Abstract
Botulinum neurotoxins (BoNTs) may affect the excitability of brain circuits by inhibiting neurotransmitter release at central synapses. There is evidence that local delivery of BoNT serotypes A and E, which target SNAP-25, a component of the release machinery specific to excitatory synapses, can inhibit seizure generation. BoNT serotype B (BoNT/B) targets VAMP2, which is expressed in both excitatory and inhibitory terminals. Here we assessed the effects of unilateral intrahippocampal infusion of BoNT/B in the rat on intravenous pentylenetetrazol (PTZ) seizure thresholds, and on the expression of spontaneous behavioral and electrographic seizures. Infusion of BoNT/B (500 and 1000 unit) by convection-enhanced delivery caused a reduction in myoclonic twitch and clonic seizure thresholds in response to intravenous PTZ beginning about 6 days after the infusion. Handling-evoked and spontaneous convulsive seizures were observed in many BoNT/B-treated animals but not in vehicle-treated controls. Spontaneous electrographic seizure discharges were recorded in the dentate gyrus of animals that received local BoNT/B infusion. In addition, there was an increased frequency of interictal epileptiform spikes and sharp waves at the same recording site. BoNT/B treated animals also exhibited tactile hyperresponsivity in comparison with vehicle-treated controls. This is the first demonstration that BoNT/B causes a delayed proconvulsant action when infused into the hippocampus. Local infusion of BoNT/B could be useful as a focal epilepsy model.
Keywords: botulinum neurotoxin, seizure, epilepsy, hippocampus, convection-enhanced delivery, pentylenetetrazol
Introduction
Botulinum neurotoxins (BoNT), a group of proteins produced by anaerobic bacteria of the Clostridium genus, are the most potent known poisons (Johnson, 1999; Rossetto et al., 2006). The toxins cause paralysis of neuromuscular and autonomic function by blocking cholinergic neurotransmission. Upon binding to motor nerve endings, BoNT molecules are internalized by receptor-mediated endocytosis and act in the neuronal cytosol as metalloendoproteases to cleave polypeptides of the SNARE complex that are required for exocytotic release of acetylcholine (Humeau et al., 2000; Simpson, 2004). The A and E serotypes irreversibly cleave synaptosomal-associated protein of 25 kDa (SNAP-25) whereas the B serotype cleaves synaptobrevin 2 (vesicle-associated membrane protein 2; VAMP2). The SNARE complex is also involved in the release of neurotransmitters other than acetylcholine, including glutamate and GABA. In comparison with the wealth of information on BoNT actions at peripheral cholinergic synapses, the effects of BoNTs on synaptic transmission in the central nervous system remain to be fully characterized. Nevertheless, there is evidence that BoNTs can inhibit neurotransmitter release from nerve terminals in the brain and spinal cord (Bigalke et al., 1981; Ashton and Dolly, 1988; McMahon et al., 1992; Capogna et al., 1997; Costantin et al., 2005; Caleo and Schiavo, 2009). For BoNTs A and E that act on SNAP-25, the toxins are more effective in blocking the release of the excitatory neurotransmitter glutamate than the inhibitory neurotransmitter GABA (Verderio et al., 2004; Matteoli et al., 2008); resistance of GABA release is believed to be due to the lack of SNAP-25 in the terminals of GABAergic neurons (Verderio et al., 2007; Garbelli et al., 2008; Benagiano et al., 2011).
Seizure susceptibility is often conceptualized as due to a pathological increase in glutamate-mediated excitation or to deficient GABA-mediated inhibition in the seizure focus and in seizure propagation pathways (Bouilleret et al., 2000; Scharfman, 2007). Selective inhibition of glutamate-mediated excitation by BoNTs could potentially cause a reduction in seizure susceptibility. In fact, in line with the ability of BoNT/E to selectively inhibit glutamate release, it has been demonstrated that intrahippocampal delivery of this BoNT serotype into the CA1 and CA3 regions of the hippocampus can inhibit epileptiform discharges and behavioral seizure activity in the kainic acid and kindling models of epilepsy (Costantin et al., 2005; Antonucci et al., 2009). Unlike SNAP-25, which is not expressed in inhibitory terminals, VAMP2, the target of BoNT/B, is colocalized with the vesicular GABA transporter in GABAergic axon terminals of rat cerebral cortex (Bragina et al., 2007; 2010). This suggests that unlike BoNT/A and BoNT/E, BoNT/B may not be selective for glutamatergic neurons. Nevertheless, there is evidence that BoNT/B can inhibit seizure activity when locally delivered into the amygdala (Gasior et al., 2013). In the present study, we sought to assess the actions of BoNT/B on seizure susceptibility following intrahippocampal delivery. We used convection-enhanced delivery (CED), which provides a means of distributing proteins, such as BoNTs, uniformly throughout a localized brain region (Rogawski, 2009). In contrast to previous studies where BoNTs had anti-seizure effects, in the hippocampus we found that BoNT/B caused a delayed reduction in seizure threshold as well as spontaneous epileptiform discharges and behavioral seizures, demonstrating that BoNT/B is proconvulsant in this brain region.
Experimental Procedures
Animals
Male Sprague-Dawley rats (Taconic Farms, Germantown, NY) weighing 225–250 g were used. Animals were randomly allocated to the test groups. A total of 24 rats were used for the intravenous PTZ test and 11 for electroencephalogram (EEG) recording. Animals were housed in groups of two, except for animals with permanent implants, which were housed individually. All rats were kept under controlled environmental conditions (22–26 °C; 40–50% humidity) with a 12-h light/dark cycle. Wood chips were used in all cages. In all rats, the body weight was recorded over the course of the experiment. Experiments were performed between 0900 and 1200. Animals were allowed at least 30 min to acclimate to the experimental room before testing. The animal facilities were fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All studies were performed under protocols approved by the Institutional Animal Care and Use Committee in strict compliance with the Guide for the Care and Use of Laboratory Animals of the National Research Council (National Academy Press, Washington, DC; http://www.nap.edu/readingroom/books/labrats/).
Pentylenetetrazol seizure threshold test
To determine seizure thresholds before and at times after administration of BoNT/B or vehicle solution, rats underwent the timed intravenous pentylenetetrazol (PTZ) seizure threshold test as described previously (Löscher, 2009; Rattka et al., 2011; Bröer et al., 2012). In brief, PTZ (8 mg/ml in saline) was infused at a constant rate of 1.0 ml/min via a 24-gauge needle inserted into the lateral tail vein of conscious, freely moving (unrestrained) rats using a 1 ml syringe (Becton, Dickinson & Co., Franklin Lakes, NJ, USA) mounted on an infusion pump (Model ‘11’ plus syringe pump; Harvard Apparatus, Holliston, MA). The needle was secured to the tail vein with a piece of adhesive tape and the animal was permitted to move inside a Makrolon® cage type III during testing. Animals always exhibited a sequence of seizure signs consisting of a myoclonic jerk (twitch) followed by clonus. The infusion was terminated immediately following the onset of the first clonic seizure, and the needle was removed from the tail vein. Rats were closely observed until they resumed normal behavior; all seizures or other abnormal behaviors occurring after termination of the infusion were recorded. Threshold values (mg/kg) were calculated from the times to the first myoclonic twitch and first clonic seizure according to the following formula: (infusion duration [s] × infusion rate [ml/min] × PTZ concentration [mg/ml] ×1000) / (60 s × weight of rat [g]). The intravenous PTZ test can be repeatedly performed in the same rat at an interval of 48 h up to 8 times with a constant threshold, allowing the assessment of changes in threshold over time (Pollack and Shen, 1985; Löscher, 2009; Bankstahl et al., 2012). In the present study, the number of intravenous PTZ seizure threshold determinations was limited to a maximum of 4 in each rat, with an interval of at least 48 h between testing. Threshold determinations were made 3 or 4 days prior to intrahippocampal BoNT/B or vehicle treatment and 1, 2, 3, 4, 7 and 14 days after treatment; not all time points were assessed in each rat. One rat died during the initial threshold determination prior to intrahippocampal infusion and was excluded from the analysis.
BoNT/B solution
The BoNT/B solution used in these experiments was Myobloc® (rimabotulinumtoxinB) at a concentration 100-fold the commercial formulation (500 unit (U)/μl) kindly provided by Solstice Neurosciences (South San Francisco, CA, USA). 1 U corresponds to the calculated median LD50 in mice as determined by Solstice; the specific activity is in the range of 70–130 U/ng. The neurotoxin, which exists in complex with noncovalently associated hemagglutinin and nonhemagglutinin proteins, is 70% nicked (intra-molecular disulfide bonds cleaved, leading to activation of the toxin) and fully unstripped (no envelope proteins removed). The neurotoxin complex is formulated with 0.05% human serum albumin, 0.01 M sodium succinate, 0.1 M NaCl in distilled water with HCl added to adjust the pH to 5.6. The vehicle solution contained the same excipients without the neurotoxin complex.
Acute intrahippocampal infusion
All surgical procedures were performed aseptically. Rats were anesthetized with ketamine (60 mg/kg, i.p.) and dexmedetomidine (0.5 mg/kg, i.p.). The animals were mounted in a stereotactic frame and a burr hole was made over the left hippocampus to target the dentate gyrus at the following coordinates with respect to bregma (Paxinos and Watson, 2007): AP −4.00 mm; ML +2.00 mm; and DV −3.5 mm. CED infusion was performed using a homemade 1 mm-step cannula constructed according to a design kindly provided by Dr. Adrian P. Kells (University of California, San Francisco). The stepped cannula design prevents backflow along the cannula (Yin et al., 2010). Cannulas and steps consisted of fused silica (Polymicro Technologies, Phoenix, AZ). The tip had an outer diameter of 164 μm and an inner diameter of 102 μm. Steps were glued to the cannula in order to achieve a sharp transition from a wider to a narrower tip. BoNT/B or vehicle solution was infused at a rate of 0.5 μl/min for a total volume of 1 or 2 μl. After completing the infusion, the cannula was left in place for 1 min to avoid backflow along the track. The cannula was removed from the brain and tested for patency by releasing a small additional drop of solution. After suturing the scalp wound, anesthesia was reversed with the α2 antagonist atipamezole (1 mg/kg, i.p.) and animals were closely observed for any behavioral alterations after recovering from anesthesia.
Permanent electrode-infusion assembly implantation and intrahippocampal infusion
Rats were anesthetized as for acute intrahippocampal infusion and were implanted with a permanent guide cannula-electrode assembly (Plastics One, Roanoke, VA), consisting of a 22-gauge, 2 mm-long polyaryletheretherketone (PEEK) tube with two 0.23-mm-diameter stainless steel electrode wires attached diametrically opposed at the periphery. The electrode wires were polyamide-insulated except for the 0.5 mm most distal extent; the tips of the wires were separated by 0.5 mm, and they projected 1 mm past the end of the guide cannula. The guide cannula-electrode assembly was fixed to a threaded central plastic pedestal. The electrode wires passed to a second threaded pedestal with pin connectors to allow a connection with a Grass CP511 AC EEG-preamplifier (Astro-Med, West Warwick, RI). When not used for infusion of BoNT/B or vehicle, the guide cannula was plugged with a dummy cannula that projected 0.5 mm past the end of the guide cannula and was secured to the pedestal with a threaded plastic cap (Plastics One). Dental acrylic cement (Lang Dental, Wheeling, IL) and stabilizing stainless steel screws (Plastics One) were used to secure the cannula-electrode assembly to the skull. The assembly was implanted dorsally into the left dentate gyrus of the hippocampus at the same stereotaxic coordinates as for acute infusion except that the distal guide cannula end was positioned at DV −1.00 mm. The electrode wires, projecting an additional 1 mm, were situated at DV −2.00 mm in the dentate gyrus. At least 5 days were allowed for recovery from surgery.
For infusion of BoNT/B or vehicle, a 28-gauge PEEK cannula was used. A stepped design was created by gluing a fused silica tip as used for acute intrahippocampal infusion inside the PEEK cannula so the tip extended 1 mm beyond the end of the PEEK cannula. The stepped cannula was inserted into the guide cannula and left in place for 1 min before the start of the infusion. The orifice of the cannula projected 2.50 mm below the end of the guide cannula (DV −3.50 mm). Infusion parameters were the same as for acute intrahippocampal infusion. The position of the cannula tip was verified histologically in 2 animals at the beginning of the study.
Behavioral testing battery
Sensory responsiveness and acoustic and tactile hyperexcitability was assessed using a simple test battery described previously (Moser et al., 1988; Rice et al., 1998). The approach-response, touch-response, finger-snap, and pick-up tests were applied and scored as follows (cf. Rattka et al., 2013): Approach-response test: A pen held vertically is moved slowly toward the face of the animal. Responses were scored as 1, no reaction; 2, the rat sniffs at the object; 3, the rat moves away from the object; 4, the rat freezes; 5, the rat jumps away from the object; and 6, the rat jumps at or attacks the object; Touch-response test: The animal is gently prodded in the rump with the blunt end of a pen. Responses were scored as 1, no reaction; 2, the rat turns toward the object; 3, the rat moves away from the object; 4, the rat freezes; 5, the rat jerks around toward the touch; 6, the rat turns away from the touch; and 7, the rat jumps with or without vocalizations; Finger-snap test: A click noise with a clicker several centimeters above the head of the animal is performed. Responses were scored as 1, no reaction; 2, the rat jumps slightly or flinches or flicks the ear (normal reaction); and 3, the rat jumps dramatically; Pick-up test: The animal is picked up by grasping around the body. Responses were scored as 1, very easy; 2, easy with vocalizations; 3, some difficulty, the rat rears and faces the hand; 4, the rat freezes (with or without vocalization); 5, difficult, the rat avoids the hand by moving away; and 6, very difficult, the rat behaves defensively, and may attack the hand.
The test battery was performed repeatedly in each animal at different time-points after BoNT/B or vehicle infusion. Three observers independently scored the response of each rat in its home cage. The scores were identical except in one instance where one observer reported a divergent score; in this case the scores were averaged. To assess whether repeated administration of the intravenous PTZ test influenced the outcome of the behavioral testing, we also conducted the behavioral tests in the 11 animals used for EEG recording, which also received BoNT/B or vehicle infusion, but did not undergo PTZ-testing. Inasmuch as the results of behavioral testing were similar in the 2 groups, the data was pooled.
EEG recording and analysis
EEG recording was conducted in freely moving animals 5–7 d after infusion using a Grass CP511 AC preamplifier (Astro-Med, West Warwick, RI, USA). Data was acquired in 5 min epochs using Axotape 9 (Axon Instruments, Foster City, CA). The sampling frequency was 2 kHz and the low- and high-pass filter was set at 50 kHz and 2 Hz, respectively. During the recording, the test animal was observed by two experimenters and any seizure behaviors were noted. The voltage records were stored digitally for later analysis, which was performed manually by S.B. and M.G. using AxoScope (Axon Instruments). Scoring of the records was conducted blind with respect to treatment group; concordance between the two scorers was verified. The total number of electrographic seizures, spikes and sharp waves occurring during the 5-min epoch was counted manually. The first 10 s of each recording was excluded to avoid artifacts due to cable adjustments and animal movements. Electrographic seizures were defined as trains of voltage fluctuations of maximum amplitude at least twice the peak-to-peak baseline that occurred at a frequency of at least 1.5 Hz and persisted for at least 5 s. Interictal spikes and sharp waves were defined as voltage fluctuations persisting 30–80 ms and 80–250 ms, respectively, of maximum amplitude at least twice baseline (see Fig. 6). Spikes and sharp waves were categorized as monophasic (positive or negative deflection), biphasic (positive and negative deflection), and polyphasic (more than 2 directional deflections). The period of electrographic seizure discharge (including 5 s prior to seizure onset) was excluded for the analysis of spikes and sharp waves.
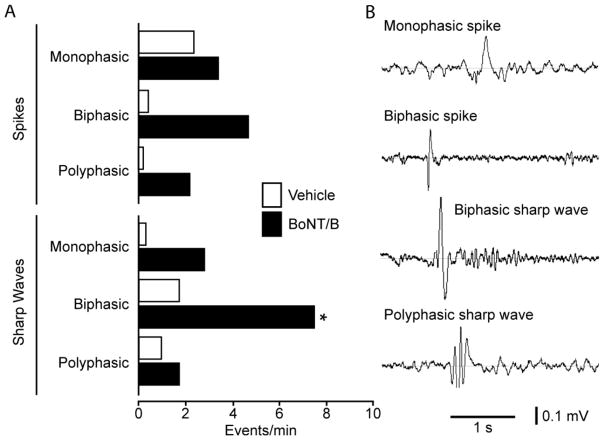
Fig. 6.
Spike and sharp wave frequencies in dentate gyrus recordings from vehicle and BoNT/B treated rats. A, bar graph indicating the frequencies of the varous spike and sharp wave types recorded in 5 min epochs from 3 control rats and 6 BoNT/B treated rats. The overall difference in the two groups is significant by two-way ANOVA for repeated measurements (P = 0.0007). Pairwise post hoc-testing with the Bonferroni method revealed that the mean values for biphasic sharp waves in the two groups were significantly different (*, P < 0.05). B, typical morphologies of some spike and sharp wave types.
Statistics
Time series mean seizure threshold values were tested for significant change by a one-way analysis of variance (ANOVA), with post hoc comparisons using t-tests with Bonferroni correction for multiple comparisons. Mean behavioral test scores were compared by two-way ANOVA, with post hoc comparisons using the Bonferroni method. All statistical analyses were performed with the Prism 5.02 software from GraphPad (La Jolla, CA, USA). P < 0.05 was considered significant.
Results
Effects of BoNT/B on intravenous PTZ seizure threshold
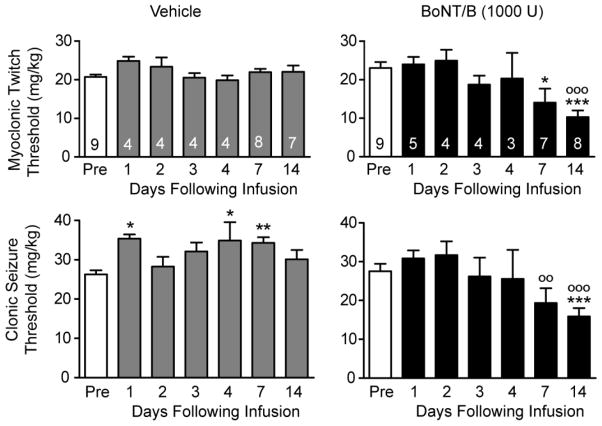
In animals that received intrahippocampal vehicle infusion, no significant change in myoclonic twitch threshold in the intravenous PTZ test was observed on any of the determination days (Fig. 1). Statistically significant increases in clonic seizure threshold were observed on days 1, 4 and 7. In contrast, animals that received BoNT/B infusion exhibited marked reductions in twitch and clonic seizure thresholds beginning on day 7. Thus, the mean preinfusion and day 7 myoclonic twitch thresholds were respectively 23.1 and 14.1 mg/kg, representing a 39% reduction (P < 0.05). The corresponding clonic seizure threshold values were 27.6 and 19.4 mg/kg, representing a 30% decrease. Although the decrease did not reach statistical significance with respect to the mean preinfusion value, there was a significant difference (P < 0.01) with respect to the corresponding value in the vehicle-treated group. Two weeks after the infusion the threshold reductions became more pronounced. Thus, on day 14 the mean myoclonic twitch and clonic seizure thresholds were 10.3 mg/kg (a 55.4% reduction with respect to the mean preinfusion value; P < 0.001) and 15.9 mg/kg (a 42.4% reduction; P < 0.01).
Fig. 1.
Effects of unilateral left intrahippocampal infusion of vehicle or BoNT/B (1000 U) on intravenous PTZ twitch and clonic seizure thresholds before (Pre) and during the 14 day period following infusion. Bars represent mean ± S.E.M. of the threshold values; the number of rats tested for both myclonic twitch and clonic seizure threshold is indicated within the bars. Asterisks (*) represent significant differences with respect to the Pre mean value; circles (○) represent significant differences with respect to the corresponding mean value in the vehicle treated group. One-way ANOVA followed by specific post hoc comparisons with Bonferroni method. */○, P < 0.05; **/○○, P < 0.01; ***/○○○, P < 0.001.
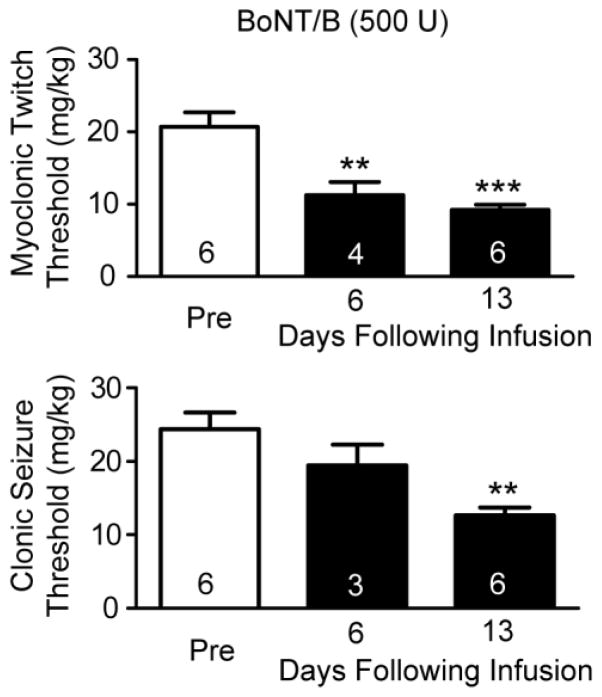
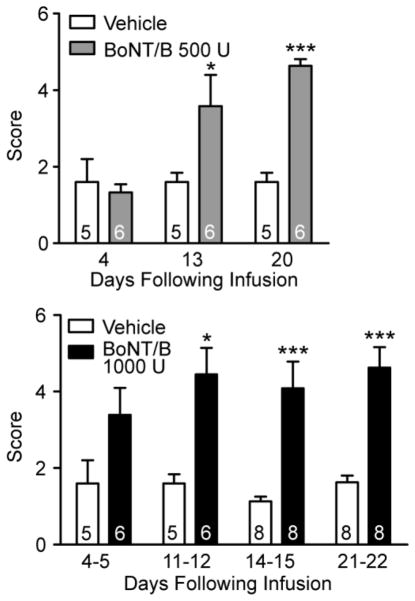
In an additional group of animals, we infused a lower dose of BoNT/B (500 U) and determined the intravenous PTZ seizure thresholds on days 6 and 13 (see Fig. 2). In this experiment the myoclonic twitch threshold was significantly reduced on both test days and the clonic seizure threshold was significantly reduced on day 13.
Fig. 2.
Effects of unilateral left intrahippocampal infusion of BoNT/B (500 U) on intravenous PTZ twitch and clonic seizure threshold before (Pre) and at 6 and 13 days following infusion. Bars represent mean ± S.E.M. of the threshold values; the number of rats tested is indicated within the bars. Asterisks (*) represent significant differences with respect to the Pre mean value. One-way ANOVA followed by specific post hoc comparisons by Bonferroni method. **, P < 0.01; ***, P < 0.001.
Health assessment
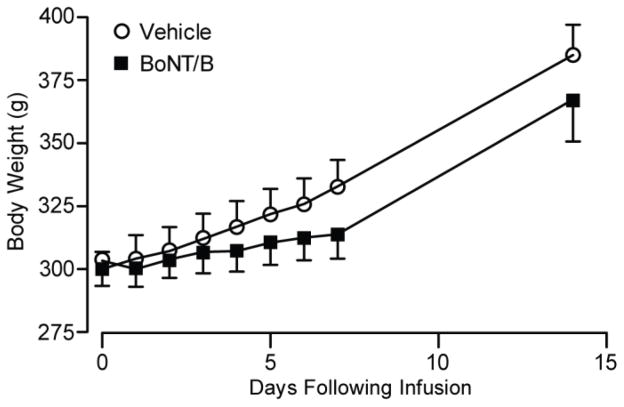
Animals receiving BoNT/B infusions were generally healthy but gained weight at a slightly reduced rate compared with those receiving vehicle (Fig. 3).
Fig. 3.
Weight development after infusion of vehicle or BoNT/B (1000 U). Data points indicate mean ± S.E.M. of the body weights of 7–13 animals in the vehicle group and 7–14 animals in the BoNT/B group. Two-way non-repeated measures ANOVA revealed a significant difference between treatments (P = 0.0435) and days following infusion (P < 0.001), but post hoc comparisons with the Bonferroni method did not show significant differences between treatments at any specific time point.
Handling-evoked and spontaneous behavioral seizures
Convulsive seizures and bouncing behavior as well as clonic movements of the forelimbs and rearing and falling were observed in BoNT/B-treated rats prior to or during handling, as early as the second day after infusion. Six out of the 9 animals treated with intrahippocampal BoNT/B (1000 U) in the experiment of Fig. 1 were observed to have spontaneous convulsive seizures in the animal facility prior to being handled for weighing. In an additional group of 6 animals chronically implanted for EEG recording and infusion (see below), two-thirds (4 out of 6) also exhibited such seizures beginning during the first week after hippocampal BoNT/B infusion. These abnormal behaviors were not observed in controls with or without chronically implanted electrode-infusion cannula assemblies.
EEG
The observation of handling-induced and spontaneous motor seizures in many of the BoNT/B-treated animals prompted us to perform a follow-up experiment to determine whether BoNT/B infusion induces electrophysiologically detectable epileptiform activity near the infusion zone. A group of 9 animals were implanted chronically with an EEG recording electrode and infusion assembly as described in Methods. EEG recording from the dentate gyrus and behavioral assessment was conducted for 5 min during the period 5 to 7 days after treatment. In 4 of 6 BoNT/B-treated rats spontaneous electrographic seizures were recorded (Fig. 4). These animals exhibited 2.3 ± 1.1 (mean ± S.E.M.) electrographic seizures during the 5 min recording period. In addition, they exhibited seizure behaviors including clonic movements of the forelimbs, rearing and falling. Vehicle-treated animals did not show seizure activity. In addition to the electrographic seizures, the EEG recordings from BoNT/B-treated rats exhibited significantly more frequent spikes and sharp waves than did vehicle-treated animals (Fig. 5). Fig. 6 compares the frequencies of spike and sharp wave types in the two groups. Every event type was more frequent in the recordings from the BoNT/B-treated animals than in the vehicle-treated animals. The overall difference between the mean values in the two groups was highly significant. Individual pair-wise comparisons demonstrated that the frequency of biphasic sharp waves in the BoNT/B group was significantly greater than in the vehicle group. An additional difference noted in the recordings in the BoNT/B group was that spike and sharp wave events tended to cluster with a frequency of 1 to 11 Hz; events in the recordings from the vehicle group did not cluster and only occurred singly.
Fig. 4.
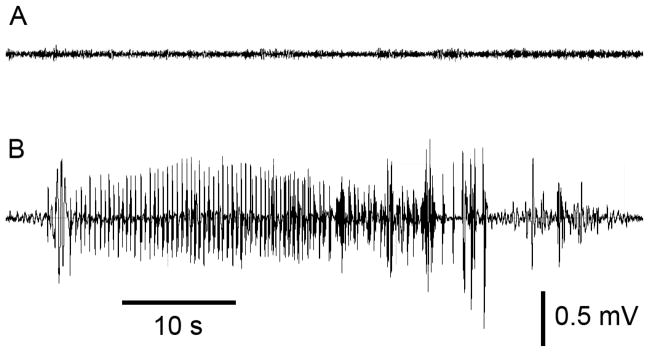
Sample EEG traces recorded from the left dentate gyrus of rats about 6 days after infusion of vehicle (A) and 1000 U BoNT/B (B). The spontaneous electrographic seizure in (B) is typical of those recorded in BoNT/B-treated animals. In the 4 animals exhibiting such activity, up to 7 such events were recorded in the 5 min recording session.
Fig. 5.
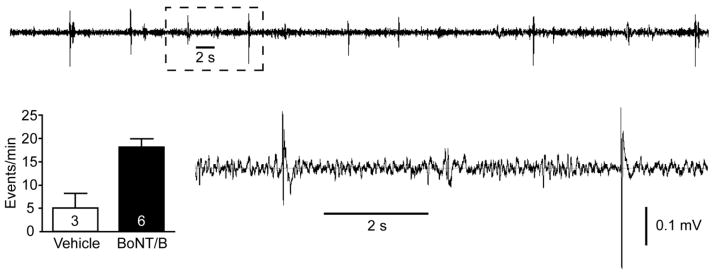
Epileptiform spikes and sharp waves 5 to 7 days after infusion of BoNT/B (1000 U) into the left hippocampus. Top trace is a segment of a typical recording from the dentate gyrus of a BoNT/B rat showing frequent spikes and sharp waves. The area marked with a dashed box is shown on an expanded scale below. The bar graph indicates the mean ± S.E.M. spike and sharp wave frequency in 5 min epochs from the vehicle and BoNT/B-treated animals. The number of animals is indicated within the bars. The difference in the means in the two groups is significant (P = 0.01; unpaired t-test).
Behavioral hyperexcitability
Behavioral hyperexcitability was assessed in all 34 rats that had received BoNT/B or vehicle infusion. BoNT/B-treated animals also were hyperkinetic and exhibited aggressive behaviors. Significant differences were observed between the BoNT/B groups and the corresponding vehicle-treated groups in the pick-up test (Fig. 7). Average scores for vehicle-treated animals ranged from 1.1 to 1.6 over the course of three weeks. These animals were easy to pick up and at times had vocalization. In the BoNT/B-treated animals, the scores ranged from 3.4 and 4.6. These animals were difficult to pick up because they moved away or jumped out of the cage. In animals that received 500 U BoNT/B, the mean pick-up score was significantly greater than the value in the vehicle control group at 13 and 20 days following treatment but not at 4 days. Similarly, in the animals that received 1000 U BoNT/B, mean pick-up score was significantly elevated at 11–12, 14–15 and 21–22 days following treatment but the increase did not reach statistical significance at 4–5 days. No significant differences between BoNT/B- and vehicle-treated animals were obtained in the approach-response, touch-response and finger-snap tests (not illustrated).
Fig. 7.
Effects of unilateral left intrahippocampal infusion of vehicle or BoNT/B on pick-up test score on various days following infusion. Upper bar chart represents single experiment with 500 U BoNT/B; lower bar chart represents pooled data from two separate experiments with 1000 U BoNT/B. Bars represent mean ± S.E.M. of the score; the number of rats is indicated within the bars. In the experiment presented in the upper panel, all animals were tested at each time-point and we performed a two-way ANOVA by rows for repeated measures. There was a significant difference with respect to treatment (P = 0.0029). In addition, there was a signficant effect of time (day of testing; P = 0.0065). In the pooled experiments presented in the lower bar chart, not all animals were tested on each time-point; therefore we performed a two-way non-repeated measures ANOVA, which revealed significant differences between treatments (P = 0.0001). ANOVA was followed by specific post hoc comparisons between the vehicle and BoNT/B treated groups with the Bonferroni method. *, P < 0.05; ***, P < 0.001.
Discussion
The main findings of the present study were (1) unilateral intrahippocampal BoNT/B caused delayed proconvulsant effects in the intravenous PTZ seizure threshold test; (2) spontaneous and handling-evoked behavioral seizures were noted in BoNT/B-treated rats; (3) spontaneous electrographic seizures were recorded from the dentate gyrus, near the site of BoNT/B infusion; (4) interictal spikes and sharp waves occurred with increased frequency in dentate gyrus recordings from BoNT/B-treated animals; and (5) BoNT/B-treatment caused an increase in tactile hyperresponsivity.
Intrahippocampal BoNT/E has previously been shown to exhibit anticonvulsant effects and also to prevent seizure-induced neuronal loss and cognitive deficits (Costantin et al., 2005; Antonucci et al., 2009). BoNT/E acts specifically on SNAP-25, which is preferentially expressed in excitatory glutamatergic nerve terminals and only affects GABA release at high doses (Verderio et al., 2004, 2007). It has therefore been proposed that the anti-seizure effects of BoNT/E are due to suppression of excitatory neurotransmission in epileptic circuits. In contrast, BoNT/B specifically targets VAMP2, which is preferentially expressed in GABAergic terminals (Bragina et al., 2007, 2010). Disinhibition by blockade of GABA-mediated inhibition could account for the novel observation reported here that intrahippocampal BoNT/B can reduce seizure threshold and elicit behavioral and electrographic seizures.
We recently reported that intra-amygdala infusion of BoNT/B in fully amygdala-kindled rats causes a prolonged elevation of the after discharge threshold and a reduction of the after discharge duration (Gasior et al., 2013). We concluded that BoNT/B can cause long-lasting inhibition of amygdala excitability. The inhibition was apparent at 3 days following infusion, which was the first time point studied, and persisted for at least 50 days. Spontaneous seizures were not observed following BoNT/B infusion into the amygdala. In contrast, the present study for the first time demonstrates that BoNT/B enhances hippocampal excitability beginning 2 to 5 days after infusion. In our prior study in amygdala-kindled rats, we did not specifically assess seizure threshold and we did not attempt to record spontaneous epileptiform discharges. Therefore, we cannot be certain that intra-amygdala BoNT/B does not induce local epileptic activity. Nevertheless, assuming that BoNT/B enhances seizures by inhibiting GABAergic inhibition, the observation that intrahippocampal BoNT/B leads to proconvulsant effects is consistent with many prior studies demonstrating that focal injection of GABAA receptor antagonists into the hippocampus leads to electrographic and behavioral seizures (Colom et al., 1991; Sierra-Paredes and Sierra-Marcuño, 1996). Tetanus toxin, a clostridal toxin that also preferentially inhibits GABA release (Bigalke et al., 1981; Collingridge and Davies, 1982; Bergey et al., 1987), is well recognized to induce seizures when injected into the hippocampus (Mellanby et al., 1977; Jefferys and Whittington, 1996; Anderson et al., 1999). Interestingly, focal injections of GABAA receptor antagonists into the amygdala have not been reported to elicit seizures (Dickinson-Anson et al., 1993; Dickinson-Anson and McGaugh, 1997) and spontaneous epileptiform discharges rarely occur in amygdala slices exposed to GABAA receptor antagonists, even though the amygdala is highly susceptible to epileptiform behavior (Gean and Chang, 1991). These observations indicate that suppression of inhibitory neurotransmission in the hippocampus causes robust proconvulsant effects whereas the amygdala is more resistant to blockade of synaptic inhibition. Therefore, it is not surprising that BoNT/B causes proconvulsant effects when administered into the hippocampus but a similar action was not observed following delivery of the neurotoxin into the amygdala.
There was a delay of 2 to 5 days or more in the onset of the reduced seizure threshold, spontaneous seizures, and behavioral hyperreactivity following intrahippocampal BoNT/B administration. It is noteworthy that there is also a delay in the occurrence of seizures following intrahippocampal tetanus toxin injection (Mellanby et al., 1977). After injection into muscle, neuromuscular paralysis by BoNT/B usually occurs within 24 to 72 h (Brin, 1997). However, in isolated muscle in vitro, 50% neuromuscular paralysis occurs within about 1 h and 90% paralysis in 2 h (Deshpande et al., 1995; Bigalke et al., 2009). In isolated neurons, blockade of neurotransmission by BoNTs also occurs on a comparable time scale (Bigalke et al., 1986; Keller et al., 2004). The onset of the proconvulsant activity of BoNT/B in the present study occurred according to a time course similar to the onset of neuromuscular paralysis. It has been proposed that the slow onset of neuromuscular paralysis that occurs when BoNTs are administered by injection into muscle is due to the presence of anatomical and perhaps other barriers that interfere with the diffusion of toxin to the site of action (Lebeda et al., 2008). The delay in the onset of action of BoNT/B in the present study is likely due to such diffusion barriers. However, we cannot exclude the possibility that the evolution of epileptic activity following intrahippocampal BoNT/B requires an epileptogenic process and is not simply the result of the pharmacological actions of the toxin. In the present study, we did not assess seizure threshold at time points greater than 2 weeks following BoNT/B so we do not know if heightened seizure susceptibility would continue for the prolonged duration (two or more months) seen in our previous study (Gasior et al., 2013). In addition, we do not know if the seizure activity would outlast the functional effects of the toxin and thus represent a true epileptogenic action.
The solution of BoNT/B used in our study contained a small amount of albumin (7.7 μM). It is recognized that application of albumin to the cortical surface can cause epileptiform activity and seizures with a latency of 4 to 7 days (Seiffert et al., 2004; Friedman et al., 2009). However, albumin is unlikely to have been responsible for the treatment effects we observed as concentrations less than 100 μM have been found to be inactive (Seiffert et al., 2004). Moreover, the vehicle solution, which failed to cause a seizure threshold reduction, also contained albumin. In fact, vehicle infusion was associated with a modest increase in clonic seizure threshold on some days, indicating an anticonvulsant action. A similar anticonvulsant action of vehicle solution noted in a previous study was attributed to a volume effect (Bröer et al., 2012).
Conclusion
Focal application of BoNTs has potential utility in the treatment of intractable localization-related epilepsy (Costantin et al., 2005; Antonucci et al., 2009; Gasior et al., 2013). This novel treatment strategy could provide an alternative to resective surgery (Rogawski, 2009). However, the present study points to a risk that BoNT/B may promote seizures. Because of the risk, this serotype is unlikely to be useful for epilepsy treatment. Indeed, our results indicate that intrahippocampal BoNT/B could represent a useful model of focal epilepsy.
Highlights.
Botulinum neurotoxins (BoNTs) may affect the excitability of brain circuits
Intrahippocampal BoNT/B caused delayed proconvulsant actions and seizures
BoNT/B differs from other BoNTs that have been found to confer seizure protection
The pro-epileptic actions of BoNT/B may be due to its ability to inhibit GABA release
Acknowledgments
We thank Jorge Sanchez for assistance with the behavioral tests and Solstice Neurosciences for providing BoNT/B and advice on its use. We also acknowledge Matthias Luz for helpful discussions. S.B. is supported by the Studienstiftung des deutschen Volkes, e.V.
Support was provided by grants from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NS072094, NS079202).
Abbreviations
- ANOVA
analysis of variance
- BoNT
botulinum neurotoxin
- CED
convection-enhanced delivery
- EEG
electroencephalogram
- PEEK
polyaryletheretherketone
- PTZ
pentylenetetrazol
- SNAP-25
synaptosomal-associated protein of 25 kDa
- U
unit
- VAMP2
vesicle-associated membrane protein 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson AE, Hrachovy RA, Antalffy BA, Armstrong DL, Swann JW. A chronic focal epilepsy with mossy fiber sprouting follows recurrent seizures induced by intrahippocampal tetanus toxin injection in infant rats. Neuroscience. 1999;92:73–82. doi: 10.1016/s0306-4522(98)00746-5. [DOI] [PubMed] [Google Scholar]
- Antonucci F, Bozzi Y, Caleo M. Intrahippocampal infusion of botulinum neurotoxin E (BoNT/E) reduces spontaneous recurrent seizures in a mouse model of mesial temporal lobe epilepsy. Epilepsia. 2009;50:963–966. doi: 10.1111/j.1528-1167.2008.01983.x. [DOI] [PubMed] [Google Scholar]
- Ashton AC, Dolly JO. Characterization of the inhibitory action of botulinum neurotoxin type A on the release of several transmitters from rat cerebrocortical synaptosomes. J Neurochem. 1988;50:1808–1816. doi: 10.1111/j.1471-4159.1988.tb02482.x. [DOI] [PubMed] [Google Scholar]
- Bankstahl M, Bankstahl JP, Bloms-Funke P, Löscher W. Striking differences in proconvulsant-induced alterations of seizure threshold in two rat models. Neurotoxicology. 2012;33:127–137. doi: 10.1016/j.neuro.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Benagiano V, Lorusso L, Flace P, Girolamo F, Rizzi A, Bosco L, Cagiano R, Nico B, Ribatti D, Ambrosi G. VAMP-2, SNAP-25A/B and syntaxin-1 in glutamatergic and GABAergic synapses of the rat cerebellar cortex. BMC Neurosci. 2011;12:118–127. doi: 10.1186/1471-2202-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey GK, Bigalke H, Nelson PG. Differential effects of tetanus toxin on inhibitory and excitatory synaptic transmission in mammalian spinal cord neurons in culture: a presynaptic locus of action for tetanus toxin. J Neurophysiol. 1987;57:121–131. doi: 10.1152/jn.1987.57.1.121. [DOI] [PubMed] [Google Scholar]
- Bigalke H, Heller I, Bizzini B, Habermann E. Tetanus toxin and botulinum A toxin inhibit release and uptake of various transmitters, as studied with particulate preparations from rat brain and spinal cord. Naunyn Schmiedebergs Arch Pharmacol. 1981;316:244–251. doi: 10.1007/BF00505657. [DOI] [PubMed] [Google Scholar]
- Bigalke H, Dressler D, Frevert J. Immunological properties of botulinum toxins. In: Truong D, Dressler D, Hallett M, editors. Manual of Botulinum Toxin Therapy. Chapter 4. Cambridge University Press; Cambridge: 2009. pp. 23–28. [Google Scholar]
- Bigalke H, Müller H, Dreyer F. Botulinum A neurotoxin unlike tetanus toxin acts via a neuraminidase sensitive structure. Toxicon. 1986;24:1065–1074. doi: 10.1016/0041-0101(86)90133-9. [DOI] [PubMed] [Google Scholar]
- Bouilleret V, Loup F, Kiener T, Marescaux C, Fritschy JM. Early loss of interneurons and delayed subunit-specific changes in GABAA-receptor expression in a mouse model of mesial temporal lobe epilepsy. Hippocampus. 2000;10:305–324. doi: 10.1002/1098-1063(2000)10:3<305::AID-HIPO11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Bragina L, Candiracci C, Barbaresi P, Giovedì S, Benfenati F, Conti F. Heterogeneity of glutamatergic and GABAergic release machineries in cerebral cortex. Neuroscience. 2007;146:1829–1840. doi: 10.1016/j.neuroscience.2007.02.060. [DOI] [PubMed] [Google Scholar]
- Bragina L, Giovedì S, Barbaresi P, Benfenati F, Conti F. Heterogeneity of glutamatergic and GABAergic release machinery in cerebral cortex: analysis of synaptogyrin, vesicle-associated membrane protein, and syntaxin. Neuroscience. 2010;165:934–943. doi: 10.1016/j.neuroscience.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Brin MF. Botulinum toxin: chemistry, pharmacology, toxicity, and immunology. Muscle Nerve Suppl. 1997;6:S146–168. [PubMed] [Google Scholar]
- Bröer S, Backofen-Wehrhahn B, Bankstahl M, Gey L, Gernert M, Löscher W. Vigabatrin for focal drug delivery in epilepsy: bilateral microinfusion into the subthalamic nucleus is more effective than intranigral or systemic administration in a rat seizure model. Neurobiol Dis. 2012;46:362–376. doi: 10.1016/j.nbd.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Caleo M, Schiavo G. Central effects of tetanus and botulinum neurotoxins. Toxicon. 2009;54:593–599. doi: 10.1016/j.toxicon.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Capogna M, Mckinney RA, O’Connor V, Gahwiler BH, Thompson SM. Ca2+ or Sr2+ partially rescues synaptic transmission in hippocampal cultures treated with botulinum toxin A and C, but not tetanus toxin. J Neurosci. 1997;17:7190–7202. doi: 10.1523/JNEUROSCI.17-19-07190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Davies J. The in vitro inhibition of GABA release by tetanus toxin. Neuropharmacology. 1982;21:851–855. doi: 10.1016/0028-3908(82)90075-2. [DOI] [PubMed] [Google Scholar]
- Colom LV, Nassif-Caudarella S, Dickson CT, Smythe JW, Bland BH. In vivo intrahippocampal microinfusion of carbachol and bicuculline induces theta-like oscillations in the septally deafferented hippocampus. Hippocampus. 1991;1:381–390. doi: 10.1002/hipo.450010406. [DOI] [PubMed] [Google Scholar]
- Costantin L, Bozzi Y, Richichi C, Viegi A, Antonucci F, Funicello M, Gobbi M, Mennini T, Rossetto O, Montecucco C, Maffei L, Vezzani A, Caleo M. Antiepileptic effects of botulinum neurotoxin E. J Neurosci. 2005;25:1943–1951. doi: 10.1523/JNEUROSCI.4402-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande SS, Sheridan RE, Adler M. A study of zinc-dependent metalloendopeptidase inhibitors as pharmacological antagonists in botulinum neurotoxin poisoning. Toxicon. 1995;33:551–557. doi: 10.1016/0041-0101(94)00188-e. [DOI] [PubMed] [Google Scholar]
- Dickinson-Anson H, McGaugh JL. Bicuculline administered into the amygdala after training blocks benzodiazepine-induced amnesia. Brain Res. 1997;752:197–202. doi: 10.1016/s0006-8993(96)01449-7. [DOI] [PubMed] [Google Scholar]
- Dickinson-Anson H, Mesches MH, Coleman K, McGaugh JL. Bicuculline administered into the amygdala blocks benzodiazepine-induced amnesia. Behav Neural Biol. 1993;60:1–4. doi: 10.1016/0163-1047(93)90638-x. [DOI] [PubMed] [Google Scholar]
- Friedman A, Kaufer D, Heinemann U. Blood-brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. Epilepsy Res. 2009;85:142–149. doi: 10.1016/j.eplepsyres.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbelli R, Inverardi F, Medici V, Amadeo A, Verderio C, Matteoli M, Frassoni C. Heterogeneous expression of SNAP-25 in rat and human brain. J Comp Neurol. 2008;506:373–386. doi: 10.1002/cne.21505. [DOI] [PubMed] [Google Scholar]
- Gasior M, Tang R, Rogawski MA. Long-lasting attenuation of amygdala-kindled seizures after convection-enhanced delivery of botulinum neurotoxins A and B into the amygdala in rat. J Pharmacol Exp Ther. 2013;346:528–534. doi: 10.1124/jpet.113.205070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gean PW, Chang FC. Bursting discharges in disinhibited amygdala slices: the role of excitatory amino acid receptors. Neuropharmacology. 1991;30:797–802. doi: 10.1016/0028-3908(91)90188-h. [DOI] [PubMed] [Google Scholar]
- Humeau Y, Doussau F, Grant NJ, Poulain B. How botulinum and tetanus neurotoxins block neurotransmitter release. Biochemie. 2000;82:427–446. doi: 10.1016/s0300-9084(00)00216-9. [DOI] [PubMed] [Google Scholar]
- Jefferys JG, Whittington MA. Review of the role of inhibitory neurons in chronic epileptic foci induced by intracerebral tetanus toxin. Epilepsy Res. 1996;26:59–66. doi: 10.1016/s0920-1211(96)00040-x. [DOI] [PubMed] [Google Scholar]
- Johnson EA. Clostridial toxins as therapeutic agents: benefits of nature’s most toxic proteins. Annu Rev Microbiol. 1999;53:551–575. doi: 10.1146/annurev.micro.53.1.551. [DOI] [PubMed] [Google Scholar]
- Keller JE, Cai F, Neale EA. Uptake of botulinum neurotoxin into cultured neurons. Biochemistry. 2004;43:526–532. doi: 10.1021/bi0356698. [DOI] [PubMed] [Google Scholar]
- Lebeda FJ, Adler M, Erickson K, Chushak Y. Onset dynamics of type A botulinum neurotoxin-induced paralysis. J Pharmacokinet Pharmacodyn. 2008;35:251–267. doi: 10.1007/s10928-008-9087-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W. Preclinical assessment of proconvulsant drug activity and its relevance for predicting adverse events in humans. Eur J Pharmacol. 2009;610:1–11. doi: 10.1016/j.ejphar.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Matteoli M, Pozzi D, Grumelli C, Condliffe SB, Frassoni C, Harkany T, Verderio C. The synaptic split of SNAP-25: Different roles in glutamatergic and GABAergic neurons? Neuroscience. 2009;158:223–230. doi: 10.1016/j.neuroscience.2008.03.014. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Foran P, Dolly JO, Verhage M, Wiegant VM, Nicholls DG. Tetanus toxin and botulinum toxins type A and B inhibit glutamate, γ-aminobutyric acid, aspartate, and met-enkephalin release from synaptosomes. Clues to the locus of action. J Biol Chem. 1992;267:21338–21343. [PubMed] [Google Scholar]
- Mellanby J, George G, Robinson A, Thompson P. Epileptiform syndrome in rats produced by injecting tetanus toxin into the hippocampus. J Neurol Neurosurg Psychiatry. 1977;40:404–414. doi: 10.1136/jnnp.40.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser VC, McCormick JP, Creason JP, Macphail RC. Comparison of chlordimeform and carbaryl using a functional observational battery. Fundam Appl Toxicol. 1988;11:189–206. doi: 10.1016/0272-0590(88)90144-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. Amsterdam: Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- Pollack GM, Shen DD. A timed intravenous pentylenetetrazol infusion seizure model for quantitating the anticonvulsant effect of valproic acid in the rat. J Pharmacol Methods. 1985;13:135–146. doi: 10.1016/0160-5402(85)90057-9. [DOI] [PubMed] [Google Scholar]
- Rattka M, Brandt C, Bankstahl M, Bröer S, Löscher W. Enhanced susceptibility to the GABA antagonist pentylenetetrazole during the latent period following a pilocarpine-induced status epilepticus in rats. Neuropharmacology. 2011;60:505–512. doi: 10.1016/j.neuropharm.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Rattka M, Brandt C, Löscher W. The intrahippocampal kainate model of temporal lobe epilepsy revisited: Epileptogenesis, behavioral and cognitive alterations, pharmacological response, and hippoccampal damage in epileptic rats. Epilepsy Res. 2013;103:135–152. doi: 10.1016/j.eplepsyres.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Rice AC, Floyd CL, Lyeth BG, Hamm RJ, Delorenzo RJ. Status epilepticus causes long-term NMDA receptor-dependent behavioral changes and cognitive deficits. Epilepsia. 1998;39:1148–1157. doi: 10.1111/j.1528-1157.1998.tb01305.x. [DOI] [PubMed] [Google Scholar]
- Rogawski MA. Convection-enhanced delivery in the treatment of epilepsy. Neurotherapeutics. 2009;6:344–351. doi: 10.1016/j.nurt.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetto O, Morbiato L, Caccin P, Rigoni M, Montecucco C. Presynaptic enzymatic neurotoxins. J Neurochem. 2006;97:1534–1545. doi: 10.1111/j.1471-4159.2006.03965.x. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. The neurobiology of epilepsy. Curr Neurol Neurosci Rep. 2007;7:348–354. doi: 10.1007/s11910-007-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, Friedman A. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24:7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Paredes G, Sierra-Marcuño G. Microperfusion of picrotoxin in the hippocampus of chronic freely moving rats through microdialysis probes: a new method of induce partial and secondary generalized seizures. J Neurosci Methods. 1996;67:113–120. [PubMed] [Google Scholar]
- Simpson LL. Identification of the major steps in botulinum toxin action. Annu Rev Pharmacol Toxicol. 2004;44:167–193. doi: 10.1146/annurev.pharmtox.44.101802.121554. [DOI] [PubMed] [Google Scholar]
- Verderio C, Grumelli C, Raiteri L, Coco S, Paluzzi S, Caccin P, Rossetto O, Bonanno G, Montecucco C, Matteoli M. Traffic of botulinum toxins A and E in excitatory and inhibitory neurons. Traffic. 2007;8:142–153. doi: 10.1111/j.1600-0854.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- Verderio C, Pozzi D, Pravettoni E, Inverardi F, Schenk U, Coco S, Proux-Gillardeaux V, Galli T, Rossetto O, Frassoni C, Matteoli M. SNAP-25 modulation of calcium dynamics underlies differences in GABAergic and glutamatergic responsiveness to depolarization. Neuron. 2004;41:599–610. doi: 10.1016/s0896-6273(04)00077-7. [DOI] [PubMed] [Google Scholar]
- Yin D, Forsayeth J, Bankiewicz KS. Optimized cannula design and placement for convection-enhanced delivery in rat striatum. J Neurosci Methods. 2010;187:46–51. doi: 10.1016/j.jneumeth.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]