Abstract
Cocaine-related fatalities can pose forensic challenges, particularly when accompanied by excited delirium (ED) syndrome and interventions by law enforcement and medical personnel. A recent report concluded that elevated heat shock protein 70 (HSP70) expression in autopsy brain samples constitutes a reliable forensic biomarker for the identification of ED as a cause of death. The present study quantified the abundance of both HSPA1A and HSPA1B gene (HSP70-encoding) transcripts in midbrain specimens from a series of cocaine-related fatalities and matched drug-free control subjects. HSP70 expression was increased significantly in cocaine abusers as a group compared to control subjects, irrespective of the presence or absence of ED. Furthermore, elevated HSP70 expression was predictive of a period of survival between cocaine use and death that included medical and/or police intervention. The present data do not support the assertion that HSP70 expression is a reliable brain biomarker for identifying ED as a cause of death.
Keywords: forensic science, forensic pathology, excited delirium, heat shock protein 70, cocaine, survival time, autopsy, human brain, microarray
Deaths associated with cocaine abuse can pose forensic challenges, particularly when sudden unexplained death follows the emergence of excited delirium (ED), a syndrome characterized by agitation, combativeness, and hyperthermia. These subjects often die in custody following confrontations with police that involve physical struggle, restraint, and/or deployment of conductive energy devices (for reviews, see 1–2). A recent study (3) reported that heat shock protein 70 (HSP70) expression was increased in the postmortem brains of cocaine subjects exhibiting ED in comparison to other (non-ED) cocaine-related deaths and drug-free controls, concluding that elevated HSP70 provides a reliable forensic biomarker for the identification of ED. As part of our broader effort to determine the profile of gene expression in cocaine abusers’ brains (4–8), we examined HSP70-related gene expression in postmortem specimens from a series of cocaine-related deaths and well-matched drug-free control subjects. HSPA1A and HSPA1B (i.e. HSP70-encoding) gene expression were significantly increased in the brains of cocaine abusers as a group compared to drug-free controls, irrespective of the presence or absence of ED. Furthermore, elevated HSP70 expression was significantly predictive of a period of survival between cocaine use and death that included medical and/or police intervention. The previous assertion that HSP70 constitutes a reliable forensic tool for the neuropathological identification of ED as a cause of death is discussed in light of these findings.
Materials and Methods
Case Acquisition and Characterization
Postmortem ventral midbrain specimens were obtained at autopsy and analyzed as described previously (4–8). Briefly, the cause and manner of death were determined by forensic pathologists after medico-legal investigations that evaluated the circumstances of death including police reports and medical records, autopsy results and toxicological data. All cases were evaluated for common drugs of abuse (including alcohol), and positive urine screens were confirmed by quantitative analysis of blood levels of cocaine and metabolites (9). The cocaine abuse cohort in the present study had a documented history of drug abuse, tested positive for cocaine and/or metabolites but negative for other drugs of abuse, and were determined to have died as a result of cocaine abuse/cocaine intoxication. Two of the cocaine-related fatalities also exhibited ED syndrome (see Table 1), as evidenced by the emergence of abnormal behaviors immediately following cocaine ingestion, followed by contact with medical and/or law enforcement personnel. One of the authors (a trained forensic pathologist) reviewed the case files of cocaine-related fatalities before final classification and inclusion of cases. Control subjects had no documented history of drug abuse, were drug-free at time of death (with the exception of a single control with a sub-intoxicating level of alcohol), and died as a result of cardiovascular disease or gunshot wounds. Cocaine abusers and drug-free controls were carefully matched for age, race, gender and sample quality measurements including brain pH and RNA integrity number (RIN), and added pair-wise to the study for a total of N=18 in each group. Subject demographics, cause of death, blood levels of cocaine and metabolites, and estimated survival time between initial contact with police and/or medical personnel and death are summarized in Table 1. Drug abuse and control groups did not differ in age, race, or gender (Table 1). Nor did the control and cocaine groups differ in terms of the postmortem sample quality indicators of brain pH (6.5±0.05 & 6.5±0.05, respectively) or RIN (6.5±0.2 vs 6.7±0.2, respectively). All data reported as means ± SEM.
TABLE 1.
Demographic characteristics and forensic data on cocaine fatalities and matched control cases.
| Case | Age | Sex/Race* | COD | Cocaine (ug/mL) |
Benzoylecgonine (ug/mL) |
Survival time (hrs) |
Case | Age | Sex/Race | COD | Cocaine (ug/mL) |
Benzoylecgonine (ug/mL) |
Survival time (hrs) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #27 | 51 | BF | ASCVD | ND† | ND | 0 | #28 | 52 | BF | Cocaine, cerebral hemorrhage |
ND | 0.077 | 0 |
| #33 | 30 | WF | GSW | ND | ND | 0 | #34 | 35 | WF | Cocaine abuse | 0.28 | 4.4 | 0 |
| #39 | 20 | WM | Dilated cardiomyopathy |
ND | ND | 0 | #40 | 25 | WM | Cocaine | 13 | 7.4 | 0 |
| #1 | 40 | BF | Dilated cardiomyopathy |
ND | ND | 0 | #2 | 34 | BF | Cocaine abuse, cardiac arrest |
ND | 0.66 | 0 |
| #7 | 47 | WM | Acute MI, ASCVD |
ND | ND | 0 | #8 | 46 | WM | Acute cocaine intoxication |
0.8 | 5.7 | 0 |
| #5 | 35 | BM | GSW (ASCVD contributing) |
ND | ND | 4 | #6 | 35 | BM | Acute cocaine intoxication |
31 | 8.9 | 0 |
| #9 | 45 | BM | MGSW | ND | ND | 0 | #10 | 46 | BM | Cocaine abuse | 0.069 | 1.5 | 0 |
| #11 | 49 | BM | MI, ASCVD | ND | ND | 0 | #12 | 49 | BM | Aortic aneurysm due to cocaine abuse |
ND | 0.42 | 0 |
| #50 | 53 | BM | ASCVD | ND | ND | 0 | #51 | 59 | BM | Cocaine abuse | ND | 0.071 | 0 |
| #52 | 51 | BM | Aortic dissection, hypertension |
ND | ND | 0 | #53 | 54 | BM | Cocaine abuse | ND | 0.09 | 0 |
| #54 | 66 | BM | MGSW | ND | ND | 0 | #55 | 64 | BM | Acute cocaine intoxication |
0.065 | 0.4 | 0 |
| #56 | 46 | BM | MGSW | ND | ND | 0 | #57 | 52 | BM | Cocaine abuse | 0.078 | 0.33 | 0 |
| #41 | 45 | WM | GSW | ND | ND | 0 | #42 | 46 | WM | Cocaine abuse‡ | ND | 0.03 | 1 |
| #3 | 33 | BM | Dilated cardiomyopathy |
ND | ND | 0 | #4 | 35 | BM | Cocaine abuse‡ | 14 | 8.1 | 1 |
| #35 | 34 | BM | MGSW | ND | ND | 0 | #36 | 34 | BM | Cocaine abuse | 0.74 | 4.3 | 1 |
| #15 | 52 | BM | Hypertensive cardiomyopathy |
ND | ND | 0 | #16 | 52 | BM | Aortic dissection due to cocaine abuse |
ND | 0.58 | 3 |
| #13 | 50 | BM | GSW | ND | ND | 0 | #14 | 52 | BM | Cocaine abuse | 0.29 | 3 | 5 |
| #37 | 45 | BM | ASCVD | ND | ND | 0 | #38 | 45 | BM | Cocaine abuse | 0.053 | 3.4 | 21 |
Cases were analyzed group-wise but listed pair-wise for ease of comparison
Control and cocaine groups did not differ in terms of age (44±3 & 45±2, respectively) or sex/race (2 BF, 1 WF, 3 WM, 12 BM in each group)
BF=black female, WF=white female, WM=white male, BM=black male
ND=not detected
Cases exhibited abnormal behaviors clearly indicative of ED syndrome
Specimen Processing, Microarray, RT-PCR, and Immunoblotting
Ventral midbrain sample processing, and subsequent gene expression analysis by microarray and quantitative real-time PCR (qRT-PCR), were implemented as recently described in detail (10). Briefly, fresh-frozen midbrain blocks were cut at a thickness of 250 microns on a cryostat, and slide-mounted for subsequent hand-dissection of dopamine cell-enriched regions of the ventral midbrain. Other specified brain regions were dissected free of surrounding tissue at the time of autopsy and frozen for subsequent use. RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol, then DNAse-treated with the Qiagen RNeasy Minikit (Qiagen, Valencia, CA, USA). Quantification of RNA was accomplished using NanoDrop ND-1000 (ThermoScientific, Waltham, MA, USA), with an initial assessment of RNA quality by agarose-formaldehyde gel electrophoresis and ethidium bromide staining.
Microarray assays (using HT-12 BeadChips; Illumina, Inc., San Diego, CA, USA) were performed by the Keck Microarray Resource as part of the NIH Neuroscience Microarray Consortium (Yale Center for Genomic Analysis, West Haven, CT, USA). The quality and quantity of each RNA sample was verified using an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) prior to labeling reactions. Biotin-labeled cRNAs were generated using the TotalPrep RNA Amplification kit (Applied Biosystems, Carlsbad, CA, USA) with 500 ng total RNA as template. Each sample was labeled in an independent reaction, using technical triplicates to obtain a measure of experimental variation. In addition, an RNA pool derived from multiple samples was hybridized across arrays to assess potential inter-array variation. Microarray controls included control RNAs spiked into RT reactions, as well as spiked-in cRNAs that matched or mismatched with BeadChips oligos. Labeled cRNAs were hybridized according to the manufacturer’s protocol and scanned on the Illumina Iscan. Initial Consortium data analysis included quantile normalization, carried out in Illumina BeadStudio.
For qRT-PCR validation experiments, 100ng RNA (aliquots of the same samples used for microarray experiments) from individual subjects was reverse-transcribed using random hexamers (Promega, Madison, WI, USA) and Omniscript RT kit (Qiagen) following the manufacturer’s protocol. Subsequent PCR reactions used SYBR Green master mix (Qiagen) following the manufacturer’s instructions. Transcript abundance in individual samples (assayed as duplicates) was quantified using the StepOne® Real-Time PCR System (Applied Biosystems) in comparison to a 6 point standard curve generated from pooled sample aliquots. PCR primer sequences (represented 5’-3’) used in validation experiments were: forward primer ACCATTGAGGAGGTAGATTAGG, reverse primer GCAAACACAGGAAATTGAGAAC for HSPA1A, and forward primer ACTGTTGGGACTCAAGGAC, reverse primer ATGAAGCCAGCTAATTACCATC for HSPA1B.
Statistical Analysis
Normalized microarray data were imported into Microsoft Excel and the statistical significance of differences in HSP70 between the cocaine and control groups was initially assessed using a group-wise t-test (α=0.05). The significance of differences among subgroups was assessed by 1-way ANOVA in GraphPad Prism (α=0.05). Pearson correlation coefficients (r) between log2 transformed microarray data and qRT-PCR data, and between array data and cocaine/metabolite levels were calculated. The performance of HSP70 RNA abundance as a diagnostic test for subject survival was calculated by Receiver Operating Characteristic curve analysis in SPSS (α=0.05).
Results
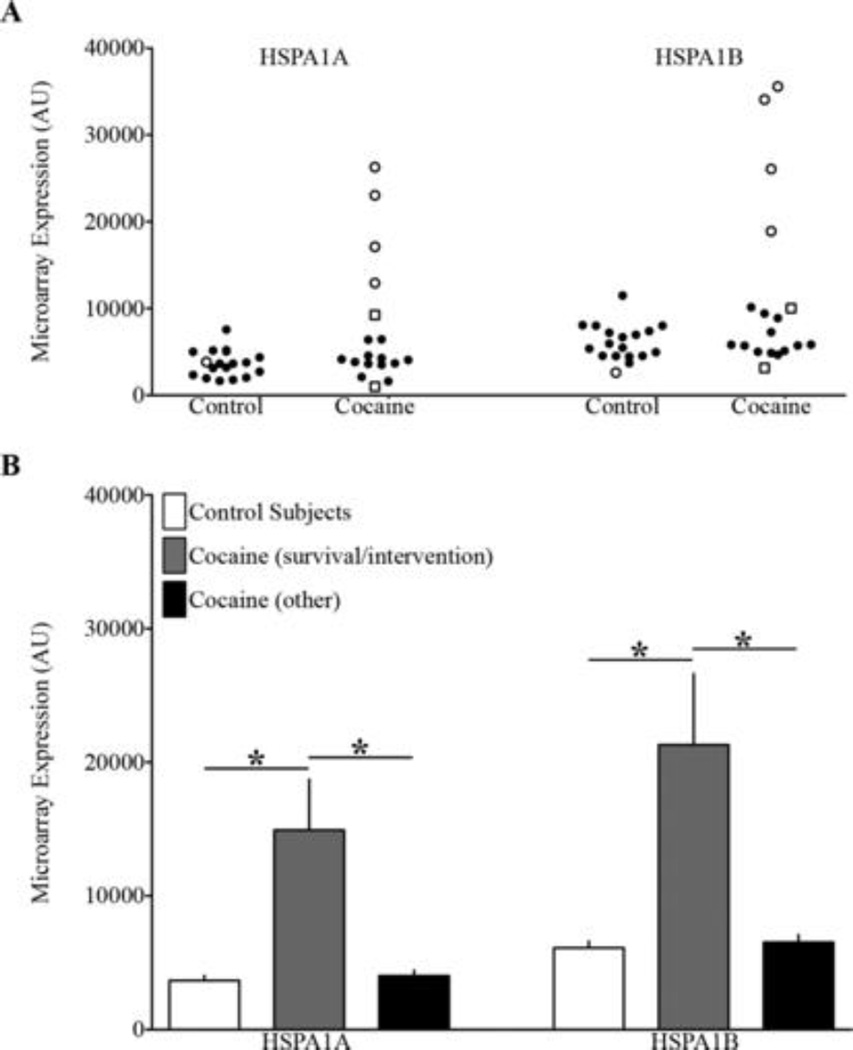
Microarray analysis was conducted on samples of ventral midbrain obtained at autopsy from a group of chronic cocaine abusers and drug-free control subjects carefully matched in terms of demographics (age, race and sex) and tissue sample quality (as reflected by brain pH and RNA integrity) (Table 1). Cocaine abusers as a group exhibited large (~2-fold) increases in the abundance of two HSP70-encoding transcripts, namely HSPA1A mRNA (7659±1741 vs. 3668±363) and HSPA1B mRNA (11459±2401 vs. 6108±494)(Figure 1A; 2-tailed p<0.04 for both transcripts). Significantly more variance in the abundance of these transcripts was evident in the cocaine cohort compared to the control subjects (Figure 1A; F-test p<0.0001). Two of the eighteen cocaine-related cases also exhibited ED syndrome prior to death (Table 1), but HSPA1A and HSPA1B transcript levels for these cases fell well within the range of values seen for other (non-ED) cocaine-related fatalities (Figure 1A). There was no significant correlation between HSPA1A or HSPA1B transcript levels and blood levels of cocaine or its metabolites (data not shown). It was noted, however, that HSPA1A and HSPA1B transcript levels were significantly higher in cocaine fatalities with a documented period of post-drug survival and contact with medical personnel and/or police before death, compared to the other cocaine-related fatalities or drug-free controls (Figure 1A&B). In fact, elevated abundance of either HSP70-encoding transcript alone was significantly predictive as a diagnostic test for a period of post-cocaine survival and intervention (p<0.03; calculated by Receiver Operating Characteristic curve analysis).
Figure 1.
Increased Heat Shock Protein 70 Gene Expression in the Brains of Cocaine-Related Fatalities may be Reflective of Postdrug Survival and Intervention rather than Excited Delirium
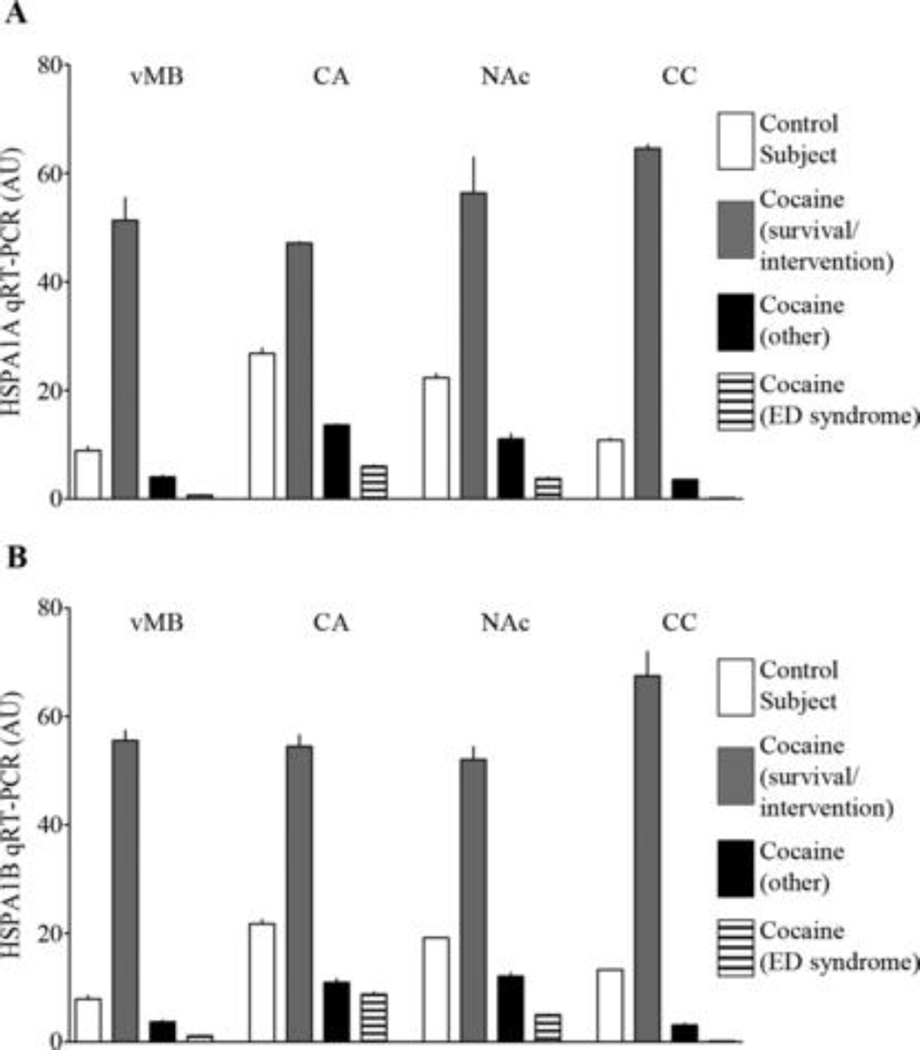
Midbrain HSPA1A and HSPA1B transcript abundances as determined by microarray correlated significantly with subsequent qRT-PCR data (Pearson r of 0.97 and 0.96, respectively; 1-tailed p<0.0001 for both transcripts; N=12); therefore qRT-PCR was used to assess HSP70 transcript abundance in several additional brain regions from four representative cases (i.e., a cocaine fatality found dead at the scene, a cocaine fatality exhibiting ED syndrome but short survival time, a cocaine fatality without ED but marked by prolonged survival time and medical interventions, and a drug-free control subject). As shown in Figure 2, HSPA1A and HSPA1B transcript levels in ventral midbrain, caudate, nucleus accumbens, and corpus callosum were elevated in the cocaine fatality with prolonged post-drug survival and intervention in comparison to the other cocaine fatalities (either ED or non-ED) and the drug-free control (Figure 2A&B), suggesting that some circumstances surrounding this death lead to a widespread induction of HSP70 gene expression throughout the CNS.
Figure 2.
Increased Heat Shock Protein 70 Gene Expression in the Brains of Cocaine-Related Fatalities may be Reflective of Postdrug Survival and Intervention rather than Excited Delirium
Discussion
Our ongoing effort to profile changes in gene expression in cocaine abusers’ brains revealed unexpectedly large, statistically significant (though variable) changes in the abundance of HSP70-related HSPA1A and HSPA1B transcripts relative to well-matched drug-free control subjects. This finding was rather unexpected, given a report that HSPA1B transcript was selectively increased in the brains of ED cases relative to other (non-ED) cocaine-related fatalities and control subjects, leading to the proposal that increased HSP70 constitutes a specific forensic biomarker for ED (3). Although our conclusions about ED must be tempered by the small number of such cases involved, our study found that HSP70 gene expression was increased significantly in cocaine abusers as a group compared to drug-free controls, irrespective of the presence or absence of ED (Figure 1). Unexpectedly, elevated HSP70 expression was instead predictive of a documented period of survival between cocaine use and death that included medical and/or police interventions, again irrespective of the emergence of ED syndrome (Figure 1B).
Although our study focused on HSP70-related changes in the dopamine cell-rich ventral midbrain (Figures 1&2), a preliminary assessment of two cocaine-responsive forebrain regions (caudate nucleus and nucleus accumbens) as well as the corpus callosum (Figure 2) suggests that cocaine-associated induction of HSP70 expression may be widespread in the CNS and thus does not account for the differences seen between our data and those of Mash et al. (3). The latter study focused on a large cohort of cocaine fatalities exhibiting ED, nearly all of whom experienced at least one force measure (e.g. mechanical restraints, conductive energy device, chemical agent), and most of whom survived 1–48 hrs after initial contact with police (3). Based on the present data, we hypothesize that the elevated HSP70 expression seen in the previous ED study (3), and also seen in a subset of our cocaine subjects, is more likely to be related to post-drug survival time and/or interventions by medical and law enforcement personnel rather than the presence or absence of ED per se. In the current study, the single drug-free control subject with a prolonged survival time and medical interventions prior to death had HSPA1A and HSPA1B transcript levels that matched our other control cases (Table 1 and Figure 1A), consistent with the notion that survival time and/or intervention(s) after cocaine insult may be key features in the induction of brain HSP70. Consistent with this interpretation, a previous forensic study has reported that the induction of HSP70 immunoreactivity in human hippocampus was associated with the length of survival after ischemic injury (11).
It is now better appreciated that HSP70 is rapidly induced in the CNS by a variety of cellular stresses (including hyperthermia and, in particular, ischemia), with its expression preceding, or even occurring in absence of, terminal physiological insult; HSP70 thus serves both biomarker and protective functions (for a review, see 12). For example, over-expression of HSP70 protects midbrain dopamine neurons against cellular stresses and neurotoxins in animal models (13–14). Although human autopsy studies obviously cannot provide such causal data, the apparent association between elevated brain HSP70 expression and prolonged survival in cocaine-related fatalities could reflect an adaptive (though ultimately unsuccessful) response to limit cocaine-induced (perhaps ischemia-related) neurotoxicity.
Footnotes
Supported by NIH Grants R01DA006470 (MJB) and F31DA032222 (MMJ).
References
- 1.Grant JR, Southall PE, Fowler DR, Mealey J, Thomas EJ, Kinlock TW. Death in custody: a historical analysis. J Forensic Sci. 2007 Sep;52(5):1177–1181. doi: 10.1111/j.1556-4029.2007.00500.x. [DOI] [PubMed] [Google Scholar]
- 2.Vilke GM, Payne-James J, Karch SB. Excited Delirium Syndrome (ExDS): redefining an old diagnosis. J Forensic Legal Med. 2012 Jan;19(1):7–11. doi: 10.1016/j.jflm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Mash DC, Duque L, Pablo J, Qin Y, Adi N, Hearn WL, et al. Brain biomarkers for identifying excited delirium as a cause of sudden death. Forensic Sci Int. 2009 Sep 10;190(1–3):e13–e19. doi: 10.1016/j.forsciint.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004 Mar;88(5):1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albertson DN, Schmidt CJ, Kapatos G, Bannon MJ. Distinctive profiles of gene expression in the human nucleus accumbens associated with cocaine and heroin abuse. Neuropsychopharmacology. 2006 Oct;31(10):2304–2312. doi: 10.1038/sj.npp.1301089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michelhaugh SK, Lipovich L, Blythe J, Jia H, Kapatos G, Bannon MJ. Mining Affymetrix microarray data for long non-coding RNAs: altered expression in the nucleus accumbens of heroin abusers. J Neurochem. 2011 Feb;116(3):459–466. doi: 10.1111/j.1471-4159.2010.07126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y, Michelhaugh SK, Schmidt CJ, Liu JS, Bannon MJ, Lin Z. Ventral midbrain correlation between genetic variation and expression of the dopamine transporter gene in cocaine-abusing versus non-abusing subjects. Addict Biol. 2011 Oct; doi: 10.1111/j.1369-1600.2011.00391.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannon MJ, Whitty CJ. Age-related and regional differences in dopamine transporter mRNA expression in human midbrain. Neurology. 1997 Apr;48(4):969–977. doi: 10.1212/wnl.48.4.969. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez A, Andollo W, Hearn WL. Analysis of cocaine and metabolites in brain using solid phase extraction and full-scanning gas chromatography/ion trap mass spectrometry. Forensic Sci Int. 1994 May 13;65(3):149–156. doi: 10.1016/0379-0738(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 10.Johnson MM, Michelhaugh SK, Bouhamdan M, Bannon MJ. The transcription factor nurr1 exerts concentration-dependent effects on target genes mediating distinct biological processes. Frontiers Neurosci. 2011 Dec 20;5:135. doi: 10.3389/fnins.2011.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubo S, Kitamura O, Orihara Y, Ogata M, Tokunaga I, Nakasono I. Immunohistochemical diagnosis and significance of forensic neuropathological changes. J Med Invest. 1998 Feb;44(3–4):109–119. [PubMed] [Google Scholar]
- 12.Hecker JG, McGarvey M. Heat shock proteins as biomarkers for the rapid detection of brain and spinal cord ischemia: a review and comparison to other methods of detection in thoracic aneurysm repair. Cell Stress Chaperones. 2011 Mar;16(2):119–131. doi: 10.1007/s12192-010-0224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manning-Bog AB, McCormack AL, Purisai MG, Bolin LM, Di Monte DA. Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration. J Neurosci. 2003 Apr 15;23(8):3095–3099. doi: 10.1523/JNEUROSCI.23-08-03095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagel F, Dohm CP, Bähr M, Wouters FS, Dietz GP. Quantitative evaluation of chaperone activity and neuroprotection by different preparations of a cell-penetrating Hsp70. J Neurosci Methods. 2008 Jun 30;171(2):226–232. doi: 10.1016/j.jneumeth.2008.03.008. [DOI] [PubMed] [Google Scholar]