Abstract
Astrocytes perform critical homeostatic physiological functions in the central nervous system (CNS) and are robustly responsive to injury, inflammation, or infection. We hypothesized that the components of the extracellular matrix (ECM), which are known to vary during development and in response to disease, determine astrocytic responses to injury and inflammation. We examined the response of primary astrocyte cultures grown on different ECM proteins to a mechanical wound (i.e., scratch). ECM substrates selected were laminin (Ln), vitronectin (Vn), fibronectin (Fn) or Tenascin C (TnC). We found that regrowth of the scratch wound was ECM dependent: recovery was arrested on fibronectin (Fn), almost complete on either Vn, Ln, or TnC. To determine whether ECM responses were also influenced by inflammation, we treated ECM plated cultures with interleukin-1β (IL-1β). We found that IL-1β arrested astrocyte growth on Ln, accelerated astrocyte growth on Fn and had no significant effect on astrocyte growth on TnC or Vn. We also determined that blocking β1integrins, the major class of receptors for all ECM proteins tested, prevented the robust response of astrocytes exposed to TnC, Ln and Vn, and also inhibited the robust effect of IL-1β to stimulate astrocyte growth on Fn. In addition, we evaluated downstream targets of integrin signaling, specifically the mammalian target of rapamycin (mTOR), and determined that activation of this pathway contributed to the response of astrocytes grown on TnC, but not on Ln, Vn or Fn. These findings provide new insights into the role of ECM as a source of heterogeneity of glial responses that may have important implications for neuropathological sequelae.
Keywords: Astrocyte, Extracellular matrix (ECM), Integrins, Mtor
1. Introduction
The extracellular microenvironment provides fundamental cues that guide cellular responses. The protein composition of the extracellular matrix (ECM) exhibits regional variation and dynamic changes related to age, injury or disease [1]. These changes in the ECM play an important role in molding central nervous system (CNS) development and influence cellular responses within the adult CNS [2]. For instance, tenascin C (TnC) is a secreted ECM glycoprotein that is expressed prominently during development and down-regulated in the mature CNS. However, TnC levels are up-regulated during neuroinflammation, specifically in response to pro-inflammatory cytokines [3]. Laminins (Ln) and fibronectins (Fn) are highly expressed during regeneration and have been shown to promote regeneration [4]. Specific changes in ECM protein expression, such as the accumulation of Fn or TnC in demyelinated plaques in multiple sclerosis, have also been shown to impede remyelination in this disease [5,6]. Similarly, up-regulation of TnC in plaques of Alzheimer's mouse brain has lead to a suggested role of TnC as an integral part of the inflammatory pathology in this disease [7]. Studies on how ECM influences the function of cells within the CNS have revealed a prominent role for ECM regulation of astrocytic functions [8,9].
Astrocytes are the most abundant cell type in the CNS and provide homeostatic and sentinel functions when they become activated in response to CNS injury, inflammation or disease [9]. Astrocytes have a spectrum of resting and activation states that are influenced by the extracellular environment [9]. This extracellular influence on the activation of astrocytes likely underlies whether astrocytes contribute to CNS pathology or promote CNS recovery [10]. ECM molecules activate intracellular signaling cascades through interactions with transmembrane protein receptors called integrins. Integrins are members of a family of glycoproteins that regulate many cellular behaviors, including proliferation and differentiation [11]. Integrin α and β subunits form heterodimers that endows a range receptor diversity and specificity for ECM proteins. Study on how different ECM molecules influence the behavior of astrocytes is expected to enable a better understanding on the role(s) of astrocytes in pathological settings.
Mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase and mTOR signaling has been implicated in regulation of cell growth, proliferation, motility, survival, as well as promoting protein synthesis, and transcriptional activation [12–14]. mTOR has two distinct complexes, mTORC1 and mTORC2, that regulate distinct signal transduction pathways. In the CNS, mTOR has been shown to enhance protein synthesis and promote neural cell differentiation, therefore, promoting an importance of mTOR signaling in the health CNS. Rapamycin is an inhibitor of mTOR activation, specifically mTORC1, and has been shown to attenuate glial cell activation [15]. Integrin signaling has been reported to engage a variety of downstream targets in the mTOR pathway. Given that astrocytes can exhibit disparate reactions to specific ECM proteins [16], we reasoned that activation of mTOR signaling may be one mechanism underlying these differences.
In this study, we hypothesized that the ECM substrate would impact the response of astrocytes to a mechanical injury and an inflammatory stimulus. For these experiments we used the canonical pro-inflammatory cytokine, interleukin-1β (IL-1β) because it is known to activate astrocytes and is prominently produced in a variety of neural injury settings [17]. We report that astrocytic behavior was dramatically influenced by the ECM protein substrate and that this determined the activation and contribution of mTOR signaling to these astrocytic responses. Together, these finding provide new information on the regulation of astrocytes that may serve to define the extracellular cues that contribute to astrocytic heterogeneity in responses to CNS injury and inflammation.
2. Material and methods
2.1. Primary glial cultures
Cultures were generated from cerebral cortices of neonatal C57BL/6 mouse pups (P0-P3), using a neural tissue dissociation kit (Miltenyi Biotec) [18]. Cells were plated into T175 flasks for up to 2 weeks before detachment using trypsin (Sigma) and then re-plated onto coverglass coated with Ln (10 μg/μL; Sigma–Aldrich), TnC, (100 μg/μL; EMD Millipore), Fn (10 μg/μL; Sigma–Aldrich), or Vn (25 μg/μL; Sigma–Aldrich) in 24-well plates. The purity of each culture system was consistent with previous reports of 90–97% GFAP + cells [19], as verified by immunocytochemistry (ICC) for GFAP for astrocytes (1:1000, Chemicon), and, Iba-1 for microglia (1:1000, WAKO).
2.2. Scratch Injury model
Confluent astrocyte monolayers were scratched once across the diameter of the coverglass using a sterile P200 pipette tip to produce an injury ~600 μm in diameter [20]. Cells were fixed at different timepoints and ICC performed. IL-1β (10 ng/ml; Peprotech) was added to the cultures as a prototypic inflammatory stimulus, as previously described [21]. The role of β1integrin was tested using a function blocking antisera (Ha2/5; 5 μg/mL) and compared with control, isotype-matched, IgG (both from BD Pharmigen). Effects of mTOR were assessed using rapamycin (5 μg; Santa Cruz Biotech) dissolved in DMSO (10 nM, Sigma). Scratch injuries were measured perpendicular to the longitudinal axis of the scratch at a minimum of three points spanning from the edges of the scratch using image analysis software (Northern Eclipse software; Empix Imaging). Western blotting was performed on cultures grown in 6 well plates coated with Ln, TnC, Fn, or Vn, and scratched 4 times per well using a sterile P200 pipette tip as described above.
2.3. Immunocytochemistry (ICC)
ICC was performed as previously described [19]. Cultures were fixed in 4% paraformaldehyde, washed and then incubated with primary fluorescent conjugated antisera for Glial Fibrillary Acidic Protein (GFAP-Cy3; 1:1000, Sigma). 4′,6-diamidino-2-phenylindole (DAPI) was added after incubation to identify nuclei. Immunoreactivity was visualized using a fluorescent microscope (Olympus, IX71) and image analysis software (Empix Imaging).
2.4. Western blot analysis
Lysates from primary cultures grown on Ln, TnC, Fn, or Vn were prepared as previously described [19]. Cells were scraped into PBS, after 0, 4, or 24 h after treatment, centrifuged (6000 × g), the pellet was resuspended in RIPA buffer [Triton X-100, NaCl, Tris–HCl base, Deoxycholic Acid, SDS, H2O] and mechanically dissociated using a motorized pellet pestle (Fisher Scientific). Each sample was prepared with 2× loading buffer [Tris–HCl, Glycerol, SDS, H2O, BPB, DTT] and 15 μg of total protein was loaded into a graduated 5–15% acrylamide gel (Biorad). After PAGE, protein was transferred onto PVDF membrane (Thermo Scientific). Blots were blocked and then incubated overnight in primary antisera. Primary antisera included pAktS473 (1:1000, Cell Signaling), panAkt (1:1000, Cell Signaling) S6KpThr389 (1:1000, Cell Signaling), p70S6Kinase (1:1000, Cell Signaling), and β-actin (1:2000, Sigma). Secondary antisera were HRP-conjugated anti-mouse (1:10,000, Vector Labs), or HRP-conjugated anti-rabbit (1:10,000, Vector Labs) for detection using ECL reagent (GE Biosciences). Exposed films were developed and quantified relative to β-actin loading for each sample [19].
2.5. Statistics
Experiments were performed in quadruplicate replicates and repeated in triplicate. Comparisons between treatments on the same substrate were made using t-tests, or one-way ANOVA to evaluate changes over time. Inter-group comparisons for differences on each ECM over time, or treatment were made two-way ANOVA. Data are presented as mean ± SEM. The null hypothesis for all experiments was P < 0.05.
3. Results
3.1. Astrocyte response to scratch is ECM dependent
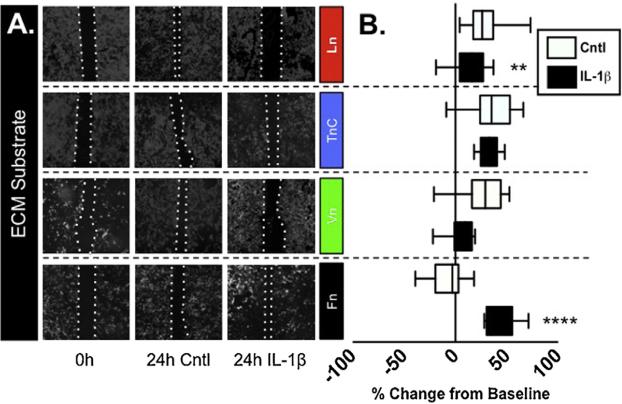
We hypothesized that the composition of the ECM may influence the behavior of astrocytes and their ability to recover from a scratch injury. This idea was based on previous studies that have demonstrated the utility of the scratch assay to explore the behavior of astrocytes [21,22], and additional studies that explored the impact of ECM on astrocyte phenotype [9]. We examined the response of astrocyte cultures to a scratch injury by assessing rate and extent of recovery. Primary murine glial cultures were grown on cover-slips that had been coated with one of the following ECM proteins (Ln, TnC, Vn, or Fn), grown to confluence, scratched and then recovery assessed 4 or 24 h later. Over the 24 h incubation, astrocytes grown on Ln, TnC or Vn exhibited significant recovery (Fig. 1). In contrast, astrocytes grown on Fn exhibited only minimal wound closure that was significantly less than all other ECM substrates tested (P < 0.0001; Fig. 1B).
Fig. 1. Astrocytic response to scratch response and IL-1β is determined by ECM substrate.
(A) Mixed glial cultures grown on cover-slips were coated with Ln, TnC, Vn, or Fn and then scratched. Scratch widths were measured after initial scratch, 4 h and 24 h. Pictures of immunocytostaining are depicted after the initial scratch and 24 h with or without IL1β by staining for the astrocytic marker, GFAP and DAPI. (B) The wound recovery was quantified to compare astrocyte wound recovery on different ECM substrates with and without the addition of IL-1β. Data are presented as box-and-whisker plots depicting the treatment median value and the interquartile range of outcomes for each treatment combination, representing n = 4/treatment repeated in triplicate. (**P < 0.01; ****P < 0.0001).
3.2. Astrocyte response to IL-1β is ECM dependent
Previous work suggests that exposure to IL-1β impairs the response of human astrocytes to wound recovery [21]. To determine whether the ECM composition influenced the effect of IL-1β, we treated cultures with IL-1β and assessed recovery on the different substrates. Consistent with previous work, treatment with IL-1β arrested the recovery of astrocytes grown on Ln (P > 0.05 vs. 0 h), which differed significantly from the robust recovery seen on Ln under control conditions (P < 0.001 vs. 24 h control; Fig. 1). When treated with IL-1β, astrocytes grown on Vn. exhibited a trend of reduced recovery but the magnitude of this effect was not statistically significant (P < 0.07 vs. 24 h control; P < 0.5 vs. 0hr control). In contrast, cultures grown on TnC, maintained their strong response in the presence of IL-1β. Most interestingly, astrocytes on Fn exhibited a minimal growth response under control conditions, but IL-1β treatment, induced a robust and significant recovery response (P < 0.001 vs. Cntl 24 h).
3.3. ECM-specific β1 integrin-mediated astrocyte responses
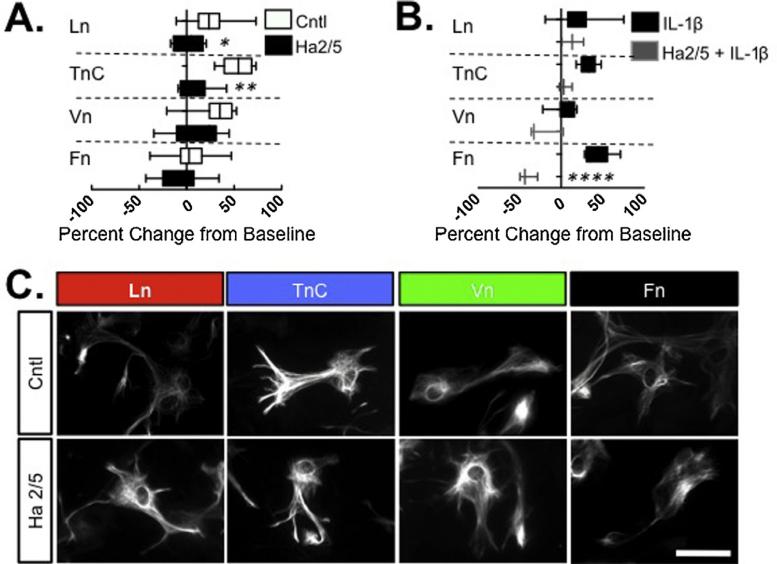
To identify potential mediators of the astrocyte responses to ECM substrates, we first examined β1integrins as the principal class of ECM receptors. To evaluate the contribution of β1integrin to astrocyte wound healing responses, we used a function-blocking antibody, Ha2/5. Astrocytes grown on TnC, Ln, or Vn exhibited an impaired scratch repair response when β1integrin was blocked, compared to IgM control-treated cultures on the same substrates (P < 0.001, TnC; P < 0.005, Ln; P < 0.04, Vn) (Fig. 2A). The contribution of β1integrin to astrocyte responses to Fn were difficult to assess as astrocytes grown on Fn exhibited only a minimal wound repair response that was not altered by treatment with Ha2/5 (Fig. 2A). However, when astrocytes grown on Fn were treated with IL-1β, which evoked a robust recovery, this IL-1β effect was completely blocked by Ha2/5 (P < 0.0001 vs. control antibody; Fig. 2B). In addition, Ha2/5 also inhibited astrocyte growth on TnC in the presence of IL-1β, (P < 0.007; Fig. 2B).
Fig. 2. Inhibition of astrocyte scratch response to ECM substrates by β1 integrin blockade.
(A) Quantification of 24 h wound recovery of mixed glial cultures were grow on cover-slips coated with Ln, TnC, Vn, or Fn and stimulated with a mechanical scratch with either with® integrin blocking antibody Ha2/5 or control IgM. Analysis of variance was used to determine whether Ha2/5 affected wound injury responses on the different ECM substrates. (B) Primary cultures were treated with Ha2/5 or Ha2/5 and IL-1β and 24 hour scratch wound recovery measured on different ECM substrates, as indicated. Following each treatment, wound recovery was measured following each treatment relative to initial wound diameter (at 0 hour) and comparisons made to controls for each treatment. (*P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001). (C) Representative photomicrographs of GFAP + astrocytes from each culture condition, as indicated, showing comparable gross morphology on each substrate with or without Ha2/5 treatment. Scale bar = 30 μm.
3.4. ECM selective activation of mTOR signaling
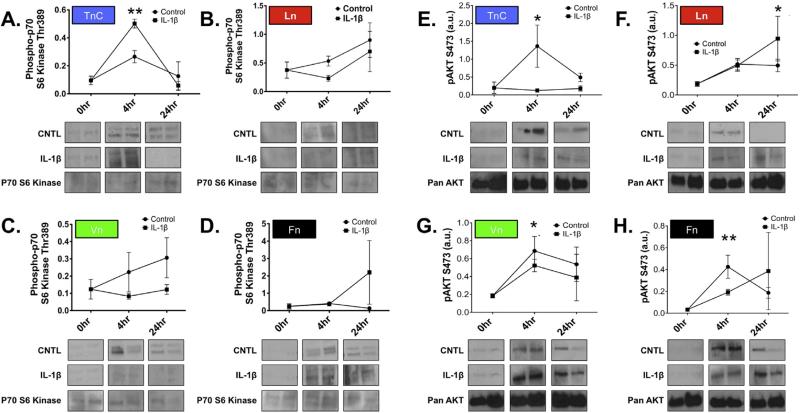
We next examined how the ECM affects putative downstream signaling of integrin receptors, specifically mTOR as an effector of cellular responses [11,12]. To determine whether mTOR activity contributed to the astrocytic wound response on different ECM substrates, we assayed astrocyte cultures by western blotting for differential activation of mTOR, specifically, phosphorylation of S6 kinase (S6k; Thr389), which is preferentially activated by mTORC1, and phosphorylation of PKB (Akt; Ser473), which is preferentially activated by mTORC2 [23].
Analysis of S6k activation in astrocytes grown on each different ECM substrate revealed a differential in response to scratch or IL-1β that depended on the substrate (Fig. 3A–D). Astrocytes grown on TnC revealed significant mTORC1 activation, in contrast, astrocytes grown on Ln, Vn, or Fn exhibited no significant change. Interestingly, the pattern of S6k activation was affected by IL-1β treatment in astrocytes grown on TnC (P < 0.006; 4hr IL-1β vs. 4 h control). We also found that S6k activation in astrocytes grown on Ln, Vn or Fn was not significantly modified by IL-1β treatment. There were no significant differences in levels of S6kinase in response to scratch on any substrate tested (Fig. 3A–D).
Fig. 3. The influence of ECM substrate on mTORC activation after scratch.
Time course analysis of S6 Kinase activation (pThr389; A–D) and Akt (pS473; E–H) following scratch wound in astrocytes grown on TnC (A and E), Ln (B and F), Vn (C and G), or Fn (D and H) with or without treatment with IL-1β. Representative immunoblots for activated S6 Kinase (pThr389) from each time point (as listed on the abscissa above each column) for Control (upper row) and IL-1β (lower row) for each ECM substrate, as indicated. Representative western blots for S6kinase from each time point show no significant change in overall levels of enzyme expression. (E–H) Representative western blots of time course analysis for pAkt activation (pS473) after scratch wound with or without treatment with IL-1β in primary astrocyte cultures. Immunoblotting for pan-Akt from each time point show no significant change in overall expression Akt expression over this time course. Quantification of western blots bands were corrected for sample loading relative to β-actin labeling of same blots (data presented as arbitrary units, a.u.) S6 Kinase data represent n = 8/ treatment/time point. (*P = 0.006) and pAkt data represent n = 8/ treatment/time point. (*P < 0.02, TnC, Ln; P < 0.04, Vn; **P < 0.005, Fn).
We then examined whether the activity of mTORC2 was also differentially regulated by ECM substrate, scratch injury, and inflammatory stimulation, using phosphorylation of Akt (ser473) [12] (Fig. 3E–H). Astrocytes grown on TnC showed significant Akt activation over time after the scratch (P < 0.02), but this effect was absent following treatment with IL-1β. Akt was also activated in astrocytes grown on Ln after scratch (P = 0.02); however, IL-1β did not modify Akt activation. Astrocytes grown on Vn or Fn also exhibited robust Akt activation in response to scratch injury (Vn, P < 0.04; Fn, P = 0.005), and IL-1β did not significantly modify this activation. We also looked at overall Akt levels, and saw no significant difference. There were no significant differences in overall Akt expression in response to scratch on any substrate tested (pan-Akt; Fig. 3E–H).
3.5. ECM specific effects of rapamycin on astrocyte scratch wound response
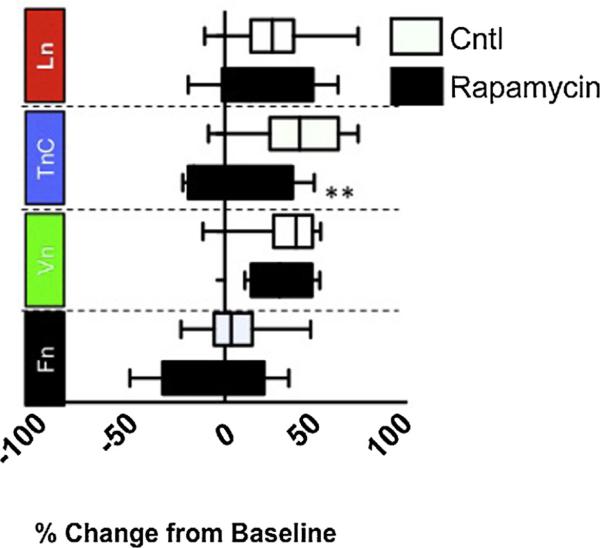
To determine whether these changes in mTOR activation on different ECM substrates functionally contributed to the differential wound responses we observed, we next examined how the ECM affects mTOR as an effector of cellular responses [11,12]. mTOR has two different complexes: mTORC1, which is inhibited by rapamycin; and mTORC2, which is not inhibited by rapamycin [12]. To determine whether mTOR activity contributed to the astrocytic wound response on ECM substrates, we treated cultures grown on different ECM substrates with rapamycin. Analysis of scratch wound recovery revealed that rapamycin blocked the response of astrocytes grown on TnC (P < 0.05) but had no effect on astrocytes grown on Vn, Fn or Ln (Fig. 4).
Fig. 4. Differential modulation of astrocyte scratch responses by rapamycin is ECM dependent.
Astrocytes grown on different ECM substrates were scratched and treated with either rapamycin (5 μg/mL) or vehicle. Measurements of scratch wound recovery after 24 h were compared with control-treated cultures on the same ECM substrate. Rapamycin was found to only impair scratch wound recovery of astrocytes grown on TnC (*P < 0.005).
4. Discussion
In this study, established the following hierarchical relationship towards improved wound regrowth on ECM substrates: TnC > Vn > Ln > Fn. Previous work has shown that the proinflammatory cytokine, IL-1β, can inhibit the proliferative and migratory responses of astrocytes in an in vitro scratch wound assay [21]. We also determined that the response of astrocytes to IL-1β was context dependent: wound-healing responses varied with the ECM substrate and ECM also impacted the response to IL-1β. Stimulation by IL-1β, also altered the following hierarchical relationship of ECM on wound regrowth: Fn > TnC > Ln > Vn. We also discovered that IL-1β arrested the astrocyte wound healing response on Ln, but markedly promoted the response on Fn. These findings complement previous observations [21,24] on ECM effects on astrocyte responses to other cytokines, including TNF [16]. Together, these data point to ECM as factors influencing the heterogeneity of astrocyte responses.
As integrins are the major class of ECM receptors, we next examined the role of β1integrins in mediating ECM dependent effects of astrocytes [4]. Interestingly, β1integrin blockade inhibited the recovery of astrocytes grown on Ln, TnC, and Vn suggesting an important role in astrocyte responses on these substrates. In addition, β1integrin blockade also inhibited the recovery of astrocyte grown on Fn when stimulated with IL-1β.
mTOR signaling has been implicated in a variety of important pathways, including autophagy, cell proliferation, cell survival, and cytokine production by astrocytes [4]. We found that mTOR played an important part in the ECM specific effects as evidenced by western blot analyses and the scratch injury response of astrocytes grown on TnC to rapamycin. These data suggest mTORC1 signaling pathway mediated the enhanced activity of astrocytes grown on TnC; but had little effect on astrocytes grown on Ln, Vn, or Fn, suggesting signaling through mTORC2, the rapamycin-independent complex. These results indicate that the failure of rapamycin to affect astrocytes grown on Ln or Vn may have been due to a specific bias toward mTORC2 signaling. While all substrates had significant activation of pAKTs473. This differential signaling resulting from the ECM suggests important differences in regulation of astrocytic responsiveness. Astrocytes grown on TnC, but not Vn, Ln, or Fn exhibited significant S6k activation over time, suggesting ECM-dependent differential regulation of mTORC1 signaling. Our data indicate a propensity of reactive astrocytes to respond to specific inflammatory signals that is directly regulated by the ECM. Future studies exploring the potential hierarchy among these ECM substrates may further define how ECM regulated heterogeneity impacts astrocyte function and reactivity and the production of factors that contributes to neuroinflammation and pathology [25,26].
We propose that the disparate reactions of astrocytes on different ECM substrates, reveals important new information about the roles of astrocytes in the CNS. In this study, we chose the ECM substrates on account of their highly dynamic expression levels during neurological diseases. For instance, the ECM composition in and around demyelinated lesions in multiple sclerosis (MS) varies with the stage of the lesion [6]: where TnC is down-regulated in active lesions; Ln, Fn and Vn are up-regulated in active lesions [5]. These ECM components have been reported to impair oligodendrocyte progenitor differentiation, and thus are considered impediments to remyelination in MS [27]. However, the presence of reactive astrocytes in all myelin lesions in MS suggests that differences in ECM content would also impact the role of astrocytes in these settings. Future studies addressing this issue may reveal how ECM regulates astrocyte functions to impact other neural cell types, including oligodendrocytes [9,19] microglia or neurons [28], and expand our growing understanding of astrocytes as active participants in neuropathology [9].
5. Conclusions
These findings complement the growing awareness that astrocyte diversity may be strongly influenced by external stimuli, including the ECM. They also provide a new perspective on the roles of the microenvironment as a significant factor influencing the diversity of astrocyte functions in CNS physiology and neuropathology.
HIGHLIGHTS.
Astrocyte wound recovery to mechanical injury varied on different ECM substrates.
Astrocyte wound responses were differentially affected by IL-1β.
β1 integrin mediated ECM dependent astrocyte wound responses.
Rapamycin blocked wound responses of astrocytes grown on TnC.
ECM changes in disease may impact astrocytic function.
Acknowledgments
We thank Anthony Sacino and Stephanie Reeves for technical assistance. We are grateful to Dr. Robert H. Miller and Dr. Holly Colognato for their helpful suggestions. This work was supported by the National Institutes of Health(NS0783392 and NS087578, SJC), National Multiple Sclerosis Society(RG5001-A-3, SJC), and the Connecticut Regenerative Medicine Research Grants Program(SCA-06-011, SJC).
Abbreviations
- ECM
extracellular matrix
- Ln
laminin
- Vn
vitronectin
- Fn
fibronectin
- TnC
tenascin C
- IL-1β
interleukin-1β
- mTOR
mamalliam target of rapamycin
References
- 1.Lau LW, Cua R, Keough MB, Haylock-Jacobs S, Yong VW. Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat. Rev. Neurosci. 2013;14:722–729. doi: 10.1038/nrn3550. [DOI] [PubMed] [Google Scholar]
- 2.Frischknecht R, Chang KJ, Rasband MN, Seidenbecher CI. Neural ECM molecules in axonal and synaptic homeostatic plasticity. Prog. Brain Res. 2014;214:81–100. doi: 10.1016/B978-0-444-63486-3.00004-9. [DOI] [PubMed] [Google Scholar]
- 3.Gaudet AD, Popovich PG. Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp. Neurol. 2014;258:24–34. doi: 10.1016/j.expneurol.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao C, Fancy SP, Franklin RJ, ffrench-Constant C. Up-regulation of oligodendrocyte precursor cell alphaV integrin and its extracellular ligands during central nervous system remyelination. J. Neurosci. Res. 2009;87:3447–3455. doi: 10.1002/jnr.22231. [DOI] [PubMed] [Google Scholar]
- 5.Gutowski NJ, Newcombe J, Cuzner ML. Tenascin-R and C in multiple sclerosis lesions: relevance to extracellular matrix remodelling. Neuropathol. Appl. Neurobiol. 1999;25:207–214. doi: 10.1046/j.1365-2990.1999.00176.x. [DOI] [PubMed] [Google Scholar]
- 6.Stoffels JM, de Jonge JC, Stancic M, Nomden A, van Strien ME, Ma D, Siskova Z, Maier O, Ffrench-Constant C, Franklin RJ, Hoekstra D, Zhao C, Baron W. Fibronectin aggregation in multiple sclerosis lesions impairs remyelination. Brain. 2013;136:116–131. doi: 10.1093/brain/aws313. [DOI] [PubMed] [Google Scholar]
- 7.Xie K, Liu Y, Hao W, Walter S, Penke B, Hartmann T, Schachner M, Fassbender K. Tenascin-C deficiency ameliorates Alzheimer's disease-related pathology in mice. Neurobiol. Aging. 2013;34:2389–2398. doi: 10.1016/j.neurobiolaging.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Harlow DE, Macklin WB. Inhibitors of myelination: ECM changes, CSPGs and PTPs. Exp. Neurol. 2014;251:39–46. doi: 10.1016/j.expneurol.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nash B, Thomson CE, Linington C, Arthur AT, McClure JD, McBride MW, Barnett SC. Functional duality of astrocytes in myelination. J. Neurosci. 2011;31:13028–13038. doi: 10.1523/JNEUROSCI.1449-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milner R, Campbell IL. The integrin family of cell adhesion molecules has multiple functions within the CNS. J. Neurosci. Res. 2002;69:286–291. doi: 10.1002/jnr.10321. [DOI] [PubMed] [Google Scholar]
- 12.Alessi DR, Pearce LR, Garcia-Martinez JM. New insights into mTOR signaling: mTORC and beyond. Sci. Signal. 2009;(2):pe27. doi: 10.1126/scisignal.267pe27. [DOI] [PubMed] [Google Scholar]
- 13.Dello Russo C, Lisi L, Feinstein DL, Navarra P. mTOR kinase, a key player in the regulation of glial functions: relevance for the therapy of multiple sclerosis. Glia. 2013;61:301–311. doi: 10.1002/glia.22433. [DOI] [PubMed] [Google Scholar]
- 14.Li CY, Li X, Liu SF, Qu WS, Wang W, Tian DS. Inhibition of mTOR pathway restrains astrocyte proliferation, migration and production of inflammatory mediators after oxygen-glucose deprivation and reoxygenation. Neurochem. Int. 2015 doi: 10.1016/j.neuint.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Norsted Gregory E, Delaney A, Abdelmoaty S, Bas DB, Codeluppi S, Wigerblad G, Svensson CI. Pentoxifylline and propentofylline prevent proliferation and activation of the mammalian target of rapamycin and mitogen activated protein kinase in cultured spinal astrocytes. J. Neurosci. Res. 2013;91:300–312. doi: 10.1002/jnr.23144. [DOI] [PubMed] [Google Scholar]
- 16.Faber-Elman A, Lavie V, Schvartz I, Shaltiel S, Schwartz M. Vitronectin overrides a negative effect of TNF-alpha on astrocyte migration. FASEB J. 1995;9:1605–1613. doi: 10.1096/fasebj.9.15.8529840. [DOI] [PubMed] [Google Scholar]
- 17.Chakraborty S, Kaushik DK, Gupta M, Basu A. Inflammasome siging at the heart of central nervous system pathology. J. Neurosci. Res. 2010;(88):1615–1631. doi: 10.1002/jnr.22343. [DOI] [PubMed] [Google Scholar]
- 18.Crocker SJ, Frausto RF, Whitton JL, Milner R. A novel method to establish microglia-free astrocyte cultures: comparison of matrix metalloproteinase expression profiles in pure cultures of astrocytes and microglia. Glia. 2008;56:1187–1198. doi: 10.1002/glia.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore CS, Milner R, Nishiyama A, Frausto RF, Serwanski DR, Pagarigan RR, Whitton JL, Miller RH, Crocker SJ. Astrocytic tissue inhibitor of metalloproteinase-1 (TIMP-1) promotes oligodendrocyte differentiation and enhances CNS myelination. J. Neurosci. 2011;31:6247–6254. doi: 10.1523/JNEUROSCI.5474-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robel S, Bardehle S, Lepier A, Brakebusch C, Gotz M. Genetic deletion of cdc42 reveals a crucial role for astrocyte recruitment to the injury site in vitro and in vivo. J. Neurosci. 2011;31:12471–12482. doi: 10.1523/JNEUROSCI.2696-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John GR, Chen L, Rivieccio MA, Melendez-Vasquez CV, Hartley A, Brosnan CF. Interleukin-1beta induces a reactive astroglial phenotype via deactivation of the Rho GTPase-Rock axis. J. Neurosci. 2004;24:2837–2845. doi: 10.1523/JNEUROSCI.4789-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishio T, Kawaguchi S, Yamamoto M, Iseda T, Kawasaki T, Hase T. Tenascin-C regulates proliferation and migration of cultured astrocytes in a scratch wound assay. Neuroscience. 2005;132:87–102. doi: 10.1016/j.neuroscience.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 23.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 24.John GR, Simpson JE, Woodroofe MN, Lee SC, Brosnan CF. Extracellular nucleotides differentially regulate interleukin-1beta signaling in primary human astrocytes: implications for inflammatory gene expression. J. Neurosci. 2001;21:4134–4142. doi: 10.1523/JNEUROSCI.21-12-04134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milner R, Campbell IL. Increased expression of the beta4 and alpha5 integrin subunits in cerebral blood vessels of transgenic mice chronically producing the pro-inflammatory cytokines IL-6 or IFN-alpha in the central nervous system. Mol. Cell. Neurosci. 2006;33:429–440. doi: 10.1016/j.mcn.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penkowa M, Giralt M, Lago N, Camats J, Carrasco J, Hernandez J, Molinero A, Campbell IL, Hidalgo J. Astrocyte-targeted expression of IL-6 protects the CNS against a focal brain injury. Exp. Neurol. 2003;181:130–148. doi: 10.1016/s0014-4886(02)00051-1. [DOI] [PubMed] [Google Scholar]
- 27.O'Meara RW, Michalski JP, Kothary R. Integrin signaling in oligodendrocytes and its importance in CNS myelination. J. Signal Transduction. 2011:354091. doi: 10.1155/2011/354091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thalhammer A, Cingolani LA. Cell adhesion and homeostatic synaptic plasticity. Neuropharmacology. 2014;78:23–30. doi: 10.1016/j.neuropharm.2013.03.015. [DOI] [PubMed] [Google Scholar]