Abstract
Gamma-aminobutyric acid (GABA) has received much attention as a health-promoting functional compound, and several GABA-enriched foods have been commercialized. In higher plants, GABA is primarily metabolized via a short pathway called the GABA shunt. The GABA shunt bypasses two steps (the oxidation of α-ketoglutarate to succinate) of the tricarboxylic acid (TCA) cycle via reactions catalyzed by three enzymes: glutamate decarboxylase, GABA transaminase, and succinic semialdehyde dehydrogenase. The GABA shunt plays a major role in primary carbon and nitrogen metabolism and is an integral part of the TCA cycle under stress and non-stress conditions. Tomato is one of the major crops that accumulate a relatively high level of GABA in its fruits. The GABA levels in tomato fruits dramatically change during fruit development; the GABA levels increase from flowering to the mature green stage and then rapidly decrease during the ripening stage. Although GABA constitutes up to 50% of the free amino acids at the mature green stage, the molecular mechanism of GABA accumulation and the physiological function of GABA during tomato fruit development remain unclear. In this review, we summarize recent studies of GABA accumulation in tomato fruits and discuss the potential biological roles of GABA in tomato fruit development.
Keywords: GABA, GABA shunt, tomato, fruit, metabolism
Introduction
Gamma-aminobutyric acid (GABA), a four-carbon non-proteinogenic amino acid, is widely found in animals, plants and bacteria. In humans, GABA functions as an inhibitory neurotransmitter in the central nervous system (Owens and Kriegstein, 2002). It has also been reported that GABA is effective at reducing blood pressure, inducing relaxation and enhancing immunity when administered orally (Inoue et al., 2003; Abdou et al., 2006). Thus, GABA has received much attention as a health-promoting functional compound, and several GABA-enriched foods have been commercialized. In higher plants, GABA is primarily metabolized via a short pathway called the GABA shunt, which bypasses two steps (the oxidation of α-ketoglutarate to succinate) of the tricarboxylic acid (TCA) cycle (Satya-Narayan and Nair, 1990; Bouché and Fromm, 2004; Figure 1). In this pathway, GABA is synthesized from glutamate in a reaction catalyzed by the enzyme glutamate decarboxylase (GAD) and subsequently catabolized to succinate through two consecutive reactions catalyzed by GABA transaminase (GABA-T) and succinic semialdehyde dehydrogenase (SSADH). Previous studies have suggested that the GABA shunt is involved in multiple physiological responses, such as the regulation of cytosolic pH, maintenance of carbon/nitrogen balance, defense against insects, protection from oxidative stress, and production of energy (Bouché and Fromm, 2004; Fait et al., 2008). Moreover, the GABA level rapidly increases in plant tissues subjected to diverse stimuli, including heat shock, mechanical stimulation, hypoxia, and phytohormones (Shelp et al., 1999).
FIGURE 1.
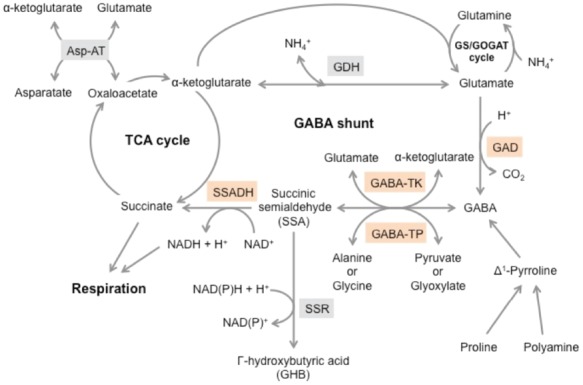
GABA metabolism and related pathways. GAD, glutamate decarboxylase; GABA-TK, α-ketoglutarate-dependent GABA transaminase; GABA-TP, pyruvate-dependent GABA transaminase; SSA, succinic semialdehyde; SSADH, succinic semialdehyde dehydrogenase; GDH, glutamate dehydrogenase; SSR, succinic semialdehyde reductase; Asp-AT, aspartate aminotransferase.
Tomato (Solanum lycopersicum) is a major crop produced worldwide. Tomato fruits are a significant food resource and have been considered an experimental model for studying the physiology, development and ripening of fleshy fruits (Steinhauser et al., 2010; Osorio et al., 2011). Tomatoes accumulate a relatively high level of GABA in the fruits (Matsumoto et al., 1997). In several cultivated tomatoes, drastic changes in GABA levels have been observed during fruit development; the GABA level increases to the mature green stage and subsequently rapidly decreases during the ripening stage (Rolin et al., 2000; Carrari et al., 2006; Akihiro et al., 2008; Saito et al., 2008; Osorio et al., 2011). In cherry tomatoes, GABA is reported to constitute up to 50% of the free amino acids at the mature green stage (Rolin et al., 2000). Despite the large accumulation, the molecular mechanism of GABA accumulation and the physiological function of this amino acid during tomato fruit development remain elusive. Elucidating these topics would help us to gain a better understanding of plant physiology, particularly in fruits. In this review, we summarize recent studies concerning GABA accumulation in tomato fruits and discuss the potential biological roles of GABA in tomato fruit development.
GABA Biosynthesis
In plants, GABA is primarily synthesized via the cytosolic enzyme GAD, which catalyzes the irreversible conversion of glutamate to GABA and CO2 (Figure 1). A plant GAD gene was first isolated from Petunia hybrida (Baum et al., 1993), and subsequently, several GAD homologues have been identified in various plant species (Ling et al., 1994; Snedden et al., 1995; Turano and Fang, 1998; Yevtushenko et al., 2003). Unlike its counterparts in animals and bacteria, most plant GADs possess a calcium/calmodulin (Ca2+/CaM) binding domain (CaMBD) at the C-terminus. In vitro studies have shown that GAD activity is stimulated through a low pH or the binding of Ca2+/CaM to the CaMBD at physiological pH (Snedden et al., 1996; Gut et al., 2009). In addition, transgenic studies revealed that the removal of the CaMBD increased GABA accumulation in plants (Baum et al., 1996; Akama and Takaiwa, 2007). Thus, it is considered that the CaMBD acts as a negative regulator/autoinhibitory domain in the absence of Ca2+/CaM, and the negative regulation is relieved through the binding of Ca2+/CaM.
In tomato, GAD gene was first cloned in 1995. Gallego et al. (1995) isolated ERT D1, a gene encoding a putative GAD protein, from a cDNA library of the pericarp of cv. “Ailsa Craig.” Similar to other plant GADs, ERT D1 protein contained a putative CaMBD. It was also revealed that ERT D1 mRNA levels peaked at the beginning of fruit ripening (Gallego et al., 1995). Subsequently, Kisaka et al. (2006) isolated GAD-19, a gene encoding another GAD protein, from tomato roots. The antisense suppression of this gene in tomato plants resulted in the production of fruits with decreased levels of GAD mRNA. However, the GABA level in these fruits was not significantly decreased compared with the WT levels, although increased levels of total free amino acids (particularly glutamate, which is the precursor to GABA) were observed (Kisaka et al., 2006). Subsequently, Akihiro et al. (2008) isolated three GAD genes, designated SlGAD1, SlGAD2, and SlGAD3, from the immature fruits of cv. “Micro-Tom.” Because the amino acid sequences of SlGAD1 and ERT D1 are precisely identical, SlGAD1 is considered an allele of ERT D1 (Akihiro et al., 2008). However, neither SlGAD2 nor SlGAD3 share precisely identical sequences with GAD-19, although blast database searches indicate that SlGAD2 has the highest homology to GAD-19 (95% identity and 98% similarity in amino acid sequences). Among the three SlGADs isolated from cv. “Micro-Tom,” SlGAD2 and SlGAD3 appear to play a major role in GABA production in tomato fruits, as the expression levels of SlGAD2 and SGAD3 are positively correlated with the GABA accumulation during fruit development (Akihiro et al., 2008). Additionally, transgenic tomato plants, in which SlGAD2 or SlGAD3 was specifically suppressed, accumulated a significantly decreased level of GABA in the fruits, whereas SlGAD1-suppressed plants produced fruits with normal levels of GABA (Takayama et al., 2015). Moreover, in triple SlGADs-suppressed plants, the fruit GABA level decreased to less than 10% of the WT level (Takayama et al., 2015), suggesting that the main route of GABA biosynthesis in tomato fruits is the decarboxylation of glutamate via GAD enzymes under normal growth conditions.
Enhanced GABA accumulation in tomato fruits has been observed in plants grown under salinity conditions or in fruits stored under 10% CO2 or under low O2 conditions after harvesting (Deewatthanawong et al., 2010; Yin et al., 2010; Mae et al., 2012). Although the expression levels of SlGAD2 and SlGAD3 were not enhanced in the fruits under salinity conditions (Yin et al., 2010), those in fruits stored under 10% CO2 or low O2 conditions were up-regulated (Deewatthanawong et al., 2010; Mae et al., 2012). These results suggest that SlGAD2 and SlGAD3 are responsive to some types of stresses. It has been suggested that stress-induced GABA accumulation in plant cells reflects increases in cytosolic H+, Ca2+ or glutamate levels, as these factors stimulate GAD activity (Shelp et al., 1999). However, in tomato, stress-induced GAD activity might also be regulated at the transcriptional level. Although it is reported that GABA can also be formed from polyamines or proline via a Δ1-pyrroline intermediate formation in response to abiotic stresses (Flores and Filner, 1985; Shelp et al., 2012; Yang et al., 2013; Signorelli et al., 2015), the contribution of these pathways in tomato fruits is still unclear.
GABA Catabolism
In many organisms, GABA is first converted to SSA via a transamination reaction through GABA-T (Figure 1). According to substrate specificity, the GABA-T enzyme can be divided into two types: α-ketoglutarate-dependent GABA-T (GABA-TK) and pyruvate-dependent GABA-T (GABA-TP). The former uses α-ketoglutarate as an amino group acceptor to generate glutamate, whereas the latter uses pyruvate to generate alanine (Bouché and Fromm, 2004). It is clear that GABA-TP also has glyoxylate-dependent GABA-T (GABA-TG) activity, which uses glyoxylate as an amino group acceptor to generate glycine (Clark et al., 2009a,b; Shimajiri et al., 2013; Trobacher et al., 2013). GABA-TK is exclusively utilized in bacteria, yeast, fungi and mammals (Satya-Narayan and Nair, 1990). However, both GABA-TK and GABA-TP activities have been detected in plant crude extracts (Shelp et al., 1995; Van Cauwenberghe and Shelp, 1999; Bartyzel et al., 2003), although only the GABA-TP gene has been isolated from plants (Van Cauwenberghe et al., 2002). Tomato is one of the species exhibiting both GABA-TK and GABA-TP activities. Although most previously investigated plants have shown lower GABA-TK activity than GABA-TP activity, Akihiro et al. (2008) detected a significantly higher level of GABA-TK activity in tomato fruits after the breaker stage. Comparison analyses between ordinary and GABA rich cultivars revealed a negative correlation between GABA contents and GABA-TK activity during fruit development (Akihiro et al., 2008). Similar trends were also observed in the tomato fruits stored under low O2 conditions, in which GABA levels were increased compared with those in fruits stored under control (air) conditions (Mae et al., 2012). These observations suggest that GABA-TK plays a major role in catabolism in tomato fruits. However, Clark et al. (2009b) presented a different view, as no GABA-TK activity was detected in assays using the cell-free extracts from the fruits of cv. “Micro-Tom,” which is the same cultivar used in Akihiro et al. (2008). Moreover, Clark et al. (2009b) observed higher levels of GABA-TP activity in tomato fruits. Thus, these authors noted the possibility that the previous study detected artificial GABA-TK activity and concluded that pyruvate/glyoxylate-dependent GABA-T activity probably accounts for the GABA catabolism observed in tomato fruits. Currently, three GABA-T genes, designated SlGABA-T1, SlGABA-T2, and SlGABA-T3, have been isolated from tomato cv. “Micro-Tom” (Akihiro et al., 2008; Clark et al., 2009b). Although the encoded proteins are localized to distinct subcellular compartments [i.e., mitochondrion (SlGABA-T1), cytosol (SlGABA-T2), or plastid (SlGABA-T3)], all three isoforms are characterized as GABA-TPs, which exhibit pyruvate/glyoxylate-dependent GABA-T activity (Clark et al., 2009b). To clarify the physiological function of these SlGABA-T isoforms in tomato fruits, Koike et al. (2013) conducted loss-of-function analyses using RNA interference (RNAi) transgenic lines with suppressed SlGABA-T genes. In this study, increased GABA accumulation was observed in the fruits of SlGABA-T1-suppressed lines (1.3–2.0 times higher in mature green fruits and 6.8–9.2 times higher in red fruits), whereas almost no correlation was observed between the GABA content and the expressions of SlGABA-T2 and SlGABA-T3 (Koike et al., 2013). Considering that the enzymatic activity of SlGABA-T1 is highest among the three isoforms in tomato fruits (Clark et al., 2009b), Koike et al. (2013) concluded that pyruvate- and glyoxylate-dependent SlGABA-T1 is the essential isoform for GABA reduction in the ripening fruits.
In plants, GABA-derived SSA is catabolized via the NAD+-dependent enzyme SSADH, which oxidizes SSA to succinate concomitantly with NADH production in mitochondria (Breitkreuz and Shelp, 1995; Busch and Fromm, 1999; Figure 1). Alternatively, SSA can also be catabolized to γ-hydroxybutyric acid (GHB) through enzymes with SSA reductase (SSR) activity (Breitkreuz et al., 2003; Hoover et al., 2007; Simpson et al., 2008; Figure 1). The former pathway provides substrates (succinate and NADH) for the mitochondrial respiratory machinery, which produces ATP as a final product (Bouché and Fromm, 2004). It is also known that SSADH activity is highly sensitive to the energy status in mitochondria (Busch and Fromm, 1999). Thus, under stress conditions in which the NAD+:NADH ratio is low, SSADH activity would be inhibited, resulting in the accumulation of SSA and feedback inhibition of GABA-T (Busch and Fromm, 1999; Van Cauwenberghe and Shelp, 1999). However, the pathway from SSA to GHB is stimulated under stress conditions and likely functions in stress tolerance through the detoxification of SSA (Breitkreuz et al., 2003; Allan et al., 2008). In tomato, one SSADH gene (SlSSADH) and two SSR genes (SlSSR1, SlSSR2) have been isolated (Akihiro et al., 2008). SlSSADH is expressed in fruits at all developmental stages, and the expression of this gene is poorly correlated with the GABA contents (Akihiro et al., 2008). However, the expression of SlSSR1 gene is slightly higher in red fruits than in breaker fruits, whereas SlSSR2 expression is higher in breaker fruits compared with red fruits (Deewatthanawong et al., 2010). However, the biochemical properties of the encoded proteins and their contribution to GABA accumulation in tomato fruits remain unclear.
The Potential Role of GABA Metabolism in Tomato Plants
In plants, GABA metabolism is involved in a wide range of physiological processes. For example, pop2, an Arabidopsis GABA-T-deficient mutant, is defective in the guidance and growth of pollen tubes (Palanivelu et al., 2003; Renault et al., 2011). Arabidopsis SSADH-deficient mutants exhibit severe dwarfism and necrotic lesions under the standard light conditions (Bouché et al., 2003). Additionally, these mutants exhibit the enhanced accumulation of reactive oxygen intermediates and cell death under environmental stresses (Bouché et al., 2003). Another Arabidopsis SSADH-deficient mutant, enf1, forms both abaxialized and adaxialized leaves (Toyokura et al., 2011). Notably, the abnormal phenotypes observed in the two different studies of ssadh mutants (Bouché et al., 2003; Toyokura et al., 2011) are both suppressed through an additional mutation in GABA-T, suggesting that these phenotypes reflect the accumulation of SSA or close derivatives, such as GHB (Ludewig et al., 2008; Toyokura et al., 2011). In tomatoes, several abnormalities have also been observed when GABA metabolism is altered. For example, SlGABA-T1-suppressed plants exhibited severe infertility, and both SlGABA-T1- and SlGABA-T3-suppressed plants exhibited dwarf phenotypes (Koike et al., 2013). Moreover, SlSSADH-suppressed plants show a dwarf phenotype, curled leaves and enhanced ROS accumulation under normal conditions (Bao et al., 2014). Interestingly, when tomato seedlings were grown under salt stress (200 mM NaCl), SlSSADH-suppressed plants exhibited significantly higher shoot biomass levels and increased chlorophyll contents and photosynthetic rates compared with control plants (Bao et al., 2014). However, SlGADs-suppressed plants and SlGABA-Ts-suppressed plants are more sensitive to salt stress, resulting in reduced biomass and the total collapse of tissue (Bao et al., 2014). These observations indicate that GABA shunt is involved in salt stress tolerance in tomato plants. Moreover, GABA shunt has been implicated in resistance against Botrytis cinerea, as GABA shunt genes are up-regulated in the leaves of the B. cinerea-resistant mutant, sitiens, and the exogenous application of GABA decreases susceptibility to B. cinerea in wild-type leaves (Seifi et al., 2013).
As described above, effects of impaired GABA metabolism on tomato plants have been increasingly reported. However, little is known about the function of GABA and the metabolism of this amino acid in fruits. Previous studies have suggested that GABA production during fruit development might contribute to the regulation of cellular pH (Rolin et al., 2000). During tomato fruit development, organic acids are continuously synthesized from unloaded sucrose, coupled with proton production. Overaccumulation of protons would cause an intracellular acidification, but the intracellular pH is probably regulated by ATP-driven proton pumps that extrude intracellular protons out of the cytoplasm, or by the proton-consuming decarboxylation of organic acids. Because GAD reaction requires protons, it might act as a sink for excess protons, preventing intracellular acidification (Rolin et al., 2000; Figure 2A). Moreover, the GAD reaction also promotes glutamate transport. In cherry tomatoes, glutamate is translocated through phloem sap and unloaded in fruits. The unloaded glutamate is subsequently transported symplastically or taken up through a proton symport mechanism across the membrane. In the latter transport mechanism, glutamate and protons are cotransported into the cytosol, thereby promoting cytoplasmic acidosis and the depolarization of the plasma membrane. Thus, continuous GABA accumulation during fruit development reflects the continuous GAD reaction, which potentially maintains glutamate transport through the consumption of excess protons (Snedden et al., 1992; Rolin et al., 2000). In addition, accumulated GABA in tomato fruits functions as an energy source, as 14C-labeled CO2 was discharged from fruits fed 14C-labeled GABA, indicating that GABA is utilized as a substrate for respiration (Yin et al., 2010; Figure 2B). Indeed, GABA shunt also functions as an alternative pathway for the production of succinate (the substrate for respiration) in tomato leaves when the enzyme of the TCA cycle is impaired (Studart-Guimarães et al., 2007). However, recent findings suggest that GABA metabolism has little effect on tomato fruit development under normal conditions, as the fruits of RNAi transgenic plants targeting the three SlGADs exhibited normal development, although the enzymatic activity of GAD and the GABA content in fruits were dramatically decreased (Takayama et al., 2015). Similarly, RNAi transgenic plants targeting SlGABA-T also produced normal fruits, although the GABA levels in red fruits were 6.8–9.2 times higher than those in wild-type controls (Koike et al., 2013). Therefore, GABA metabolism in tomato fruits might be involved in stress tolerance, similar to other plants. Another possibility is that GABA contributes to tomato seed dispersal through changes in the amino acid composition during fruit development. Because GABA functions in defense against pests and pathogens (Bown et al., 2006; Seifi et al., 2013), GABA accumulation in fruits at the early developmental stage might protect immature seeds (Figure 2A). However, the GABA levels in fruits rapidly decline during the ripening stage, when seeds have already matured. In parallel, the levels of glutamate and/or aspartate, which provide the “Umami taste,” dramatically increase during the ripening stage. These changes in the amino acid composition might attract insects and animals, resulting in successful seed dispersal (Figure 2B). The increases in glutamate and/or aspartate during fruit ripening have been well characterized in various cultivars (Rolin et al., 2000; Akihiro et al., 2008; Koike et al., 2013). The increase in glutamate probably reflects the increase in glutamate dehydrogenase (GDH) and GABA-TK activities during the ripening stage and the decreased consumption of glutamate through GAD, which is almost undetectable in ripe fruits (Sorrequieta et al., 2010; Ferraro et al., 2015; Figure 1). On the other hand, aspartate is synthesized from glutamate through aspartate aminotransferase (Figure 1). In GABA-rich cultivars, lower levels of glutamate and aspartate have been observed in ripening fruits (Akihiro et al., 2008), suggesting that GABA catabolism contributes to the accumulation of glutamate and glutamate-derived aspartate in ripening fruits. Furthermore, Snowden et al. (2015) recently identified a tonoplast-localized glutamate/aspartate/GABA exchanger (SlCAT9) in tomato fruits. As overexpression of the SlCAT9 gene strongly influences the accumulation of glutamate, aspartate, and GABA during tomato fruit development, it is suggested that the intracellular transport of amino acids between vacuole and cytosol is also a major determinant of their accumulation in ripening fruits (Snowden et al., 2015). Although the pathway involving the conversion from GABA to glutamate remains uncertain, GABA catabolism might play a crucial role in the determination of tomato fruit taste during ripening.
FIGURE 2.
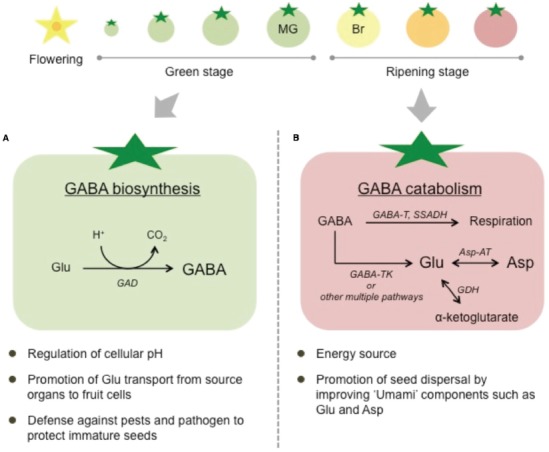
Potential roles of GABA in tomato fruits. (A) Fruits at the early developmental (green) stage when GABA is biosynthesized. (B) Fruits at the ripening stage when GABA is catabolized. MG, mature green; Br, breaker; GAD, glutamate decarboxylase; GABA-T (K), (α-ketoglutarate-dependent) GABA transaminase; SSADH, succinic semialdehyde dehydrogenase; Asp-AT, aspartate aminotransferase; Glu, glutamate; Asp, aspartate.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was funded in part by the Research and Development Program for New Bio-industry initiatives (BRAIN) to HE. We also thank all members our laboratory for helpful discussions throughout the work.
References
- Abdou A. M., Higashiguchi S., Horie K., Kim M., Hatta H., Yokogoshi H. (2006). Relaxation and immunity enhancement effects of gamma-aminobutyric acid (GABA) administration in humans. Biofactors 26, 201–208. 10.1002/biof.5520260305 [DOI] [PubMed] [Google Scholar]
- Akama K., Takaiwa F. (2007). C-terminal extension of rice glutamate decarboxylase (OsGAD2) functions as an autoinhibitory domain and overexpression of a truncated mutant results in the accumulation of extremely high levels of GABA in plant cells. J. Exp. Bot. 58, 2699–2707. 10.1093/jxb/erm120 [DOI] [PubMed] [Google Scholar]
- Akihiro T., Koike S., Tani R., Tominaga T., Watanabe S., Iijima Y., et al. (2008). Biochemical mechanism on GABA accumulation during fruit development in tomato. Plant Cell Physiol. 49, 1378–1389. 10.1093/pcp/pcn113 [DOI] [PubMed] [Google Scholar]
- Allan W. L., Simpson J. P., Clark S. M., Shelp B. J. (2008). Gamma-hydroxybutyrate accumulation in Arabidopsis and tobacco plants is a general response to abiotic stress: putative regulation by redox balance and glyoxylate reductase isoforms. J. Exp. Bot. 59, 2555–2564. 10.1093/jxb/ern122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H., Chen X., Lv S., Jiang P., Feng J., Fan P., et al. (2014). Virus-induced gene silencing reveals control of reactive oxygen species accumulation and salt tolerance in tomato by γ-aminobutyric acid metabolic pathway. Plant Cell Environ. 38, 600–613. 10.1111/pce.12419 [DOI] [PubMed] [Google Scholar]
- Bartyzel I., Pelczar K., Paszkowski A. (2003). Functioning of the γ-aminobutyrate pathway in wheat seedlings affected by osmotic stress. Biol. Plant. 47, 221–225. 10.1023/B:BIOP.0000022255.01125.99 [DOI] [Google Scholar]
- Baum G., Chen Y., Arazi T., Takatsuji H., Fromm H. (1993). A plant glutamate decarboxylase containing a calmodulin binding domain. Cloning, sequence, and functional analysis. J. Biol. Chem. 268, 19610–19617. [PubMed] [Google Scholar]
- Baum G., Lev-Yadun S., Fridmann Y., Arazi T., Katsnelson H., Zik M., et al. (1996). Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J. 15, 2988–2996. [PMC free article] [PubMed] [Google Scholar]
- Bouché N., Fait A., Bouchez D., Møller S. G., Fromm H. (2003). Mitochondrial succinic-semialdehyde dehydrogenase of the γ-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc. Natl. Acad. Sci. U.S.A. 100, 6843–6848. 10.1073/pnas.1037532100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché N., Fromm H. (2004). GABA in plants: just a metabolite? Trends Plant Sci. 9, 110–115. 10.1016/j.tplants.2004.01.006 [DOI] [PubMed] [Google Scholar]
- Bown A. W., MacGregor K. B., Shelp B. J. (2006). Gamma-aminobutyrate: defense against invertebrate pests? Trends Plant Sci. 11, 424–427. 10.1016/j.tplants.2006.07.002 [DOI] [PubMed] [Google Scholar]
- Breitkreuz K. E., Allan W. L., Van Cauwenberghe O. R., Jakobs C., Talibi D., André B., et al. (2003). A novel γ-hydroxybutyrate dehydrogenase. Identification and expression of an Arabidopsis cDNA and potential role under oxygen deficiency. J. Biol. Chem. 278, 41552–41556. 10.1074/jbc.M305717200 [DOI] [PubMed] [Google Scholar]
- Breitkreuz K. E., Shelp B. J. (1995). Subcellular compartmentation of the 4-aminobutyrate shunt in protoplasts from developing soybean cotyledons. Plant Physiol. 108, 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch K. B., Fromm H. (1999). Plant succinic semialdehyde dehydrogenase. Cloning, purification, localization in mitochondria, and regulation by adenine nucleotides. Plant Physiol. 121, 589–597. 10.1104/pp.121.2.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrari F., Baxter C., Usadel B., Urbanczyk-Wochniak E., Zanor M., Nunes-Nesi A., et al. (2006). Integrated analysis of metabolite and transcript levels reveals the metabolic shifts that underlie tomato fruit development and highlight regulatory aspects of metabolic network behavior. Plant Physiol. 142, 1380–1396. 10.1104/pp.106.088534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. M., Di Leo R., Dhanoa P. K., Van Cauwenberghe O. R., Mullen R. T., Shelp B. J. (2009a). Biochemical characterization, mitochondrial localization, expression, and potential functions for an Arabidopsis γ-aminobutyrate transaminase that utilizes both pyruvate and glyoxylate. J. Exp. Bot. 60, 1743–1757. 10.1093/jxb/erp044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. M., Di Leo R., Van Cauwenberghe O. R., Mullen R. T., Shelp B. J. (2009b). Subcellular localization and expression of multiple tomato γ-aminobutyrate transaminases that utilize both pyruvate and glyoxylate. J. Exp. Bot. 60, 3255–3267. 10.1093/jxb/erp161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deewatthanawong R., Rowell P., Watkins C. B. (2010). γ-Aminobutyric acid (GABA) metabolism in CO2 treated tomatoes. Postharvest Biol. Technol. 57, 97–105. 10.1016/j.postharvbio.2010.03.007 [DOI] [Google Scholar]
- Fait A., Fromm H., Walter D., Galili G., Fernie A. R. (2008). Highway or byway: the metabolic role of the GABA shunt in plants. Trends Plant Sci. 13, 14–19. 10.1016/j.tplants.2007.10.005 [DOI] [PubMed] [Google Scholar]
- Ferraro G., D’Angelo M., Sulpice R., Stitt M., Valle E. M. (2015). Reduced levels of NADH-dependent glutamate dehydrogenase decrease the glutamate content of ripe tomato fruit but have no effect on green fruit or leaves. J. Exp. Bot. 66, 3381–3389. 10.1093/jxb/erv150 [DOI] [PubMed] [Google Scholar]
- Flores H. E., Filner P. (1985). Polyamine catabolism in higher plants: characterization of pyrroline dehydrogenase. Plant Growth Regul. 3, 277–291. 10.1007/BF00117586 [DOI] [Google Scholar]
- Gallego P. P., Whotton L., Picton S., Grierson D., Gray J. E. (1995). A role for glutamate decarboxylase during tomato ripening: the characterization of a cDNA encoding a putative glutamate decarboxylase with a calmodulin-binding site. Plant Mol. Biol. 27, 1143–1151. 10.1007/BF00020887 [DOI] [PubMed] [Google Scholar]
- Gut H., Dominici P., Pilati S., Astegno A., Petoukhov M. V., Svergun D. I., et al. (2009). A common structural basis for pH- and calmodulin-mediated regulation in plant glutamate decarboxylase. J. Mol. Biol. 392, 334–351. 10.1016/j.jmb.2009.06.080 [DOI] [PubMed] [Google Scholar]
- Hoover G. J., Van Cauwenberghe O. R., Breitkreuz K. E., Clark S. M., Merrill A. R., Shelp B. J. (2007). Characteristics of an Arabidopsis glyoxylate reductase: general biochemical properties and substrate specificity for the recombinant protein, and developmental expression and implications for glyoxylate and succinic semialdehyde metabolism in planta. Can. J. Bot. 85, 883–895. 10.1139/B07-081 [DOI] [Google Scholar]
- Inoue K., Shirai T., Ochiai H., Kasao M., Hayakawa K., Kimura M., et al. (2003). Blood-pressure-lowering effect of a novel fermented milk containing gamma-aminobutyric acid (GABA) in mild hypertensives. Eur. J. Clin. Nutr. 57, 490–495. 10.1038/sj.ejcn.1601555 [DOI] [PubMed] [Google Scholar]
- Kisaka H., Kida T., Miwa T. (2006). Antisense suppression of glutamate decarboxylase in tomato (Lycopersicon esculentum L.) results in accumulation of glutamate in transgenic tomato fruits. Plant Biotechnol. 23, 267–274. 10.5511/plantbiotechnology.23.267 [DOI] [Google Scholar]
- Koike S., Matsukura C., Takayama M., Asamizu E., Ezura H. (2013). Suppression of γ-aminobutyric acid (GABA) transaminases induces prominent GABA accumulation, dwarfism and infertility in the tomato (Solanum lycopersicum L.). Plant Cell Physiol. 54, 793–807. 10.1093/pcp/pct035 [DOI] [PubMed] [Google Scholar]
- Ling V., Snedden W. A., Shelp B. J., Assmann S. M. (1994). Analysis of a soluble calmodulin binding protein from fava bean roots: identification of glutamate decarboxylase as a calmodulin-activated enzyme. Plant Cell 6, 1135–1143. 10.1105/tpc.6.8.1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig F., Hüser A., Fromm H., Beauclair L., Bouché N. (2008). Mutants of GABA transaminase (POP2) suppress the severe phenotype of succinic semialdehyde dehydrogenase (ssadh) mutants in Arabidopsis. PLoS ONE 3:e3383. 10.1371/journal.pone.0003383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mae N., Makino Y., Oshita S., Kawagoe Y., Tanaka A., Aoki K., et al. (2012). Accumulation mechanism of γ-aminobutyric acid in tomatoes (Solanum lycopersicum L.) under low O2 with and without CO2. J. Agric. Food Chem. 60, 1013–1019. 10.1021/jf2046812 [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Ohno K., Hiraoka Y. (1997). Studies on the utilization of functional food materials containing high levels of gamma-aminobutyric acid (Part1). Ehime Kougi Kenkyu Houkoku (In Japanese). 35, 97–100. [Google Scholar]
- Osorio S., Alba R., Damasceno C. M., Lopez-Casado G., Lohse M., Zanor M. I., et al. (2011). Systems biology of tomato fruit development: combined transcript, protein, and metabolite analysis of tomato transcription factor (nor, rin) and ethylene receptor (Nr) mutants reveals novel regulatory interactions. Plant Physiol. 157, 405–425. 10.1104/pp.111.175463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens D. F., Kriegstein A. R. (2002). Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 3, 715–727. 10.1038/nrn919 [DOI] [PubMed] [Google Scholar]
- Palanivelu R., Brass L., Edlund A. F., Preuss D. (2003). Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 114, 47–59. 10.1016/S0092-8674(03)00479-3 [DOI] [PubMed] [Google Scholar]
- Renault H., El Amrani A., Palanivelu R., Updegraff E. P., Yu A., Renou J. P., et al. (2011). GABA accumulation causes cell elongation defects and a decrease in expression of genes encoding secreted and cell wall-related proteins in Arabidopsis thaliana. Plant Cell Physiol. 52, 894–908. 10.1093/pcp/pcr041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolin D., Baldet P., Just D., Chevalier C., Biran M., Raymond P. (2000). NMR study of low subcellular pH during the development of cherry tomato fruit. Aust. J. Plant Physiol. 27, 61–69. 10.1071/pp99051 [DOI] [Google Scholar]
- Saito T., Matsukura C., Sugiyama M., Watahiki A., Ohshima I., Iijima Y., et al. (2008). Screening for γ-aminobutyric acid (GABA)-rich tomato varieties. J. Japan. Soc. Hort. Sci. 77, 242–250. 10.2503/jjshs1.77.242 [DOI] [Google Scholar]
- Satya-Narayan V., Nair P. M. (1990). Metabolism, enzymology and possible roles of 4-aminobutyrate in higher plants. Phytochemistry 29, 367–375. 10.1016/0031-9422(90)85081-P [DOI] [Google Scholar]
- Seifi H. S., Curvers K., De Vleesschauwer D., Delaere I., Aziz A., Höfte M. (2013). Concurrent overactivation of the cytosolic glutamine synthetase and the GABA shunt in the ABA-deficient sitiens mutant of tomato leads to resistance against Botrytis cinerea. New Phytol. 199, 490–504. 10.1111/nph.12283 [DOI] [PubMed] [Google Scholar]
- Shelp B. J., Bown A. W., McLean M. D. (1999). Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 4, 446–452. 10.1016/S1360-1385(99)01486-7 [DOI] [PubMed] [Google Scholar]
- Shelp B. J., Bozzo G. G., Trobacher C. P., Zarei A., Deyman K. L., Brikis C. J. (2012). Hypothesis/review: contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci. 193–194, 130–135. 10.1016/j.plantsci.2012.06.001 [DOI] [PubMed] [Google Scholar]
- Shelp B. J., Walton C. S., Snedden W. A., Tuin L. G., Oresnik I. J., Layzell D. B. (1995). Gaba shunt in developing soybean seeds is associated with hypoxia. Physiol. Plant. 94, 219–228. 10.1111/j.1399-3054.1995.tb05304.x [DOI] [Google Scholar]
- Shimajiri Y., Ozaki K., Kainou K., Akama K. (2013). Differential subcellular localization, enzymatic properties and expression patterns of γ-aminobutyric acid transaminases (GABA-Ts) in rice (Oryza sativa). J. Plant Physiol. 170, 196–201. 10.1016/j.jplph.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Signorelli S., Dans P. D., Coitiño E. L., Borsani O., Monza J. (2015). Connecting proline and γ-aminobutyric acid in stressed plants through non-enzymatic reactions. PLoS ONE 10:e0115349. 10.1371/journal.pone.0115349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J. P., Di Leo R., Dhanoa P. K., Allan W. L., Makhmoudova A., Clark S. M., et al. (2008). Identification and characterization of a plastid-localized Arabidopsis glyoxylate reductase isoform: comparison with a cytosolic isoform and implications for cellular redox homeostasis and aldehyde detoxification. J. Exp. Bot. 59, 2545–2554. 10.1093/jxb/ern123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedden W. A., Arazi T., Fromm H., Shelp B. J. (1995). Calcium/calmodulin activation of soybean glutamate decarboxylase. Plant Physiol. 108, 543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedden W. A., Chung I., Pauls R. H., Bown A. W. (1992). Proton/l-glutamate symport and the regulation of intracellular pH in isolated mesophyll cells. Plant Physiol. 99, 665–671. 10.1104/pp.99.2.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedden W. A., Koutsia N., Baum G., Fromm H. (1996). Activation of a recombinant petunia glutamate decarboxylase by calcium/calmodulin or by a monoclonal antibody which recognizes the calmodulin binding domain. J. Biol. Chem. 271, 4148–4153. 10.1074/jbc.271.8.4148 [DOI] [PubMed] [Google Scholar]
- Snowden C. J., Thomas B., Baxter C. J., Smith J. A., Sweetlove L. J. (2015). A tonoplast Glu/Asp/GABA exchanger that affects tomato fruit amino acid composition. Plant J. 81, 651–660. 10.1111/tpj.12766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrequieta A., Ferraro G., Boggio S. B., Valle E. M. (2010). Free amino acid production during tomato fruit ripening: a focus on l-glutamate. Amino Acids 38, 1523–1532. 10.1007/s00726-009-0373-1 [DOI] [PubMed] [Google Scholar]
- Steinhauser M., Steinhauser D., Koehl K., Carrari F., Gibon Y., Fernie A. R., et al. (2010). Enzyme activity profiles during fruit development in tomato cultivar and Solanum pennellii. Plant Physiol. 153, 80–98. 10.1104/pp.110.154336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studart-Guimarães C., Fait A., Nunes-Nesi A., Carrari F., Usadel B., Fernie A. R. (2007). Reduced expression of succinyl-coenzyme A ligase can be compensated for by up-regulation of the γ-aminobutyrate shunt in illuminated tomato leaves. Plant Physiol. 145, 626–639. 10.1104/pp.107.103101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama M., Koike S., Kusano M., Matsukura C., Saito K., Ariizumi T., et al. (2015). Tomato glutamate decarboxylase genes SlGAD2 and SlGAD3 play key roles in regulating γ-aminobutyric acid levels in tomato (Solanum lycopersicum). Plant Cell Physiol. 10.1093/pcp/pcv075 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Toyokura K., Watanabe K., Oiwaka A., Kusano M., Tameshige T., Tatematsu K., et al. (2011). Succinic semialdehyde dehydrogenase is involved in the robust pattering of Arabidopsis leaves along the adaxial-abaxial axis. Plant Cell Physiol. 52, 1340–1353. 10.1093/pcp/pcr079 [DOI] [PubMed] [Google Scholar]
- Trobacher C. P., Clark S. M., Bozzo G. G., Mullen R. T., DeEll J. R., Shelp B. J. (2013). Catabolism of GABA in apple fruit: subcellular localization and biochemical characterization of two γ-aminobutyrate transaminases. Postharvest Biol. Technol. 75, 106–113. 10.1016/j.postharvbio.2012.08.005 [DOI] [Google Scholar]
- Turano F. J., Fang T. K. (1998). Characterization of two glutamate decarboxylase cDNA clones from Arabidopsis. Plant Physiol. 117, 1411–1421. 10.1104/pp.117.4.1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauwenberghe O. R., Makhmoudova A., McLean M. D., Clark S., Shelp B. J. (2002). Plant pyruvate-dependent gamma-aminobutyrate transaminase: identification of an Arabidopsis cDNA and its expression in Escherichia coli. Can. J. Bot. 80, 933–941. 10.1139/b02-087 [DOI] [Google Scholar]
- Van Cauwenberghe O. R., Shelp B. J. (1999). Biochemical characterization of partially purified gaba:pyruvate transaminase from Nicotiana tabacum. Phytochemistry 52, 575–581. 10.1016/S0031-9422(99)00301-5 [DOI] [Google Scholar]
- Yang R., Guo Q., Gu Z. (2013). GABA shunt and polyamine degradation pathway on γ-aminobutyric acid accumulation in germinating fava bean (Vicia faba L.) under hypoxia. Food Chem. 136, 152–159. 10.1016/j.foodchem.2012.08.008 [DOI] [PubMed] [Google Scholar]
- Yevtushenko D. P., McLean M. D., Peiris S., Van Cauwenberghe O. R., Shelp B. J. (2003). Calcium/calmodulin activation of two divergent glutamate decarboxylases from tobacco. J. Exp. Bot. 54, 2001–2002. 10.1093/jxb/erg210 [DOI] [PubMed] [Google Scholar]
- Yin Y. G., Tominaga T., Iijima Y., Aoki K., Shibata D., Ashihara H., et al. (2010). Metabolic alterations in organic acids and γ-aminobutyric acid in developing tomato (Solanum lycopersicum L.) fruits. Plant Cell Physiol. 51, 1300–1314. 10.1093/pcp/pcq090 [DOI] [PubMed] [Google Scholar]


