Abstract
Background and Aims
Portal hypertension is characterized by reduced hepatic eNOS activity. Asymmetric-dimethylarginine (ADMA), an eNOS inhibitor, is elevated in cirrhosis and correlates with severity of portal hypertension. Dimethylargininedimethylaminohydrolase-1 (DDAH-1) is the key enzyme metabolizing hepatic ADMA. This study characterized DDAH-1 in cirrhosis, and explored hepatic DDAH-1 reconstitution through FXR agonism and DDAH-1 gene therapy.
Methods
DDAH-1 Immunohistochemistry was conducted on human cirrhosis and healthy liver tissue. Subsequently, sham-operated or bile-duct-ligated (BDL) cirrhosis rats were treated with FXR agonist Obeticholic acid (OA, 5mg/kg) or vehicle for 5 days. Further animals underwent hydrodynamic injection with DDAH-1-expressing plasmid or saline control. Groups: Sham+saline, BDL+saline, BDL+DDAH-1-plasmid. Portal pressure (PP) measurements were performed. Plasma ALT was measured by Cobas-Integra; DDAH-1 expression by qPCR and Western blot; eNOS activity by radiometric assay.
Results
Immunohistochemistry and Western-blotting confirmed hepatic DDAH-1 was restricted to hepatocytes, and expression decreased significantly in cirrhosis. In BDL rats, reduced DDAH-1 expression was associated with elevated hepatic ADMA, reduced eNOS activity and high PP. OA treatment significantly increased DDAH-1 expression, reduced hepatic tissue ADMA, and increased liver NO generation. PP was significantly reduced in BDL+OA vs. BDL+vehicle (8±1 vs. 13.5±0.6 mmHg; p<0.01) with no change in MAP. Similarly, DDAH-1 hydrodynamic injection significantly increased hepatic DDAH-1 gene and protein expression, and significantly reduced PP in BDL+DDAH-1 vs. BDL+ saline (p<0.01).
Conclusion
This study demonstrates DDAH-1 is a specific molecular target for portal pressure reduction, through actions on ADMA-mediated regulation of eNOS activity. Our data support translational studies targeting DDAH-1 in cirrhosis and portal hypertension.
Keywords: Portal hypertension, ADMA, DDAH-1, Nitric oxide
INTRODUCTION
Sinusoidal portal hypertension, as a consequence of chronic liver disease, heralds the onset of the most lethal complications of cirrhosis. The pathobiology of portal hypertension is characterised by elevated intrahepatic resistance to flow and increased splanchnic blood flow. Intrahepatic resistance occurs not only because of hepatic fibrosis, but also due to sinusoidal endothelial dysfunction and increased intrahepatic vascular tone[1]. Nitric oxide (NO) is an essential regulator of intrahepatic vascular tone. In cirrhosis, hepatic NO levels are significantly reduced, with associated elevated sinusoidal vascular resistance[2].
Asymmetric dimethylarginine (ADMA) is a competitive endogenous inhibitor of endothelial nitric oxide synthase (eNOS) associated with eNOS dysfunction in decompensated cirrhosis[3] and acute liver failure[4]. Indeed, we recently demonstrated elevated plasma and hepatic ADMA levels in patients with cirrhosis and superimposed alcoholic hepatitis, associated with significantly reduced hepatic eNOS activity, increased portal pressure and increased mortality[5]. ADMA is formed ubiquitously within cells through proteolysis, and acts in a paracrine fashion to inhibit the action of all nitric oxide synthases[6]. Hepatic ADMA levels are predominantly controlled through elimination by the enzyme dimethylarginine dimethylaminohydrolase-1 (DDAH-1), hence hepatic DDAH-1 dysfunction is thought to be a key pathogenic mechanism for ADMA accumulation[7].
The Farnesoid X receptor (FXR) is part of a family of nuclear hormone receptors that have an important role in bile, lipid and glucose homeostasis[8]. In addition, FXR modulates the transcription of many inflammatory and cell-cycle control genes and is particularly abundant in the liver, kidney and intestine. Recently, DDAH-1 was identified as an FXR target gene from studies in diabetic Zucker rats, where a synthetic FXR agonist was shown to significantly increase hepatic DDAH-1 gene expression[9].
The aim of this study was to determine if DDAH-1 expression was altered in cirrhotic rodents, and if so, to investigate the potential therapeutic benefit of augmenting hepatic DDAH-1 with an FXR agonist on hepatic ADMA levels, eNOS activity and portal pressure. Subsequently, to confirm that the portal pressure lowering effects of FXR agonism were mediated through a DDAH-1 mechanism, a gene therapy approach was used to similarly augment hepatic DDAH-1 levels and confirm a therapeutic lowering of portal pressure.
MATERIALS AND METHODS
Human tissue samples
Human liver samples were obtained with local ethical committee approval and all patients gave written informed consent. Transjugular liver biopsy specimens were obtained from a series of patients with alcoholic cirrhosis and portal hypertension, and fixed in formalin for subsequent histological evaluation. Further control liver samples from patients without liver disease were also obtained for histology.
Histological evaluation
Human liver sections were embedded, cut and stained with hematoxylin and eosin (H&E) using standard techniques, and immuno-staining with anti-DDAH-1 antibodies (Abcam, UK) was performed as previously described[10].
Animals and Bile Duct Ligation Model
All animal experiments were conducted in accordance with the UK Animals in Scientific Procedures Act 1986. Male Sprague-Dawley rats (Charles-Rivers), weighing 220±25g, were housed in a temperature and humidity controlled environment. Animals had access to food and water ad libitum, with a light/dark cycle of 12 hours. The bile duct ligation (BDL) model of cirrhosis was used in this study, since this replicates advanced cirrhosis, demonstrating portal hypertension and extra-hepatic organ failure[11]. BDL and sham surgery was performed under induction anaesthesia with 5% isoflurane and maintenance with 2% isoflurane, as previously described[11]. To confirm findings in the BDL model, hepatic DDAH-1 was also characterised in the carbon tetrachloride (CCl4) model previously described in Sprague-Dawley rats. [12].
Study Design
-
(i)
Three weeks after BDL and sham surgery, rats were orally administered the FXR agonist Obeticholic Acid (OA) (Intercept Pharmaceuticals, USA) at 5mg/kg in vehicle (corn oil) for 5 days, or vehicle alone. Three groups were studied: (a) Sham+vehicle, b) BDL+vehicle, c) BDL+OA. At the end of the study, at week 4, all rats underwent haemodynamic measurements and were then sacrificed.
-
(ii)
A further group of Sprague Dawley rats underwent hydrodynamic injection of plasmid DNA, through modification of the method described by Maruyama et al, previously showing efficient temporary hepatic transgene expression in rodents [13]. Rapid, high-volume, high-pressure injection is thought to lead to transgene expression by causing retrograde flow in the inferior vena cava and hepatic vein, leading to transient permeation of cell membranes and subsequent uptake and expression of plasmid DNA. As fibrosis in the BDL model typically progresses from the peri-portal region, hydrodynamic retrograde injection of plasmid may in theory lead to permeation of peri-venous hepatocytes, where fibrosis is typically less advanced, leading to passive uptake of plasmid DNA (which is also much smaller than the diameter of viral vector particles).
Endotoxin-free plasmid DNA (1mg) was dissolved in 15mls of 0.9% sterile saline solution and warmed to 37°C. Following cannulation of the right jugular vein, hydrodynamic injection of the saline-DNA solution, or of saline alone, was performed over a period of 15 seconds. The DDAH-1 expressing plasmid was pCMVSport6_HsDDAH-1 (Source Bioscience, UK). Three groups were studied: (a) sham+saline injection, (b) BDL+saline injection, (c) BDL+DDAH-1 expressing plasmid. Similarly, haemodynamic measurements were performed up to 5 days following plasmid injection, at 4 weeks post BDL surgery.
Hemodynamic measurements
Portal pressure and systemic hemodynamics were measured 4 weeks following surgery. Briefly, measurements were made under anaesthesia. Portal pressure (PP) was assessed by direct cannulation of the main portal vein, and mean arterial pressure (MAP) by cannulation of the right carotid artery. All measurements were transduced to a Powerlab (4SP) linked to a computer with Chart v5.0.1 software. The mean of three readings taken one minute apart was recorded.
Plasma biochemistry
Plasma samples were analysed for ALT, albumin and bilirubin (Cobas Integra 400, Roche-diagnostics, Burgess Hill, West Sussex, UK).
Rat liver cell isolation
Hepatocytes and non-parenchymal cells were isolated from Sprague Dawley rats by collagenase perfusion at Yale Cell Isolation Core Facility (New Haven, Connecticut, U.S.A.) as described[14]. These cells were lysed in a lysis buffer immediately after the isolation and stored until used for Western blot analysis.
Western Blot
Proteins were isolated from snap frozen liver tissue using standard techniques. Equal amounts of protein extract were denatured and separated on 4-12% NuPAGE Bis-Tris Gels and transferred on to PVDF membranes (Lifetech, UK), which were then probed with anti-DDAH-1 (Abcam), anti-DDAH-2 (Abcam) and anti-tubulin (Millipore) monoclonal antibodies by standard techniques. The bands were visualized using an enhanced ECL detection kit (Amersham, UK) and quantified by densitometry.
Quantitative PCR
RNA was extracted from whole liver tissue following homogenization, using Trizol (Invitrogen), and was reverse transcribed to cDNA using Superscript II reverse transcriptase (Invitrogen) according to manufacturers instructions. Quantitative PCR (qPCR) was performed using Taqman (Life Technologies) FAM labeled probes to DDAH-1 (transcripts NM_012137.3 and NM_001134445.1) and to the housekeeping control gene peptidylprolyl isomerase A (PPIA) (transcript NM_017101.1). Quantitative PCR was performed using the Taqman Universal PCR Mastermix (Life Technologies) according to manufacturer's instructions. Samples were processed in triplicate, and analysed by the 2–[delta][delta]Ct method.
Tissue NOS activity
A previously determined method of 14C L-arginine conversion to 14C L-citrulline was used to measure eNOS activity, with slight modification[15]. (See supplemental methods).
Statistics
Data was analysed using GraphPad Prism v5.0a (GraphPad Software, Inc., San Diego, CA). Data are expressed as mean ± SEM. Two-tailed unpaired t-tests were used to define differences between means of normally distributed data of equal variance. For data that was not normally distributed, a Mann-Whitney test was used; p<0.05 was taken to be statistically significant.
RESULTS
DDAH-1 is Expressed in Hepatocytes and Levels are Decreased in Cirrhosis
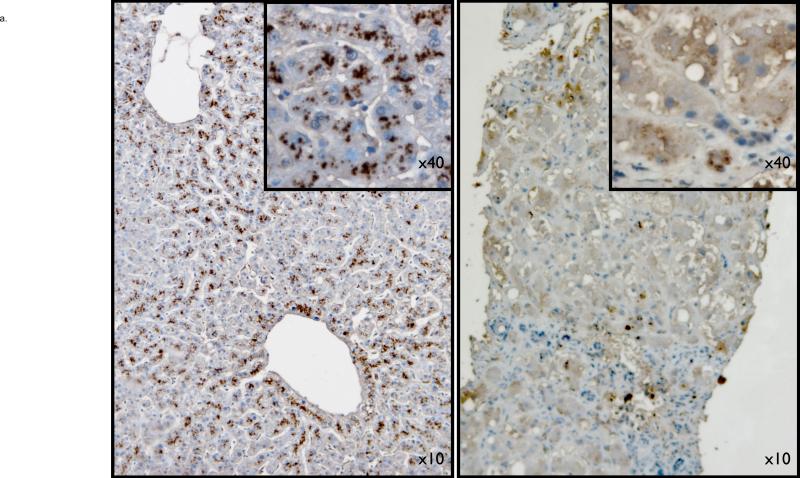
The precise cellular localisation of DDAH-1 has not been previously determined, in part due to insufficient specificity of commercially available antibodies for rodent DDAH-1. Therefore, to answer this question, we performed immunohistochemistry on human liver tissue from both healthy and cirrhotic liver specimens. Figure 1a from a healthy liver specimen demonstrates normal liver parenchyma, with hepatocyte DDAH-1 protein expression, predominantly in zone 3. In cirrhotic samples from patients with decompensated alcoholic liver disease (but no histological alcoholic hepatitis), a clear loss of parenchymal architecture is seen along with the presence of fibrous bands. DDAH-1 remains localised to hepatocytes, although expression levels appear decreased.
Figure 1.
1a: top panel- Hepatic DDAH-1 is located within the cytoplasm of hepatocytes of human liver; DDAH-1 levels are markedly decreased in cirrhotic liver (right side image) compared to a healthy liver (left side image).
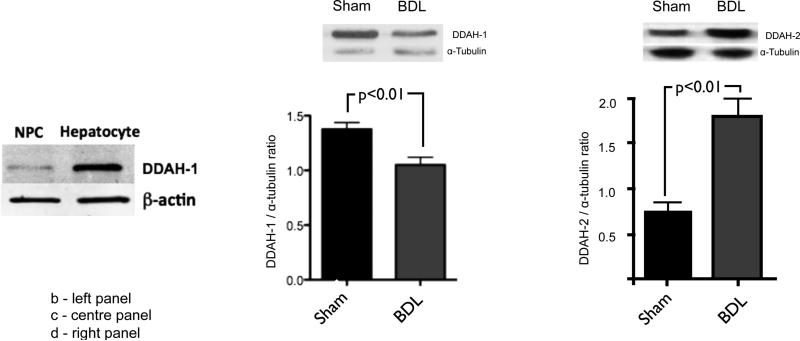
Similarly, in the BDL rat model of cirrhosis, DDAH-1 is predominantly located in the hepatocyte cell fraction from rat liver as compared with the non-hepatocyte cell (NPC) fraction (1b: left panel), and DDAH-1 protein levels are markedly reduced in BDL rat liver compared to sham (1c: centre panel). By contrast, hepatic DDAH-2 protein levels are increased in BDL rat liver compared to sham (1d: right panel).
To confirm these findings in a rodent model, we performed cell separation and western blot on liver tissue from healthy control rats. In agreement with the human immunohistochemistry data, figure 1b demonstrates that DDAH-1 is predominantly present in the parenchymal liver cell fraction. To confirm that levels of DDAH-1 are reduced in cirrhosis, we performed western blot on liver tissue from BDL and sham operated rats. Figure 1c confirms that hepatic DDAH-1 protein expression is decreased in the BDL model of cirrhosis. These findings were also replicated in the CCl4 rat model of cirrhosis (supplementary figure 1). By contrast hepatic DDAH-2 protein expression was elevated in BDL rats compared to sham (figure 1d). Supplementary figure 2 demonstrates advanced fibrosis in BDL rats in which the DDAH isoenzymes were assessed compared to the normal histological appearance in sham.
The FXR Agonist Obeticholic Acid Increases Hepatic DDAH-1 Expression and Reduces Portal Pressure in Cirrhotic Rats
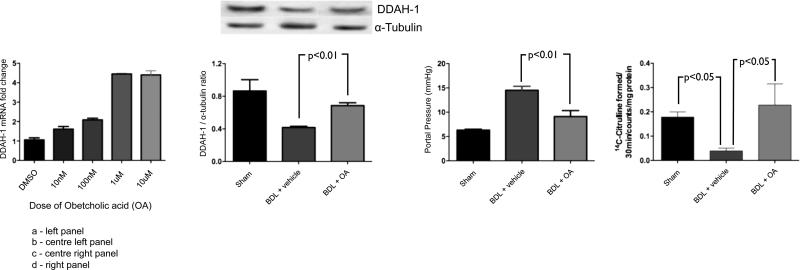
To determine if the FXR agonist, OA, also augments DDAH-1 expression in human hepatocytes, HepG2 cells were exposed to increasing concentrations of OA for 24 hours. As expected, OA increased DDAH-1 gene expression in a dose-dependent fashion up to a concentration of 1uM (figure 2a).
Figure 2.
The FXR agonist Obeticholic Acid (OA): induces DDAH-1 mRNA expression in HepG2 cells in vitro (2a: left panel), and when used in vivo it significantly increases hepatic DDAH-1 protein expression in BDL rats (2b: centre panel), and significantly reduces portal pressure in BDL rats (2c: centre right panel). eNOS activity was significantly reduced in BDL rats compared to sham animals. This reduction in eNOS activity is restored to sham levels after OA therapy (2d: right panel)
Subsequently, OA or vehicle was used to treat sham operated or BDL animals in the three groups: a) Sham+vehicle, b) BDL+vehicle, c) BDL+OA. BDL rats had significantly increased bilirubin and aminotransferase levels compared with sham rats, and these parameters reduced significantly following OA therapy (table 1). Similar to previous experiments, hepatic DDAH-1 expression was decreased in BDL cirrhotic rats as compared to sham rats (figure 2b), and, as previously described, BDL rats had significantly elevated portal pressure compared to sham (figure 2c) and significantly decreased MAP (table 1). Additionally, hepatic tissue ADMA levels were significantly increased in BDL rats compared to sham (496±24 nmol/mg liver tissue vs 71±12 nmol/mg tissue respectively, p=0.04) However, of interest, there was no significant difference found in plasma ADMA levels between BDL and sham (0.99±0.12 vs 0.74±0.08 μmol/L, p=0.6). Moreover, hepatic tissue eNOS activity was markedly reduced in BDL rats compared with sham animals (mean values 0.04±0.01 vs. 0.18±0.02 14C-Citrulline counts formed/30 minutes/mg protein respectively, p< 0.05). This change in activity occurred in the absence of any significant change in eNOS protein expression (data not shown) as has been previously described.
Five days of treatment with OA in BDL rats led to a significant increase in hepatic DDAH-1 expression compared with BDL+vehicle (figure 2b), and a significant reduction in portal pressure in the BDL+OA group compared with the BDL+vehicle group (figure 2c) with no significant change in mean arterial pressure (table 1). Five days of treatment with OA was also associated with a significant improvement in hepatic eNOS activity in the BDL+OA group as compared with the BDL+vehicle group (figure 2d). This was associated with a significant reduction in hepatic ADMA compared to BDL+vehicle (320±21 vs. 496±24 nmol/mg liver tissue, p=0.03). However, no change was noted in histological fibrosis over the 5 days of therapy (reticulin and sirius red stains- data not shown).
Gene Delivery with DDAH-1 Also Increases Hepatic DDAH-1 Expression and Reduces Portal Pressure in Cirrhotic Rats
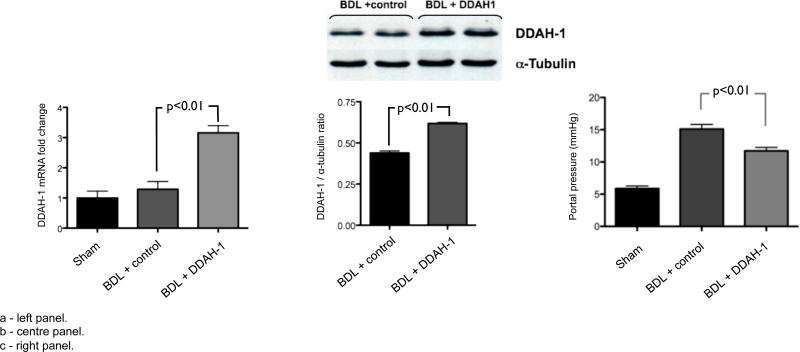
As previously, the sham+saline group had significantly lower portal pressure compared to the BDL+saline group (figure 3c). Hydrodynamic injection with DDAH-1 led to a significant increase in both hepatic gene expression (figure 3a) and protein expression of DDAH-1 (figure 3b). Following hydrodynamic injection with DDAH-1, there was no change in plasma bilirubin or aminotransferase levels compared to BDL rats given saline [bilirubin: 131.1(7.3) vs. 138.2(6.5), p=0.8; ALT: 90.3(3.3) vs. 91.6(3.8) IU/ml, p=0.4]. Hydrodynamic injection of DDAH-1 resulted in significantly decreased portal pressure in BDL cirrhotic rats compared to control saline injection (figure 3c). As noted previously with OA treatment, there was no significant change in mean arterial pressure with DDAH-1 gene therapy [90.3 (3.3) mmHg vs. 91.6 (3.8), BDL-DDAH-1 vs. BDL-Saline].
Figure 3.
Hydrodynamic gene delivery of DDAH-1 expressing plasmid into BDL rats leads to increased DDAH-1 mRNA (3a: left panel), and protein (3b: centre panel), and decreased portal pressure (3c: right panel) relative to control plasmid.
DISCUSSION
This study explored the augmentation of DDAH-1 through FXR signalling and DDAH-1 gene therapy to determine the effect on portal pressure in cirrhotic rats. The principal findings in this study are: (i) DDAH-1 expression in the liver is predominantly located in hepatocytes, and this expression is significantly decreased in man and rodent models of cirrhosis; (ii) Augmentation of DDAH-1 in cirrhotic rats with a highly selective FXR agonist results in a marked increase in DDAH-1 expression and is associated with increase in liver NO generation, reduction in hepatic ADMA and a significant reduction of portal pressure without a change in MAP; (iii) Increasing hepatic DDAH-1 expression through a gene therapy approach also results in a similar lowering of portal pressure, again with no effect on MAP nor liver biochemistry.
Mechanistic studies over several decades have highlighted the importance of NO in maintaining hepatic vascular tone, and demonstrated that reduced intrahepatic NO generation is important in the development of increased intrahepatic resistance and portal hypertension [16-18]. Paradoxically, eNOS expression increases within the liver in cirrhosis, despite the aforementioned decrease in hepatic NO generation[4, 19]. This suggests that mechanisms exist to inhibit NOS activity or/and scavenge the NO that is generated, and several inhibitors of NOS, including Caveolin-1 [20], ADMA [5] and NOSTRIN [21], have been identified as playing a role in portal hypertension.
ADMA is an important endogenous inhibitor of NOS and has been identified as the main determinant of endothelial dysfunction in conditions such as reno-vascular disease and pulmonary hypertension[22]. Several studies have shown that ADMA levels are also markedly increased in the plasma of patients with liver disease and correlate with severity of portal hypertension[5], onset of organ dysfunction and outcome[23]. It follows that strategies to lower hepatic ADMA levels may lead to a reduction in portal pressure.
The primary route of elimination of hepatic ADMA is through hydrolysis by the enzyme DDAH-1. Although two isoforms of the DDAH enzyme exist, elegant work by Pope et al confirmed that DDAH-1 is the major isoform involved in ADMA metabolism (the liver being a major site of its expression) [24], though DDAH-2 (predominantly expressed in the heart) may also act to augment local NO bioavailability through alternative mechanisms[25]. A contemporaneous study to this paper by Verbeke et al also used the FXR agonist OA to demonstrate a therapeutic effect for portal hypertension in two rodent models of cirrhosis[26]. This group found that a high dose of OA in BDL rats led to an increase in hepatic DDAH-2 expression by Western blot in BDL rats, along with a decrease in portal pressure. This is at odds with several authors in the literature who demonstrate a clear effect of FXR signalling on DDAH-1 expression. Hu et al identified that the DDAH-1 gene has a FXR response element within the first intron, and that administration of the FXR agonist GW4064 to Zucker rats resulted in a 6-fold increase in DDAH-1 expression with a reduction in plasma ADMA levels[9]. A more recent study by Ghebremariam et al assessed the effect of the FXR agonist OA (deployed in the current study) in a high salt diet hypertensive rodent model, confirming a significant increase in hepatic DDAH-1 expression following OA[27]. By contrast, we have shown that DDAH-2 levels are increased in BDL rats compared to sham animals, which is in keeping with its purported role in promoting eNOS expression in model systems such as BDL rats. Moreover, there is no functional FXR responsive element within the DDAH-2 gene and no precedent for FXR agonism leading to increased DDAH-2 expression, including in whole-genome expression analysis of FXR agonist treated animals. Therefore, for the purposes of this study and for potential translation to a therapy that would lower hepatic ADMA, we chose to characterise hepatic DDAH-1 expression in cirrhosis, and the effects of FXR agonism on the DDAH-1 pathway.
In this study, we show for the first time that liver DDAH-1 is predominantly expressed in hepatocytes, demonstrating that this is the site of cellular hepatic ADMA metabolism in health. Our data also shows that there is a significant reduction in hepatic DDAH-1 protein expression in two different models of cirrhosis, the BDL and CCl4 treated rats, in which portal hypertension is well described. A more detailed characterization in the BDL rat showed that reduced DDAH-1 expression was associated with increased hepatic ADMA, reduced hepatic eNOS activity despite increased eNOS protein expression and elevated portal pressure. Augmentation of DDAH-1 using the selective FXR agonist, OA, resulted in a significant increase in hepatic DDAH-1, reduced liver ADMA and increased hepatic eNOS activity compared to disease control. This in turn was associated with a significant lowering of portal pressure. It is likely, therefore, that increasing hepatocyte ADMA metabolic capacity by augmenting DDAH-1 reduces the availability of free liver ADMA, hence decreasing inhibition of local eNOS-mediated NO generation in neighbouring sinusoidal endothelial cells in a paracrine fashion. This assertion is supported by previous studies suggesting that endothelial cells have the ability to markedly increase their uptake of ADMA in pathophysiological states, such as ischaemic myocardium, further demonstrating that plasma ADMA concentrations considerably underestimate the degree of ADMA generation within organ parenchyma[28].
The FXR receptor, as part of a nuclear transcription factor super-family, has major roles controlling the transcription of genes regulating bile acid, glucose and triglyceride metabolism in the liver[29, 30]. Furthermore, increasing evidence suggests that FXR activation also has anti-inflammatory activity, both in the gut and the liver[31].
In the current study, we show that application of OA has a dose-dependent effect on increasing DDAH-1 transcription in HepG2 cells, and following in vivo application in BDL rats for only 5 days of therapy, results in a marked increase in DDAH-1 protein expression. Treated animals also show a significant decrease in hepatic ADMA concentrations which is associated with reduced portal pressure but no significant change in systemic ADMA or mean arterial pressure. This is an important observation suggesting that modulation of hepatocyte DDAH-1 can regulate local liver ADMA metabolism and thereby hepatic eNOS activity, without effects on systemic NO generation or blood pressure, which would be consistent with the observation of Ghebremariam and colleagues in their hypertensive model discussed above[27].
Having shown that DDAH-1 augmentation through FXR signalling is associated with a reduction in portal pressure and decreased hepatic ADMA levels, a specific DDAH-1 gene therapy approach was used to confirm the causal link between DDAH-1 expression and improvement in portal hypertension. This approach was adopted because, despite extensive efforts through compound screening and drug discovery, no specific agonist for DDAH-1 exists to date. Our data shows a clear correlation between increase in DDAH-1 gene and protein expression following hydrodynamic gene therapy and a reduction in portal pressure in BDL animals. This supports the assertion that DDAH-1 is key in the local regulation of hepatic ADMA and eNOS activity in cirrhosis, and that maintaining hepatic DDAH-1 through liver specific targeting provides a novel approach to therapy in portal hypertension. Although the method of hydrodynamic delivery is clearly not translatable, it provides strong evidence to support a rationale for gene therapy approaches to augment DDAH-1 in advanced cirrhosis.
Interestingly, our results demonstrate a decrease in serum bilirubin and ALT with OA treatment alongside a decrease in portal pressure, consistent with a modulatory effect on hepatic inflammation possibly through SOCS3 signalling as described by Xu et al[32]. By contrast, when a specific DDAH-1 gene therapy strategy was used, a similar (though less marked) reduction in portal pressure was noted without a decrease in bilirubin or ALT. This highlights that FXR has pluripotent actions on cirrhotic liver which may compliment actions on portal pressure reduction, although induction of DDAH-1 and thereby lowering of ADMA is likely to be a major component of this effect observed in the short duration of treatment used in this study.
FXR ligands reduce the activation of hepatic stellate cells thereby decreasing profibrogenic responses, as evidenced by reduced fibrosis in chronic treatment of BDL rats[33]. Though this might explain a reduction in portal pressure with sustained treatment, it is unlikely to have a significant impact on portal pressure reduction in this study given the short 5-day duration of therapy, as noted by the lack of change in liver architecture. Similarly, FXR ligand binding has been shown to enhance rat eNOS gene promoter activation. However, it is well established that hepatic eNOS is up-regulated in BDL rats and thus further augmentation of eNOS protein is unlikely to counteract the effects of reduced eNOS activity demonstrated in the literature and in this study [34].
In conclusion, this study provides strong evidence to suggest hepatic DDAH-1 is reduced in cirrhosis, thus offering an explanation for the observed decrease in hepatic eNOS activity underpinning portal hypertension through increased intrahepatic resistance. We demonstrate that augmenting hepatic DDAH-1 can abrogate portal hypertension without deleterious effects on mean arterial pressure. Further studies are required to delineate the mechanism for reduced hepatic DDAH-1 in cirrhosis, and to explore whether this is due to altered DDAH-1 post- transcriptional regulation, as suggested in other conditions with high local oxidative stress and inflammation[35].
Supplementary Material
Acknowledgement
Obeticholic acid was gifted by Intercept Pharmaceuticals, San Diego, CA through an MTA with UCL. There was no financial remuneration for either the delivery of the study nor for the investigators.
Grant Support: UCL-Business Proof of Concept award to RPM
National Institutes of Health DK082600 to YI
Abbreviations
- ADMA
Asymmetric Dimethylarginine
- DDAH-1
Dimethylarginine Dimethylaminohydrolase 1
- eNOS
Endothelial nitric oxide synthase
- FXR
Farnesoid X Receptor
- HVPG
Hepatic venous pressure gradient
- MAP
Mean arterial pressure
- NO
Nitric oxide
- OA
Obeticholic acid
Footnotes
Author Contributions:
RPM: concept, design and supervision of study; analysis and interpretation of data; drafting of the manuscript
GM acquisition of data; analysis and interpretation of data; drafting of the manuscript
VB acquisition of data; analysis and interpretation of data; drafting of the manuscript
FM acquisition of data and technical support
ND acquisition of data and technical support
VS acquisition of data, technical support and interpretation of data
YI acquisition of data; analysis and interpretation of data; drafting of the manuscript
RJ analysis and interpretation of data; drafting of the manuscript
Conflict of Interest Statement:
The authors have no conflict of interests to declare
REFERENCES
- 1.Roskams T, Baptista A, Bianchi L, Burt A, Callea F, Denk H, et al. Histopathology of portal hypertension: a practical guideline. Histopathology. 2003;42:2–13. doi: 10.1046/j.1365-2559.2003.01464.x. [DOI] [PubMed] [Google Scholar]
- 2.Iwakiri Y, Grisham M, Shah V. Vascular biology and pathobiology of the liver: Report of a single-topic symposium. Hepatology. 2008;47:1754–1763. doi: 10.1002/hep.22203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lluch P, Torondel B, Medina P, Segarra G, Del Olmo JA, Serra MA, et al. Plasma concentrations of nitric oxide and asymmetric dimethylarginine in human alcoholic cirrhosis. Journal of hepatology. 2004;41:55–59. doi: 10.1016/j.jhep.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Mookerjee RP, Dalton RN, Davies NA, Hodges SJ, Turner C, Williams R, et al. Inflammation is an important determinant of levels of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine (ADMA) in acute liver failure. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2007;13:400–405. doi: 10.1002/lt.21053. [DOI] [PubMed] [Google Scholar]
- 5.Mookerjee RP, Malaki M, Davies NA, Hodges SJ, Dalton RN, Turner C, et al. Increasing dimethylarginine levels are associated with adverse clinical outcome in severe alcoholic hepatitis. Hepatology. 2007;45:62–71. doi: 10.1002/hep.21491. [DOI] [PubMed] [Google Scholar]
- 6.Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O'Hara B, et al. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med. 2007;13:198–203. doi: 10.1038/nm1543. [DOI] [PubMed] [Google Scholar]
- 7.Jacobi J, Sydow K, von Degenfeld G, Zhang Y, Dayoub H, Wang B, et al. Overexpression of dimethylarginine dimethylaminohydrolase reduces tissue asymmetric dimethylarginine levels and enhances angiogenesis. Circulation. 2005;111:1431–1438. doi: 10.1161/01.CIR.0000158487.80483.09. [DOI] [PubMed] [Google Scholar]
- 8.Trauner M, Baghdasaryan A, Claudel T, Fickert P, Halilbasic E, Moustafa T, et al. Targeting nuclear bile acid receptors for liver disease. Dig Dis. 2011;29:98–102. doi: 10.1159/000324141. [DOI] [PubMed] [Google Scholar]
- 9.Hu T, Chouinard M, Cox AL, Sipes P, Marcelo M, Ficorilli J, et al. Farnesoid X receptor agonist reduces serum asymmetric dimethylarginine levels through hepatic dimethylarginine dimethylaminohydrolase-1 gene regulation. J Biol Chem. 2006;281:39831–39838. doi: 10.1074/jbc.M606779200. [DOI] [PubMed] [Google Scholar]
- 10.Pullamsetti S, Kiss L, Ghofrani HA, Voswinckel R, Haredza P, Klepetko W, et al. Increased levels and reduced catabolism of asymmetric and symmetric dimethylarginines in pulmonary hypertension. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:1175–1177. doi: 10.1096/fj.04-3223fje. [DOI] [PubMed] [Google Scholar]
- 11.Wright G, Davies NA, Shawcross DL, Hodges SJ, Zwingmann C, Brooks HF, et al. Endotoxemia produces coma and brain swelling in bile duct ligated rats. Hepatology. 2007;45:1517–1526. doi: 10.1002/hep.21599. [DOI] [PubMed] [Google Scholar]
- 12.Huang HC, Haq O, Utsumi T, Sethasine S, Abraldes JG, Groszmann RJ, et al. Intestinal and plasma VEGF levels in cirrhosis: the role of portal pressure. Journal of cellular and molecular medicine. 2012;16:1125–1133. doi: 10.1111/j.1582-4934.2011.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maruyama H, Higuchi N, Nishikawa Y, Kameda S, Iino N, Kazama JJ, et al. High-level expression of naked DNA delivered to rat liver via tail vein injection. The journal of gene medicine. 2002;4:333–341. doi: 10.1002/jgm.281. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Soroka CJ, Mennone A, Rahner C, Harry K, Pypaert M, et al. Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology. 2006;131:878–884. doi: 10.1053/j.gastro.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knowles RG, Merrett M, Salter M, Moncada S. Differential induction of brain, lung and liver nitric oxide synthase by endotoxin in the rat. The Biochemical journal. 1990;270:833–836. doi: 10.1042/bj2700833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittal MK, Gupta TK, Lee FY, Sieber CC, Groszmann RJ. Nitric oxide modulates hepatic vascular tone in normal rat liver. Am J Physiol. 1994;267:G416–422. doi: 10.1152/ajpgi.1994.267.3.G416. [DOI] [PubMed] [Google Scholar]
- 17.Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344–351. doi: 10.1016/s0016-5085(98)70487-1. [DOI] [PubMed] [Google Scholar]
- 18.Shah V, Haddad FG, Garcia-Cardena G, Frangos JA, Mennone A, Groszmann RJ, et al. Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. The Journal of clinical investigation. 1997;100:2923–2930. doi: 10.1172/JCI119842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goh BJ, Tan BT, Hon WM, Lee KH, Khoo HE. Nitric oxide synthase and heme oxygenase expressions in human liver cirrhosis. World journal of gastroenterology : WJG. 2006;12:588–594. doi: 10.3748/wjg.v12.i4.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah V, Toruner M, Haddad F, Cadelina G, Papapetropoulos A, Choo K, et al. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology. 1999;117:1222–1228. doi: 10.1016/s0016-5085(99)70408-7. [DOI] [PubMed] [Google Scholar]
- 21.Mookerjee RP, Wiesenthal A, Icking A, Hodges SJ, Davies NA, Schilling K, et al. Increased gene and protein expression of the novel eNOS regulatory protein NOSTRIN and a variant in alcoholic hepatitis. Gastroenterology. 2007;132:2533–2541. doi: 10.1053/j.gastro.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 22.Millatt LJ, Whitley GS, Li D, Leiper JM, Siragy HM, Carey RM, et al. Evidence for dysregulation of dimethylarginine dimethylaminohydrolase I in chronic hypoxia-induced pulmonary hypertension. Circulation. 2003;108:1493–1498. doi: 10.1161/01.CIR.0000089087.25930.FF. [DOI] [PubMed] [Google Scholar]
- 23.Nijveldt RJ, Teerlink T, Van Der Hoven B, Siroen MP, Kuik DJ, Rauwerda JA, et al. Asymmetrical dimethylarginine (ADMA) in critically ill patients: high plasma ADMA concentration is an independent risk factor of ICU mortality. Clinical nutrition. 2003;22:23–30. doi: 10.1054/clnu.2002.0613. [DOI] [PubMed] [Google Scholar]
- 24.Pope AJ, Karrupiah K, Kearns PN, Xia Y, Cardounel AJ. Role of dimethylarginine dimethylaminohydrolases in the regulation of endothelial nitric oxide production. The Journal of biological chemistry. 2009;284:35338–35347. doi: 10.1074/jbc.M109.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasegawa K, Wakino S, Tanaka T, Kimoto M, Tatematsu S, Kanda T, et al. Dimethylarginine dimethylaminohydrolase 2 increases VEGF expression through Sp1 transcription factor in endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1488–1494. doi: 10.1161/01.ATV.0000219615.88323.b4. [DOI] [PubMed] [Google Scholar]
- 26.Verbeke L, Farre R, Trebicka J, Komuta M, Roskams T, Klein S, et al. Obeticholic acid, a farnesoid-X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2013 doi: 10.1002/hep.26939. [DOI] [PubMed] [Google Scholar]
- 27.Ghebremariam YT, Yamada K, Lee JC, Johnson CL, Atzler D, Anderssohn M, et al. FXR agonist INT-747 upregulates DDAH expression and enhances insulin sensitivity in high-salt fed Dahl rats. PloS one. 2013;8:e60653. doi: 10.1371/journal.pone.0060653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai AL, et al. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. The Journal of biological chemistry. 2007;282:879–887. doi: 10.1074/jbc.M603606200. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizzo G, Disante M, Mencarelli A, Renga B, Gioiello A, Pellicciari R, et al. The farnesoid X receptor promotes adipocyte differentiation and regulates adipose cell function in vivo. Molecular pharmacology. 2006;70:1164–1173. doi: 10.1124/mol.106.023820. [DOI] [PubMed] [Google Scholar]
- 31.Adorini L, Pruzanski M, Shapiro D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug discovery today. 2012;17:988–997. doi: 10.1016/j.drudis.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Xu Z, Huang G, Gong W, Zhou P, Zhao Y, Zhang Y, et al. FXR ligands protect against hepatocellular inflammation via SOCS3 induction. Cellular signalling. 2012;24:1658–1664. doi: 10.1016/j.cellsig.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L, et al. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497–1512. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Wilson A, Kuruba R, Zhang Q, Gao X, He F, et al. FXR-mediated regulation of eNOS expression in vascular endothelial cells. Cardiovascular research. 2008;77:169–177. doi: 10.1093/cvr/cvm016. [DOI] [PubMed] [Google Scholar]
- 35.Luo Z, Teerlink T, Griendling K, Aslam S, Welch WJ, Wilcox CS. Angiotensin II and NADPH oxidase increase ADMA in vascular smooth muscle cells. Hypertension. 2010;56:498–504. doi: 10.1161/HYPERTENSIONAHA.110.152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.