations at first contact care level for undiagnosed CL. However, continued surveillance for the changing clinical trends and updating the scoring system would remain important.
Acknowledgements
Authors are thankful to Dr N. Ekneligoda and Mr S. Weerasinghe for secretarial and technical assistance. Financial assistance through grants from University of Colombo (AP/3/2/2014/RG/13) and NIAID/NIH, USA (1 R01 AI099602-01) is acknowledged.
Disclaimer Statements
Contributors H.V.Y.D. Siriwardana: Concept and design of the paper, original project design, proposal writing, ethical application, project conduct, data collection, laboratory testing, data entry and analysis, manuscript writing. U. Senerath: Checked the statistical validity of analyzed data and results, agreed to the final manuscript. P.H. Chandrawansa: Clinical case detection and case referral to the PI, read and agreed to the final manuscript. N.D. Karunaweera: Guided the PI in all the aspects from project proposal to manuscript preparation, agreed to the final manuscript.
Funding Financial assistance for this project was provided by University of Colombo (AP/3/2/2014/RG/13), and NIAID/ NIH, USA (1 R01 AI099602-01).
Conflicts of interest There is no conflict of interest.
Ethics approval Ethical clearance was obtained from the Ethics Review Committee of Faculty of Medicine, University of Colombo, Sri Lanka.
References
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P. Leishmaniasis. Worldwide and global estimates of its incidence. PLoS ONE. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. 2010. Control of the Leishmaniases. Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases; pp. 1–185. Technical Report Series. NO. 949. [Google Scholar]
- 3.WHOGlobal Plan to Combat Neglected Tropical Diseases 2008–2015. 2007. Available from http://whqlibdoc.who.int/hq/2007/who_cds_ntd_2007.3_eng.pdf [Google Scholar]
- 4.Athukorale DN, Seneviratne JK, Ihalamulla RL, Premaratne UN. Locally acquired cutaneous leishmaniasis in Sri Lanka. J Trop Med Hyg. 1992;95:432–3. [PubMed] [Google Scholar]
- 5.Siriwardana HVYD, Udagedara C, Karunaweera ND. Study on clinical patterns, risk factors and efficacy of cryo therapy in cutaneous leishmaniasis in Sri Lanka. Cey Med J. 2003;48(1):10–12. doi: 10.4038/cmj.v48i1.3386. [DOI] [PubMed] [Google Scholar]
- 6.Karunaweera ND, Pratlong F, Siriwardana HVYD, Ihalamulla RL, Dedet JP. Sri Lankan cutaneous leishmaniasis is caused by Leishmaniadonovani zymodeme MON-37. Trans R Soc Trop Med Hyg. 2003;97(4):380–1. doi: 10.1016/s0035-9203(03)90061-7. [DOI] [PubMed] [Google Scholar]
- 7.Siriwardana HV, Noyes HA, Beeching NJ, Chance ML, Karunaweera ND, Bates PA. Leishmaniadonovani and cutaneous leishmaniasis. Sri Lanka. Emerg Infect Dis. 2007;13(3):476–8. doi: 10.3201/eid1303.060242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siriwardana HVYD, Thalagala N, Karunaweera ND. Clinical and epidemiological studies on the cutaneous leishmaniasis caused by Leishmania (Leishmania) donovani in Sri Lanka. Ann Trop Med Parasitol. 2010;104(3):213–23. doi: 10.1179/136485910X12647085215615. [DOI] [PubMed] [Google Scholar]
- 9.Rajapaksa US, Ihalamulla RL, Udagedera C, Karunaweera ND. Cutaneous leishmaniasis in Southern Sri Lanka. Emerg Infect Dis. 2007;101(8):799–803. doi: 10.1016/j.trstmh.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Ranasinghe S, Zhang WW, Wickremasinghe R, Abeygunasekera P, Chandrasekharan V, Athauda S et al. Leishmania donovani zymodeme MON-37 isolated from an autochthonous visceral leishmaniasis patient in Sri Lanka. Pathog Glob Health. 2012;106(7):421–4. doi: 10.1179/2047773212Y.0000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abeygunesekara PH, Costa YJ, Seneviratne N, Ranatunga N, Wijesundera MdeS. Locally acquired visceral leishmaniasis in Sri Lanka. Cey Med J. 2007;52(1):30–1. doi: 10.4038/cmj.v52i1.1047. [DOI] [PubMed] [Google Scholar]
- 12.Rathnayake D, Ranawake RR, Sirimanna G, Siriwardhane Y, Karunaweera N, De Silva R. Co-infection of mucosal leishmaniasis and extra pulmonary tuberculosis in a patient with inherent immune deficiency. Int J Dermatol. 2010;49(5):549–51. doi: 10.1111/j.1365-4632.2010.04376.x. [DOI] [PubMed] [Google Scholar]
- 13.Karunaweera ND. Leishmania donovani causing cutaneous leishmaniasis in Sri Lanka, a wolf in sheep's clothing? Trends Parasitol. 2009;25:458–63. doi: 10.1016/j.pt.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 14.2009. Faculty of Medicine, University of Colombo, Sri Lanka. National action plan for leishmaniasis control in Sri Lanka. In: First international colloquium on leishmaniasis, Colombo, Sri Lanka. Abstracts and action plan. [Google Scholar]
- 15.Stockdale L, Newton R. A review of preventative methods against human leishmaniasis infection. PLoS Negl Trop Dis. 2013;7(6):e2278. doi: 10.1371/journal.pntd.0002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karunaweera ND, Siriwardana HVYD, Karunanayake PH. Management of leishmaniasis. Sri Lanka Prescriber. 2013;21(2):1–5. [Google Scholar]
- 17.Ranawaka RR, Weerakoon HS, de Silva SH. Randomized, double-blind, controlled, comparative study on intralesional 10% and 15% hypertonic saline versus intra-lesional sodium stibogluconate in Leishmania donovani cutaneous leishmaniasis. Int J Dermatol. 2015;54(5):555–63. doi: 10.1111/ijd.12685. [DOI] [PubMed] [Google Scholar]
- 18.Ranawaka RR, Weerakoon HS, Opathella N. Liquid nitrogen cryotherapy on Leishmania donovani cutaneous leishmaniasis. J Dermatolog Treat. 2011;22(4):241–5. doi: 10.3109/09546631003762654. [DOI] [PubMed] [Google Scholar]
- 19.Gordon C, Alimuddin Z, editorsLeishmaniasis Dedet JP, Pratlong F.Manson's Tropical Diseases 21st edLondon: Saunders; 2002. p. 1339–64. [Google Scholar]
- 20.Ho TS, Wang SM, Lin YS, Liu CC. Clinical and laboratory predictive markers for acute dengue infection. J Biomed Sci. 2013;20:75. doi: 10.1186/1423-0127-20-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis JS, McGloughlin S, Tong SY, Walton SF, Currie BJA. novel clinical grading scale to guide the management of crusted scabies. PLoS Negl Trop Dis. 2013;7(9):e2387. doi: 10.1371/journal.pntd.0002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosh SK, De S, Sarkar U, Ghosh M, Chatterjee MK, Samanta S. Clinical profile of dengue during 2005 outbreak in Kolkata and predictive markers of dengue haemorrhagic fever. J Indian Med Assoc. 2011;109(11):790–3. [PubMed] [Google Scholar]
- 23.Diniz JL, Costa MO, Gonçalves DU, Braz J. Muco cutaneous leishmaniasis: clinical markers in presumptive diagnosis. Otorhinolaryngol. 2011;77(3):380–4. doi: 10.1590/S1808-86942011000300018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rathakrishnan A, Klekamp B, Wang SM, Komarasamy TV, Natkunam SK, Sathar J et al. Clinical and immunological markers of dengue progression in a study cohort from a hyperendemic area in Malaysia. PLoS One. 2014;9(3):e92021. doi: 10.1371/journal.pone.0092021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siriwardana HVYD. Western Province, Sri Lanka: University of Colombo; 2008. (Study on the clinical epidemiology of leishmaniasis in Sri Lanka and the molecular identification of the parasite [PhD thesis]). [Google Scholar]