Abstract
Objective
While forgetfulness is widely reported by breast cancer survivors, studies documenting objective memory performance yield mixed, largely inconsistent, results. Failure to find consistent, objective memory issues may be due to the possibility that cancer survivors misattribute their experience of forgetfulness to primary memory issues rather than to difficulties in attention at the time of learning.
Methods
To clarify potential attention issues, factor scores for Attention Span, Learning Efficiency, Delayed Memory, and Inaccurate Memory were analyzed for the CVLT-II in 64 clinically-referred breast cancer survivors with self-reported cognitive complaints; item analysis was conducted to clarify specific contributors to observed effects and contrasts between learning and recall trials were compared to normative data. Performance on broader cognitive domains is also reported.
Results
The Attention Span factor, but not Learning Efficiency, Delayed Memory, or Inaccurate Memory factors, was significantly affected in this clinical sample. Contrasts between trials were consistent with normative data and did not indicate greater loss of information over time than in the normative sample.
Conclusions
Results of this analysis suggest attentional dysfunction may contribute to subjective and objective memory complaints in breast cancer survivors. These results are discussed in the context of broader cognitive effects following treatment for clinicians who may see cancer survivors for assessment.
Keywords: Cancer, Memory, CVLT-II
Background
Breast cancer is the most prevalent of all cancers [1] and the objective cognitive effects of breast cancer treatment have been widely studied over the past two decades. Cross-sectional and longitudinal neuroimaging and neurocognitive studies have detected a range of subtle neuropsychological deficits [2]. Animal studies of chemotherapy-induced cognitive impairment have demonstrated changes in learning and memory [3]. Taken together, the accumulated neuropsychological, neuroimaging, and animal research has provided breast cancer survivors and neuropsychologists with confirmation of a subtle profile of cancer and cancer treatment associated cognitive change [4].
Forgetfulness is a common complaint in cancer survivors. While breast cancer survivors do self-report memory difficulties [5, 6], objective findings of a primary memory impairment are inconsistent. Four early meta-analyses reported significant effects in multiple domains, including in visual and verbal memory, but these analyses included either studies with multiple cancer types and therapies [7], or included within the analysis studies that measured cognitive function during active treatment [7–10]. In contrast, the most recent meta-analysis that included only post-treatment breast cancer survivors found cross-sectional and longitudinal effects in verbal and visuospatial functioning but not in primary memory measures [11]. Specific to the current study, that meta-analysis did not find a significant attention effect, but did not analyze individual components of serial verbal list learning measures as we have done in the current analysis.
Given the increased numbers and longevity of breast cancer survivors, as well as better patient education regarding cognitive effects that may follow treatment, it is imperative that clinicians gain a clearer understanding of the relatively subtle pattern of findings that may follow treatment. The inconsistent finding of memory dysfunction in research samples is particularly problematic given that forgetfulness is a typical subjective complaint in clinically referred patients. There is a possibility that only a subset of survivors may experience cognitive dysfunction following treatment and these relatively subtle effects are not detected within larger research groups of unaffected survivors following treatment. Failure to find consistent, objective memory issues may be also due to the possibility that cancer survivors misattribute their experience of forgetfulness to primary memory issues rather than to difficulties in attention at the time of learning. This distinction will bear on treatment recommendations, including pharmacologic therapy as well as cognitive rehabilitation strategies and interventions following treatment.
This retrospective study investigated whether memory difficulties reported by patients in a clinically referred sample were confirmed in objective testing and to what extent attentional dysfunction might play a role in reported complaints. We chose to focus on a clinically referred sample, the majority of which complained of forgetfulness, since they are arguably the most representative of the kinds of cognitive complaints and issues that cancer survivors report and exhibit following treatment. Further, given that patients in this sample reported cognitive dysfunction severe enough to be referred for comprehensive neuropsychological assessment, objective memory dysfunction should be more likely to be detected if present. By examining performance patterns on the California Verbal Learning Test–Second Edition [12] (CVLT-II), we sought to determine whether the most common symptom reported by clinically referred patients, memory difficulties, was substantiated. The CVLT-II was chosen as it is a standard part of clinical neuropsychological assessment and provides quantitative information at each phase of learning and memory (learning, retention and retrieval). Exploratory and confirmatory factor analysis has identified latent constructs measured by the CVLT–II [13, 14]. Among competing factor structures, these studies found strongest support for a four-factor solution that includes Attention Span, Learning Efficiency, Delayed Memory, and Inaccurate Memory. Hypotheses for this study are informed by clinical observation that suggests generally intact delayed memory in cancer survivors following treatment, little loss of information over time, and specific weakness in initial encoding of to-be-learned information. Utilizing this four-factor model, we hypothesized that clinically referred patients would exhibit a significant weakness in the Attention Span factor, with preserved Delayed Memory performance. Further, we hypothesized that at the individual item level, Trial 1 performance, specifically, would be significantly lower than the age-matched cohort, with preserved performance on the Long Delay Free Recall Trial similar to that of the normative sample. Finally, with regard to information loss over time, we predicted no significant difference in contrasts between Trial 5, Short Delay Free Recall and Long Delay Free Recall compared to CVLT-II normative contrast data [12]. Our hypotheses are based on the association of Trial 1 performance with single trial learning and reliance on brief auditory attention, the decreased influence of attention on Trial 5 performance, and hypothesized intact retention and retrieval over short and long delays (Short Delay Free Recall and Long Delay Free Recall). To further contextualize performance on the CVLT-II, we also report results of remaining neuropsychological measures administered as part of the clinical evaluation to further clarify cognitive performance in this sample.
Methods
Participants in this retrospective study included 64 female breast cancer patients who were administered the CVLT-II as part of a larger battery of tests during clinical neuropsychological evaluation conducted between 2009 and 2013 at the Memorial Sloan-Kettering Cancer Center Neuropsychology Service. IRB approval was obtained by MSKCC to retrospectively analyze clinically referred patients’ neuropsychological batteries. Patients were either self-referred or referred by their oncologist or psychiatrist. Patients who were untestable in the clinicians’ judgment due to acute depression or anxiety, had a chronic mental illness such as bipolar disorder or schizophrenia, a formal diagnosis of ADHD or a learning disorder, or a premorbid neurologic syndrome affecting cognition were not included in this sample. Patients were not excluded based on treatment modality.
Measures and Procedure
California Verbal Learning Test – Second Edition [12]
a measure of serial verbal list learning and recall. In the learning phase of the task, individuals are read 16 words and asked to immediately recall these words over five repeated trials (Trials 1–5). Individuals are then read and asked to repeat a distractor list (List B), and are then asked to freely recall items from the first list (Short Delay Free Recall) followed by a cued recall trial (Short Delay Cued Recall). After a 20-minute delay, individuals are again asked to freely recall the first list (Long Delay Free Recall) followed by a second cued recall trial (Long Delay Cued Recall). Finally, individuals are given a yes/no recognition trial (Recognition) in which they are to identify word list items from the first word list.
Premorbid estimation of ability was assessed using one of three measures: Test of Premorbid Functioning [15]; North American Adult Reading Test [16]; or the Reading subtest of the Wide Range Achievement Test-4 (WRAT-4) [17]. Patients also completed the Beck Depression Inventory – FastScreen for Medical Patients[18]. Cut-off scores for the BDI FastScreen consist of: 0–3 minimal; 4–8 mild; 9–12 moderate; and 13–21 severe depression.
Remaining tests in the neuropsychological battery consisted of: a measure of confrontation naming, The Boston Naming Test (n = 54) [19]; a measure of phonemic fluency, FAS-COWAT (n = 59) [20]; a measure of semantic fluency, Animal Naming (n = 59) [20]; measures of psychomotor speed, visual scanning, and set shifting, Trail Making Tests A and B (n = 58; n = 57) [21]; measures of psychomotor speed, WAIS-IV Coding (n = 58), Symbol Search; measures of auditory attention and working memory, Digit Span (n = 59), Arithmetic (n = 54) [22]; a measure of visuospatial learning and recall, the Rey-Osterrieth Complex Figure Test Immediate and Delayed Recall (n = 42) [23]; and a measure of verbal learning and memory, the WMS-IV Logical Memory I and II (n = 58) [24].
Analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) and the Microsoft Excel package was used for data visualization.
For CVLT-II analysis, all individual raw scores were entered into the CVLT-II scoring software and compared to an age and gender adjusted normative cohort. For the factor model analysis, participants’ normatively transformed z-scores were grouped and entered according to Donders’ four factor model [13], consisting of Attention Span, Learning Efficiency, Delayed Memory, and Inaccurate Memory. Factors consisted of the following individual scores – Attention Span: Trial 1; List B; Percent Recall from Middle Trials 1 – 5; Learning Efficiency: Trial 5; Semantic Clustering; Across Trial Consistency; Delayed Memory: Short Delay Free Recall; Short Delay Cued Recall; Long Delay Free Recall; Long Delay Cued Recall; Recognition Hits; Inaccurate Memory: Intrusions (from all free recall and cued trials); False Positives (from recognition trial). For Intrusions and False Positives, z-scores were reversed for statistical analysis and data visualization. Resulting factor scores were entered into a series of one-sample t-tests with a comparison value of 0, representing the mean performance of the normative cohort. For the secondary individual item analysis, z-scores for each item were entered into a series of one sample t-tests with a comparison value of 0.
For contrast score analysis, previously unpublished raw contrast data between Trial 5, SDFR and LDFR trials from the original CVLT-II standardization sample were requested and acquired from the test publisher (total n = 1087; women 20 – 79 years of age n = 566). Mean and standard deviation of contrast scores (SDFR – Trial 5; LDFR – SDFR; LDFR – Trial 5) for each age band in the normative sample were generated. Subject by subject z-scores were calculated for our Clinical group based on the mean contrast and standard deviation values of age matched normative subject performance. Group comparisons were made at z = −1.5 or greater to assess differences in performance between groups. A z contrast of −1.5 or greater was chosen due to the limited reliability of contrast measures [25], since a more extreme contrast score would be less likely to have resulted from a spurious variation in performance on a given trial.
For analysis of individual measures in the neuropsychological battery, the Boston Naming Test, Trail Making Tests A and B, FAS-COWAT, and Animal Naming, raw scores were compared to normative data corrected for education, age, ethnicity and gender [26]. For WAIS-IV and WMS-IV subtests, raw scores were compared to normative data corrected for age and education using the demographic correction option available through the scoring software [27] . For the Rey-Osterrieth Complex Figure Test, raw scores were compared to normative data corrected for age [23]. All test scores were transformed into z-scores for statistical analysis and data visualization. Resulting scores were entered into one sample t-tests with a comparison value of 0 representing the mean performance of the normative cohort.
Results
Subject characteristics are presented in Table 1. Results of statistical analysis of factor scores, individual items, and contrasts of CVLT-II variables are presented in Table 2. Results of statistical analysis for all remaining neuropsychological measures are presented in Table 3.
Table 1.
Demographic and Medical Status of Clinically Referred Breast Cancer Patients.
| Demographics | Mean (SD) or N (%) or Range |
|---|---|
| Age, y | 47.79 (13.37) |
| Education, y | 16.76 (2.05) |
| Race | |
| Caucasian | 48 (75%) |
| Asian | 7 (11%) |
| African American | 8 (13%) |
| Hispanic | 1 (1%) |
| Premorbid Estimate (Standard Score) | 110 (9.31)† |
| Time between CT and testing (CT treated) | Median = 18 (0–163) |
| BDI Fast Screen Score (Raw Score) | 4.03 (3.39) |
| Memory Complaint | 56 (88%)†† |
|
| |
| Medical Variables | N (%) |
|
| |
| Disease Stage | |
| 0 | 1 (2%) |
| I | 23 (36%) |
| II | 19 (30%) |
| III | 10 (16%) |
| IV | 2 (3%) |
| No stage recorded | 9 (13%) |
| Surgery | |
| Lumpectomy | 15 (23%) |
| Mastectomy | 41 (73%) |
| Bilateral/Radical | 8 (13%) |
| Radiation | 41 (64%) |
| Chemotherapy and Radiation | 34 (53%) |
| Chemotherapy Length (in months) | 5.80 (0–27) |
| Chemotherapy | |
| ACT | 30 (47%) |
| Other | 10 (16%) |
| CMF | 10 (16%) |
| None | 13 (20%) |
| Chemotherapy regimen not recorded | 1(1%) |
| Endocrine Therapy at Time of NP Testing | 39 (61%) |
| Tamoxifen (Novadex)* | 17(27%)* |
| Arimidex (Anastrozole) | 15 (23%) |
| Letrozole (Femara) | 7 (11%) |
| Menopausal Status | |
| Premenopausal | 31 (48%) |
| Perimenopausal | 4 (6%) |
| Menopause | 25 (40%) |
| No Status recorded | 4 (6%) |
Two participants were also receiving Lupron
Based on either the WRAT-Reading; Wechsler Test of Adult Reading; or Test of Premorbid Functioning
Based on subject report of primary complaint
Table 2.
Results of one-sample t-tests on CVLT-II Factor scores and individual items
| Mean | 95% Confidence Interval | Df | t | p | ≤-1.5z (%) | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
|
| |||||||
| Attention | −0.284 | −0.495 | −0.072 | 63 | −2.68 | 0.009 | -- |
| Trial 1 | −0.305 | −0.594 | −0.016 | 63 | −2.11 | 0.039 | 20% |
| List B | −0.188 | −0.487 | 0.112 | 63 | −1.25 | 0.216 | 20% |
| Middle Region | −0.359 | −0.652 | −0.067 | 63 | −2.46 | 0.017 | -- |
|
| |||||||
| Learning Efficiency | 0.133 | −0.088 | 0.353 | 63 | 1.20 | 0.233 | -- |
| Trial 5 | −0.008 | −0.281 | 0.265 | 63 | −0.06 | 0.955 | 12% |
| Semantic Clustering | 0.070 | −0.261 | 0.402 | 63 | 0.42 | 0.673 | -- |
| Recall Consistency | 0.336 | 0.107 | 0.565 | 63 | 2.93 | 0.005 | -- |
|
| |||||||
| Delayed Memory | −0.089 | −0.334 | 0.156 | 63 | −0.73 | 0.47 | -- |
| SDFR | 0.055 | −0.241 | 0.350 | 63 | 0.37 | 0.713 | 14% |
| SDCR | −0.070 | −0.392 | 0.251 | 63 | −0.44 | 0.664 | 20% |
| LDFR | 0.031 | −0.225 | 0.288 | 63 | 0.24 | 0.808 | 12% |
| LDCR | −0.117 | −0.374 | 0.139 | 63 | −0.91 | 0.365 | 14% |
| Hits | −0.344 | −0.595 | −0.092 | 63 | −2.73 | 0.008 | |
|
| |||||||
| Inaccurate Memory | −0.063 | −0.338 | 0.213 | 63 | −0.45 | 0.652 | -- |
| Intrusions | −0.023 | −0.325 | 0.278 | 63 | −0.16 | 0.877 | -- |
| False Positives | −0.102 | −0.423 | 0.220 | 63 | −0.63 | 0.531 | -- |
|
| |||||||
| Comparison of Contrast Scores between Patients and Normative Sample | N | df | χ2 | p | |||
|
| |||||||
| SDFR-Trial 5 | 489 | 1 | 0.031 | 0.859 | |||
| LDFR-Trial 5 | 489 | 1 | 0.090 | 0.760 | |||
| LDFR-SDFR | 489 | 1 | 0.001 | 0.96 | |||
Note. SDFR: Short Delay Free Recall; SDCR: Short Delay Cued Recall; LDFR: Long Delay Free Recall; LDCR: Long Delay Cued Recall; d′: Recognition Sensitivity
Table 3.
Results of one-sample t-tests on neuropsychological measures
| Mean | 95% Confidence Interval
|
df | t | p | ≤-1.5z (%) | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
|
| |||||||
| Boston Naming | .18889 | −0.098 | 0.476 | 53 | 1.32 | .192 | 9% |
| Animal Naming | −.17797 | −0.452 | 0.096 | 58 | −1.30 | .199 | 12% |
| FAS COWAT | −.10678 | −0.359 | 0.145 | 58 | −0.85 | .399 | 5% |
| Trails A | −.28448 | −0.567 | −0.002 | 57 | −2.02 | .048 | 12% |
| Trails B | .00877 | −0.257 | 0.275 | 56 | 0.07 | .948 | 11% |
| WAIS-IV Coding | .07241 | −0.182 | 0.327 | 57 | 0.57 | .571 | 7% |
| WAIS-IV Symbol Search | .02881 | −0.259 | 0.316 | 58 | 0.20 | .842 | 8% |
| WAIS-IV Digit Span | .12542 | −0.121 | 0.372 | 58 | 1.02 | .313 | 5% |
| WAIS-IV Arithmetic | −.03519 | −0.282 | 0.212 | 53 | −0.29 | .776 | 7% |
| Rey-O CFT Immediate | −.01905 | −0.439 | 0.400 | 41 | −0.09 | .927 | 14% |
| Rey-O CFT Delayed | −.13571 | −0.517 | 0.246 | 41 | −0.72 | .477 | 19% |
| WMS-IV Logical Memory I | −.22586 | −0.543 | 0.091 | 57 | −1.43 | .159 | 14% |
| WMS-IV Logical Memory II | −.01207 | −0.290 | 0.266 | 57 | −0.09 | .931 | 9% |
As a group, participants were well educated and had above average cognitive functioning (as indicated by premorbid estimates). They tended to be Caucasian (75%) and ranged in age from 21 to 79. The median time between finishing chemotherapy and the neuropsychological evaluation was 18 months (Range 0–163 months). While two patients had Stage IV disease at the time of the neuropsychological evaluation, metastases to liver, lymph nodes and bone were recorded but none to the brain. Memory complaints were pervasive (88%) as indicated by the primary complaint of the patient; other complaints included distractibility, language functioning, and psychomotor slowing. Additional information is shown in Table 1. Psychotropic medications were being taken by 56% of the sample and included 54% taking an antidepressant and anxiolytic medications, 33% taking solely an antidepressant, 21% taking solely an anxiolytic, and 2% taking a stimulant. However, the mean BDI Fast Screen for Medical Patients score indicated only mild symptoms of depression.
CVLT-II Four-Factor Analysis
Analysis of performance on the four factors of Attention Span, Learning Efficiency, Delayed Memory and Inaccurate Memory revealed significantly lower performance on the Attention Span factor in the Clinical group compared to an age and gender matched normative cohort (t(63) = −2.68; p = .009) (Table 2; Figure 1a), as predicted. Analysis of remaining factor scores on Learning Efficiency, Delayed Memory and Inaccurate Memory failed to reveal any significant difference in factor level performance ([t(63) = 1.20; p = .233]; [t(63) = −0.73; p = .47]; and [t(63) = −0.45; p = .65]) respectively.
Figure 1.
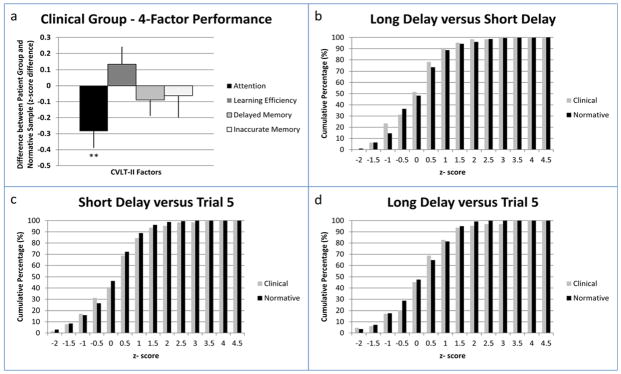
(a) Mean performance for Attention, Learning Efficiency, Delayed Memory, and Inaccurate Memory Factors. (b–d) Cumulative percentage at each z-score for Clinical and Normative groups. Note. ** = p ≤ .01.
Individual Item Analysis
Analysis of individual constituent scores of each factor revealed significant differences between the Clinical and normative groups (Table 2). Within the Attention factor, Trial 1 performance (t(63) = −2.11; p = .039) and Middle Region Recall (t(63) = −2.46; p = .017) were both significantly lower in the clinically referred group. Within the Learning Efficiency factor, Across Trial Consistency (t(63) = 2.93; p = .005) was significantly higher in the Clinical group. Within the Delayed Memory factor, Recognition Hits (t(63) = −2.73; p = .008) was significantly lower in the clinically referred group. Within the Inaccurate Memory factor, no difference was found in either False Positives or Intrusions.
CVLT-II Contrast Score Analysis
Normatively adjusted contrast z-scores between Trial 5, SDFR, and LDFR were calculated and compared to base rates of contrast scores in the normative sample (Table 2; Figure 1b–d). No significant differences were exhibited between SDFR and Trial 5, LDFR and Trial 5, or LDFR and SDFR at z = −1.5 or greater between normative and Clinical groups ([χ2(1, N = 489) = 0.031, p = .859]; [χ2(1, N = 489) = 0.09, p = .76]; [χ2(1, N = 489) = 0.001, p = .96] respectively).
Neuropsychological Battery Analysis
For analysis of performance on individual neuropsychological measures, only performance on the Trail Making Test A (t(57) = −2.02; p = .048) was significantly lower in the Clinical group (Table 3). Of note, given the prediction of preserved recall in this sample, analysis of the Rey-Osterrieth Complex Figure Test Immediate Recall (t(41) = −0.09; p = .927), Delayed Recall (t(41) = −0.72; p = .477), WMS-IV Logical Memory I (t(57) = −1.43; p = .159) and Logical Memory II subtests (t(57) = −0.09); p = .931), did not reveal any significant difference from normative cohorts.
Conclusions
The purpose of this retrospective clinical study was to examine the CVLT-II performance profiles of clinically referred breast cancer survivors who underwent neuropsychological assessment prompted by subjective cognitive complaints. Based on previous clinical observations that attentional issues might be a primary area of dysfunction in this cohort, as well as inconclusive findings for memory dysfunction in previous research samples, we specifically sought to examine the relative influence of attention and memory function on CVLT-II performance. We predicted that clinically referred patients would exhibit specific relative weakness in the Attention Factor of the CVLT-II and Trial 1 performance with preserved function in the Delayed Memory Factor and intact retention of information over time. These predictions were confirmed in our analysis, with patients exhibiting significantly decreased performance on the Attention Factor, Trial 1, and Middle Region Recall with preserved delayed memory performance and no evidence of information decay over time exceeding that of the normative sample. These results indicate that, in clinically referred breast cancer survivors, the role of attention, specifically, may be important in reports of memory dysfunction.
In addition to these expected findings, analysis of individual items that load on factors outside of Attention found significantly higher across-trial consistency and significantly lower hits on the recognition trial in our Clinical group compared to the normative sample. Given that these items load on factors that were found not to be significant, interpretation of these individual items is qualified and clinical significance is unclear. Greater across trial consistency indicates that the Clinical group was more consistent in specific item recall over each of the five learning trials than in the normative sample. This may be related to the tendency of the Clinical group to recall significantly greater items from the beginning of the list. While not a focus of the primary analysis, secondary analysis of the primacy variable, i.e., the tendency to recall words from the beginning of the list, was significantly greater in our clinical group (t(63) = 2.839; p = .006), indicating a primacy effect. This would in turn increase the consistency of item recall across learning trials. True-positive identification, or hits, on the recognition trial was significantly below the normative sample. This indicates that while the Clinical group exhibited normal range free recall performance, they did not benefit to the same extent from additional prompts in recognition format. Significantly, recognition sensitivity (d′) was not significantly different from the normative sample. Taking these two findings together, this suggests that the Clinical group may have been more conservative in endorsing recognition list items.
The results of this analysis have implications for clinical assessment, hypothesis testing, study design, and interpretation of results in research focusing on cognitive outcomes of cancer treatment. First, despite subjective complaints of forgetfulness in our Clinical group, objective memory dysfunction was not exhibited on the CVLT-II Delay trials, Rey-Osterrieth Complex Figure Trials, or WMS-IV Logical Memory subtests. While a memory effect was absent, results from the learning trials of the CVLT-II indicate that attentional/learning processes were significantly affected. One explanation may be that patients misinterpret difficulties in recollection as due to forgetting rather than to suboptimal attention at the time of initial exposure. One clinical implication of this finding is that rehabilitation of cognitive issues might focus on attention dysfunction, either through the use of attentional strategies or pharmacologic treatment, in addition to rehabilitation that focuses on retention and recall aids. Attentional dysfunction is also important to underscore for affected patients since attention difficulties may be considered more tractable than primary memory issues in treatment. With regard to research design, absence of objective memory problems in our Clinical group is important for future research in this area in that it would argue for inclusion of measures focused on attention and the process of acquisition of new information in studies designed to clarify cognitive outcomes of cancer treatment. Several previous studies focus on single-trial recall measures or do not analyze multi-trial learning items when they are included in a research battery. Use of single-trial learning measures, such as the Rey-Osterrieth Complex Figure Test or Logical Memory subtests, does not allow for the separation of attentional and recall processes, and as a result may conflate the effects of learning and later recall. Similarly, even in cases in which a recall measure is used that contains multiple learning trials (CVLT-II; HVLT), aggregating these measures in a memory factor that includes only delayed recall performance precludes analysis of earlier learning processes. The International Cognition and Cancer Task Force has previously recommended use of the HVLT in studies investigating treatment related cognitive difficulties [28] and a similar analysis as applied here to the CVLT can be performed to investigate attentional contributions to memory dysfunction. Furthermore, results of this study support the use of an empirically derived factor approach to analysis of individual measures, as well as analysis of secondary variables contained in those measures. In analyzing our data as discrete factors, we were able to achieve greater sensitivity to potential effects in learning and memory. Finally, analysis of secondary variables, such as the Middle Region Recall score which in our analysis revealed a strong effect, may also improve sensitivity to subtle changes in cognitive performance.
In regard to performance on the broader neurocognitive battery, we note that performance on the Trail Making Test Part A was significantly lower than the normative cohort. Due to the number of comparisons available in our battery, this result is of unclear significance. To the extent that this finding is not spurious, this result would be consistent with results found in previous research samples, in which performance on speeded measures appear to be significantly affected [29]. The finding of psychomotor slowing, together with the primary attention finding exhibited on the CVLT-II, may suggest that memory complaints in this cohort are due to an interaction of psychomotor slowing and attentional dysfunction. Of note to our central question of whether actual memory dysfunction is exhibited in this clinical sample, in addition to preserved memory performance on the CVLT-II, preserved memory function was also found on the Rey-Osterrieth Immediate and Delayed Recall Trials as well as on the Logical Memory subtests of the WMS-IV. Interestingly, performance on primary measures of attention (Digit Span; Arithmetic) was not significantly affected, and this could be understood as arguing against an attentional deficit in learning and memory performance. However, prior research on the relation between Digit Span and Arithmetic subtests with CVLT-II Trial 1 performance fails to find a significant relationship between performance on these tasks [27], and this was the case in our clinical sample as well.* Previous research in clinical and healthy groups, including Alzheimer’s samples, has similarly highlighted differences specifically between digit repetition and immediate word recall performance [30–32], although reasons for this disagreement remain unclear. Cherry et al. [32] speculated that immediate word recall tasks present more items than can be recalled in a single exposure and that “beyond-span” items compete for limited attention. Further they point out that digit repetition may be easier due to the fact that digits are shorter (one to two syllables), are hierarchical, and come from a single closed class of stimuli. Thus, clinically referred patients in our study may be able to compensate for relatively easier Digit Span trials but fail to compensate once stimulus demands increase in Trial 1, with the result that the attentional network is overloaded.
Strengths of this study include the focus on clinically referred breast cancer patients who self-report cognitive difficulties since completion of treatment, as well as factor and item specific analysis that allows for the investigation of learning and memory processes individually. There are limitations as well. First, we relied upon published normative data to generate cognitive profiles of our Clinical group. While the CVLT-II is adjusted for age and gender, no adjustment for education is applied in our generally well-educated sample, and this may have inflated performance estimates. Arguing against this, performance is not generally increased above normative values, and instead exhibits specific, significantly lower scores on attentional items (Attention Factor; Trial 1; Middle Region Recall) with preserved recall performance (SDFR; LDFR). We note also that in memory measures that do include correction for education, Logical Memory I and Logical Memory II, Clinical group performance is again not significantly different from the normative sample. To what extent clinical sample performance is indicative of performance in research cohorts, or of breast cancer survivors more generally, is unclear, and it is important to note that patients included in this study were self-selected as a result of cognitive concerns potentially biasing our results in the direction of finding increased cognitive dysfunction. Arguing against this, tested levels of performance in our Clinical group are similar to subtle findings in attention and processing speed in previous studies. The role of other factors that may be contributory also needs to be addressed in relation to the findings we report here. In addition to prior cancer treatment, which included chemotherapy exposure, the majority of our patients presented on active hormone treatments. There is some evidence that Tamoxifen may have an effect on cognitive function [33, 34], while aromatase inhibitors, such as Anastrozole and Letrozole, are less clear in their effects [35, for review]. Menopausal phase may also have some effect on cognition, although most patients in our sample were premenopausal and the literature is mixed as to the influence of menopausal phase on cognition [36–38]. Given stress and concern following diagnosis and treatment, many patients are treated for mood symptoms. In our sample, pharmacologic treatment was mixed, although scores on concurrently administered measures of mood were not elevated in our sample, and clinical judgment was exercised such that evaluations were not administered to patients who presented with significant depression or anxiety. The effects of age and the potential for neurological comorbidities are also possible, although only nine patients were older than 65 in our sample, arguing against the probability that a subset of our patients were exhibiting mild cognitive impairment or an incipient dementing illness associated with advanced age. Finally, we note that while primary analysis relied only upon a limited number of statistical comparisons, secondary analyses that contextualized these results added to the number of statistical comparisons and, therefore, potentially to a risk for Type I errors.
The results of this study address a significant gap in the research literature on cognitive outcomes of cancer treatment, specifically, the performance profiles of clinically referred cancer survivors following treatment and the role of attention in memory complaints. Our results are compelling as much for what abilities were preserved following treatment as for what abilities were affected. The pattern is most suggestive of subtle attentional dysfunction exhibited in the attention/learning variables of the CVLT-II, with preserved memory at longer delays on multiple measures of memory. In regard to study design, analysis, and interpretation of future research, results of this study suggest that inclusion of serial learning memory measures, a factor approach to analysis, and a focus on attentional function will be important in clarifying cognitive dysfunction. In regard to clinical care, results of this study are suggestive of what cognitive domains might be targeted for pharmacologic treatment, for direct remediation, and for formulation of strategies, i.e., attentional and learning interventions, that might be most useful to cancer survivors more generally.
Acknowledgments
Funding
No funding was received for this research.
This research was unfunded, and the authors have no conflicts of interest to report. The information in this manuscript is an original contribution to the literature and has not been previously published, either in print or electronically. The authors thank the data licensing staff at Pearson for permission to use previously unpublished contrast data from the original standardization sample. The authors gratefully acknowledge the assistance of Raffi Leicht.
Footnotes
([Digit Span: r(59) = .143, p = .280]; [Arithmetic: r(54) = .045, p = .745])
Contributor Information
James C. Root, Assistant Attending Neuropsychologist, Neurocognitive Research Laboratory, Department of Psychiatry and Behavioral Sciences, Memorial Sloan Kettering Cancer Center, Assistant Professor, Weill Cornell Medical College.
Elizabeth Ryan, Assistant Attending Neuropsychologist, Neurocognitive Research Laboratory, Department of Psychiatry and Behavioral Sciences, Memorial Sloan Kettering Cancer Center, Assistant Professor, Weill Cornell Medical College.
Gregory Barnett, Brown University School of Medicine.
Charissa Andreotti, Postdoctoral Fellow, Neurocognitive Research Laboratory, Department of Psychiatry and Behavioral Sciences, Memorial Sloan Kettering Cancer Center.
Kemi Bolutayo, Department of Psychiatry and Behavioral Sciences, Memorial Sloan Kettering Cancer Center.
Tim Ahles, Attending Psychologist, Neurocognitive Research Laboratory, Department of Psychiatry and Behavioral Sciences, Memorial Sloan Kettering Cancer Center, Professor, Weill Cornell Medical College.
References Cited
- 1.Howlader N, Noone A, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) 2012 Available from: http://seer.cancer.gov/csr/1975_2009_pops09/
- 2.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30(30):3675–86. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seigers R, Fardell JE. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci Biobehav Rev. 2011;35(3):729–41. doi: 10.1016/j.neubiorev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Hurria A, Somlo G, Ahles T. Renaming “chemobrain”. Cancer Investigation. 2007;25(6):373–7. doi: 10.1080/07357900701506672. 782023378 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Schagen SB, van Dam FS, et al. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85(3):640–50. doi: 10.1002/(SICI)1097-0142(19990201)85:3<640::AID-CNCR14>3.0.CO;2-G. [pii] [DOI] [PubMed] [Google Scholar]
- 6.Shilling VJV. Self-reported cognitive problems in women receiving adjuvant therapy for breast cancer. Eur J Oncol Nurs. 2007;11(1):6–15. doi: 10.1016/j.ejon.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Anderson-Hanley C, Sherman ML, et al. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. J Int Neuropsychol Soc. 2003;9(7):967–82. doi: 10.1017/S1355617703970019. S1355617703970019 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Falleti MG, Sanfilippo A, et al. The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: a meta-analysis of the current literature. Brain Cogn. 2005;59(1):60–70. doi: 10.1016/j.bandc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Jansen CE, Miaskowski C, et al. A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer. 2005;104(10):2222–33. doi: 10.1002/cncr.21469. [DOI] [PubMed] [Google Scholar]
- 10.Stewart A, Bielajew C, et al. A meta-analysis of the neuropsychological effects of adjuvant chemotherapy treatment in women treated for breast cancer. Clin Neuropsychol. 2006;20(1):76–89. doi: 10.1080/138540491005875. [DOI] [PubMed] [Google Scholar]
- 11.Jim HS, Phillips KM, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol. 2012;30(29):3578–87. doi: 10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delis DC, Kramer JH, et al. Adult Version Manual. 2. The Psychological Corporation; San Antonio, TX: 2000. California Verbal Learning Test. [Google Scholar]
- 13.Donders J. A confirmatory factor analysis of the California Verbal Learning Test--Second Edition (CVLT-II) in the standardization sample. Assessment. 2008;15(2):123–31. doi: 10.1177/1073191107310926. [DOI] [PubMed] [Google Scholar]
- 14.DeJong J, Donders J. A confirmatory factor analysis of the California Verbal Learning Test--Second Edition (CVLT-II) in a traumatic brain injury sample. Assessment. 2009;16(4):328–36. doi: 10.1177/1073191109336989. [DOI] [PubMed] [Google Scholar]
- 15.Pearson. Advanced Clinical Solutions for the WAIS-IV and the WMS-IV (ACS) Pearson; San Antonio: 2009. [Google Scholar]
- 16.Friend KB, Grattan L. Use of the North American Adult Reading Test to estimate premorbid intellectual function in patients with multiple sclerosis. J Clin Exp Neuropsychol. 1998;20(6):846–51. doi: 10.1076/jcen.20.6.846.1110. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson GS, Robertson GJ. WRAT4: Wide Range Achievement Test. Psychological Assessment Resources; 2006. [Google Scholar]
- 18.Beck AT, Steer RA, Brown GK. BDI - FastScreen for Medical Patients. Pearson; 2000. [Google Scholar]
- 19.Kaplan E, Goodglass H, Weintrab S. The Boston naming test. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- 20.Spreen O, Strauss E. A compendium of neuropsychological tests. Oxford University Press; New York: 1991. [Google Scholar]
- 21.Reitan RM. Validity of the Trail-Making Test. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- 22.Wechsler D. Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV) Pearson; San Antonio: 2008. [Google Scholar]
- 23.Meyers J, Meyers K. Rey Complex Figure Test and Recognition Trial. PAR; Odessa: 1995. [Google Scholar]
- 24.PsychCorp. WMS-IV Technical Manual. Pearson; San Antonio: 2009. [Google Scholar]
- 25.Crawford JR, Sutherland D, Garthwaite PH. On the reliability and standard errors of measurement of contrast measures from the D-KEFS. J Int Neuropsychol Soc. 2008;14(6):1069–73. doi: 10.1017/S1355617708081228. [DOI] [PubMed] [Google Scholar]
- 26.Heaton RK, Miller SW, et al. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults (HRB) PAR; Odessa: 2004. [Google Scholar]
- 27.PsychCorp. WAIS-IV Technical Manual. Pearson: San Antonio; 2008. [Google Scholar]
- 28.Wefel JS, Vardy J, et al. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–8. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 29.Ahles TA, Saykin AJ, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28 (29):4434–40. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teng EL, Wimer C, et al. Alzheimer’s dementia: performance on parallel forms of the dementia assessment battery. J Clin Exp Neuropsychol. 1989;11(6):899–912. doi: 10.1080/01688638908400943. [DOI] [PubMed] [Google Scholar]
- 31.Murdock BB., Jr Recent developments in short-term memory. Br J Psychol. 1967;58(3):421–33. doi: 10.1111/j.2044-8295.1967.tb01099.x. [DOI] [PubMed] [Google Scholar]
- 32.Cherry BJ, Buckwalter JG, Henderson VW. Better preservation of memory span relative to supraspan immediate recall in Alzheimer’s disease. Neuropsychologia. 2002;40(7):846–52. doi: 10.1016/s0028-3932(01)00173-7. [DOI] [PubMed] [Google Scholar]
- 33.Schilder CM, Seynaeve C, et al. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol. 2010;28(8):1294–300. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- 34.Schilder CM, Eggens PC, et al. Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC-chemotherapy: cross-sectional findings from the neuropsychological TEAM-side study. Acta Oncol. 2009;48(1):76–85. doi: 10.1080/02841860802314738. 902183905 [pii] [DOI] [PubMed] [Google Scholar]
- 35.Phillips KA, Ribi K, Fisher R. Do aromatase inhibitors have adverse effects on cognitive function? Breast Cancer Res. 2011;13(1):203. doi: 10.1186/bcr2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuh JL, Wang SJ, et al. A longitudinal study of cognition change during early menopausal transition in a rural community. Maturitas. 2006;53(4):447–53. doi: 10.1016/j.maturitas.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Weber MT, Rubin LH, Maki PM. Cognition in perimenopause: the effect of transition stage. Menopause. 2013 doi: 10.1097/gme.0b013e31827655e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer PM, Powell LH, et al. A population-based longitudinal study of cognitive functioning in the menopausal transition. Neurology. 2003;61(6):801–6. doi: 10.1212/01.wnl.0000079051.91602.e2. [DOI] [PubMed] [Google Scholar]


