Abstract
Signals from rod photoreceptors bias (shift) the hues determined by cone photoreceptors for extrafoveal mesopic stimuli, creating green, blue, and red rod hue biases at long, middle, and short wavelengths, respectively. The fovea contains far fewer rods and S cones but may not be immune to rod hue biases. Here, we determine the biases found for mesopic foveal stimuli presented on a CRT display. The rod green bias was observed at unique yellow for all but one observer with 2° tests and persisted for most observers with 0.5° tests. The rod red bias typically seen at unique blue in extrafoveal studies was not apparent for either size of foveal test stimulus, and it was sometimes replaced by a rod green bias. The rod blue bias typically seen at unique green and unique red in extrafoveal studies was weak on average and inconsistent for both sizes of foveal test stimuli. Thus, small mesopic foveal stimuli permit rod influence on M- and L-cone color pathways but disadvantage rod influence on S-cone pathways, perhaps because of the sparseness of foveal S-cones. However, some observers did show idiosyncratic foveal rod hue biases that do not follow the general trends.
1. INTRODUCTION
Past studies have demonstrated rod influence on hue perception at mesopic light levels with large extrafoveal stimuli that effectively stimulate rods as well as cones [1–3]. Three types of rod hue biases have been extensively studied using extrafoveal stimuli: a rod green bias (rods enhance green versus red, increasing L/(L + M) troland ratios of unique yellow), a rod blue bias (rods enhance blue versus yellow, reducing S-cone troland levels of unique red and green), and a rod red bias (rods enhance red versus green, reducing L/(L + M) troland ratios of unique blue). The rod green bias likely reveals interaction of signals from rods and L and M cones in midget ganglion pathways, while the rod blue bias and rod red bias likely reveal interaction of signals from rods and S cones in small-bistratified ganglion pathways [4,5].
It might be assumed that the sparseness or absence of foveal rod photoreceptors would protect foveal stimuli from rod hue biases. Indeed, several studies have reported not finding foveal rod hue biases (e.g., [6]). However, some evidence of rod hue biases was reported in two older studies [7,8]. Unfortunately, these studies employed few observers (three per study), tested for rod influence on only two of the four unique hues (green and yellow), and produced results that varied among individual observers. Thus, it was not possible to discern a reliable or comprehensive description of foveal rod hue biases.
Another issue is that both earlier studies employed stimuli composed of monochromatic lights presented in Maxwellian view. The use of CRT displays is far more common in modern research on color vision. However, the phosphors for these displays have broader emission spectra that, especially for mixtures of phosphor outputs, have lower spectral purity and perceptual saturation than monochromatic lights. Prior work has demonstrated that spectral purity of test lights affects the magnitude of extrafoveal rod hue biases and does so to different degrees for the three specific rod hue biases [9]. However, extrapolation of the results from that study, which used mixtures of spectral lights, to what might be expected for mixtures of CRT phosphors is not straightforward.
This laboratory recently published the first study of rod hue biases on stimuli presented on a CRT display [10]. For large, extrafoveal stimuli, all three rod hue biases were found, and the rod blue bias was confirmed for unique red as well as unique green for the CRT display. That study also included a preliminary demonstration of foveal rod hue biases for three observers and two sizes of foveal test stimuli (1° and 2° diameter). Rod green bias at unique yellow and rod blue bias at unique green were the most commonly encountered rod influences, but high variability among the small number of observers prevented confident determination of general trends from those results.
The goal of the present study is to determine the incidence, direction, and magnitude of rod hue biases on all four unique hues for mesopic foveal stimuli of 0.5° and 2.0° diameter presented on a CRT display. We improve on past studies by employing a larger number of observers (eight) to better distinguish general trends from individual variations. We also include a smaller test stimulus (0.5° diameter) than previously used for a CRT display in order to assess rod hue biases on the central foveal region.
2. METHODS
A. Observers
Eight color-normal observers, as assessed by the Nagel Anomaloscope and Ishihara Pseudoisochromatic Plates, volunteered for the experiment. Participants consisted of six females and two males between the ages of 19–22. All of the observers had previously participated in other studies of rod influence on hue and were aware of the studies’ outcomes. However, most were naïve to any expectations about potential outcomes of the present study. All procedures and consent forms were approved by the Institutional Review Board at the University of Washington.
B. Apparatus and Stimuli
A CRT display (ViewSonic G90fB) having a frame rate of 60 Hz was used and controlled by a computer that provided 8-bit precision for each phosphor. The full screen subtended 33° by 25° (w × h) at a distance of 57 cm. The observer was seated with head positioned in a chin and forehead rest. A filter holder was positioned next to the observer’s eye, so neutral-density filters covered a field of view that extended beyond the full monitor screen. All test stimuli were presented at isoluminance within a condition. Across observers, test-stimulus light levels ranged from 0.05–0.17 (CIE2°) or 0.03–0.08 (CIE10°) cd/m2 and were controlled using neutral density filters of optical densities of 2.0–2.5 log units. The background was black (zero level for each gun, at least a factor of 104 cd/m2 below test level). Low mesopic test stimulus levels, such as the present, have been demonstrated to maximize rod influence but still allow sufficient cone function for reliable hue-balance settings for most observers [10–12]. A black foam board surrounded the CRT display, to eliminate stray light from the room. The light output of each phosphor of the monitor was measured by means of a Photo Research SpectraScan PR-650 spectroradiometer and linearized by the MatLab software that controlled gun voltages. Observers viewed the screen monocularly, through the natural pupil of the right eye. The left eye was patched during all stimulus presentations. Test stimuli of 0.5° or 2.0° diameter were surrounded by a square grid of four 0.25° fixation dots placed 4° from fixation, which flashed alternately with the test stimuli, and was set to 50% of the test-stimulus luminance. Observers were instructed to fixate the center of the dot array prior to and during the test presentation but actual fixation was not monitored. Test stimuli were presented for 1 s with 3-s inter-trial interval.
Cone troland values were calculated from the color-step measurements using the Smith–Pokorny cone fundamentals [13], the 1924 CIE Vλ, and a nominal pupil diameter for all observers of 6 mm. S-cone trolands, which are arbitrarily related to L- and M-cone trolands, were calculated using the scaling factor suggested by [14].
C. Procedures
Observers were instructed to adjust the hue of the test stimulus to a designated unique hue (red, green, blue, or yellow). Thus, observers adjusted the balance of red versus green to yield a red-green null for unique blue and unique yellow. Similarly, observers adjusted the balance of blue versus yellow to yield a blue-yellow null for unique red and unique green. The observer controlled the direction of chromaticity change and signaled their unique hue choice by pressing a keyboard. The chromaticity of the test stimulus varied in 217, 343, or 494 steps (depending on condition) around the perimeter of the triangle formed by the R, G, and B phosphors. The test stimulus was initially assigned a random chromaticity from a large range of steps centered around the expected unique hue settings. Initial key presses produced multistep changes, the magnitude of which decreased over the first three reversals of direction until, after three reversals, the change remained at one step. Observers made their judgments on the basis of the hue seen at the conclusion of the 1s test stimulus presentation. Each condition consisted of five test stimulus settings back to back. The starting chromaticity for each trial was random and independent of prior settings.
Rod influence was assessed by the difference between settings for each unique hue measured under bleached (minimizes rod influence) and dark-adapted conditions (maximizes rod influences). For the dark-adapted condition, the observer dark adapted the right eye with an eye patch for at least 15 min. For the bleached condition, the observer exposed their eye to a xenon flash of longstanding use in our laboratory (0.5 J, 3.3 ms, from a Quantum Q-Flash, model T) and made judgments from 3 to 8 min following the flash exposure. The bleaching flash subtended 17° diameter and was centered at the test location of the fovea. The minimum interval between successive bleaching flashes was 10 min.
Observers made settings for all four unique hues for both dark-adapted and bleached conditions on the same day. The order of unique hue testing was reversed on half the sessions, as was the order of bleached and dark-adapted conditions. On a given day, bleached and dark-adapted conditions were separately grouped into two blocks. Each condition was tested in four different sessions run on different days.
3. RESULTS
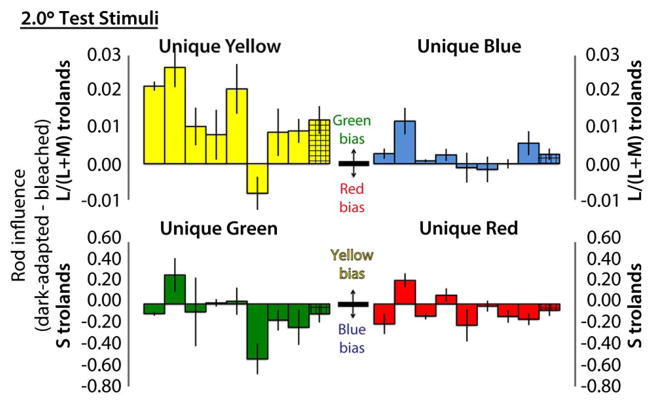
Rod influence, computed as dark-adapted minus bleached cone troland values, is shown for 2.0° and 0.5° stimuli in Figs. 1 and 2, respectively. Rod influence is expressed as a change of L/(L + M) trolands for unique yellow and blue (red–green balances) and as a change of S-cone trolands for unique red and green (blue–yellow balances). Interpretation of each direction of change (e.g., rod green bias) is shown in the center of each panel. Differences between hue settings under bleached and dark-adapted conditions were calculated for each session. Solid bars show the mean and standard error of these differences over the four sessions for individual observers, presented in the same order in each panel. Hatched bars at the right of each cluster show the mean and standard error across all observers.
Fig. 1.
Rod influence on all four unique hues for 2.0°-diameter foveal test stimuli, for individual observers (solid bars) and across-observer means (hatched bars). See text for format details. A relatively large rod green bias is shown by seven of eight observers on unique yellow. Average rod hue biases on the other unique hues are smaller and show greater variation in magnitude and direction.
Fig. 2.
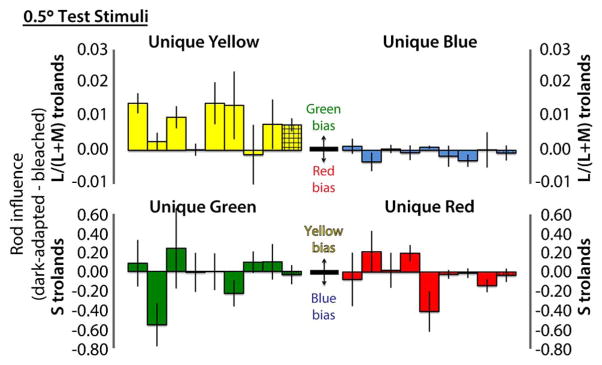
Rod influence on all four unique hues for 0.5°-diameter foveal test stimuli., for individual observers (solid bars) and across-observer means (hatched bars). Average rod green bias on unique yellow was reduced compared to the 2.0° test conditions, and fewer observers showed a convincing effect. Average rod hue biases on the other three unique hues were eliminated, although some observers still showed idiosyncratic effects.
With 2.0°-diameter foveal test stimuli (Fig. 1), seven of eight observers showed convincing rod green bias for unique yellow. Generally smaller, more variable effects were found for unique blue, green, and red. On average though, unique blue showed a rod green bias, and unique red and unique green showed a blue bias.
With 0.5°-diameter foveal test stimuli (Fig. 2), fewer observers showed the rod green bias, and the average magnitude was reduced. For the other unique hues, average rod hue biases were eliminated, although some observers still showed idiosyncratic individual effects, which in a few cases appear larger than those found for the 2.0° stimuli.
4. DISCUSSION
A rod green bias at unique yellow was the largest and most common foveal rod hue bias found on the CRT display for both 2.0° and 0.5° diameter test stimuli. Even though reduction of the test diameter to 0.5° reduced average rod green bias, it was still found for at least half the observers.
This foveal rod green bias was observed in a higher proportion of observers than in prior foveal studies [7,8,10]. Likely, our testing of a larger number of observers—eight compared to three in the prior studies—provided a more precise incidence estimate and reduced the role of chance sampling distortions. Possibly also some aspect of the free viewing of a CRT display, may have facilitated the occurrence of rod green bias relative to past studies, which used monochromatic lights presented in Maxwellian view. This seems less likely because the broader-spectrum and reduced purity of the CRT phosphors and the greater variation of retinal illuminance without control of observer pupil size might be expected to reduce, not increase, the magnitude of effects. In any case, we believe that the present results provide the best estimates to date of the incidence and magnitude of foveal rod hue biases.
Foveal stimuli disadvantaged the rod blue bias (seen at unique green and unique red) and rod red bias (seen at unique blue) that have been observed for extrafoveal stimuli and that are presumably mediated by interaction of rod signals with S-cone signals. These results are consistent with past studies of foveal rod influences [7,8,10].
Foveal rod blue bias was shown by only about half the observers for 2° stimuli, and fewer for 0.5° stimuli. Both magnitude and direction of interaction varied among observers and between test sizes for several observers. The overall mean effects across observers were barely larger than the standard error of the mean for 2° stimuli and smaller than the standard error for the 0.5° stimuli.
Rod red bias at unique blue was essentially eliminated by the present foveal stimuli: in only two instances across all observers and conditions was its magnitude even barely larger than the standard error bars. Instead, we were more likely to see a rod green bias at unique blue. Extrafoveal studies have shown that green and red rod hue biases compete with each other at unique blue, apparently because the short-wavelength stimulation allows rod influence through both L-M-cone pathways (producing the green bias also seen at unique yellow) and S-cone pathways (producing the red bias seen only at unique blue). Brief durations of extrafoveal test stimuli have been shown to disadvantage the rod red bias and uncover the rod green bias [15–17], suggesting that the rod red bias has a slower time course than the quicker green bias. The reason that foveal stimulus placement also disadvantages the rod red bias is not clear. On the one hand, S-cones are relatively sparse within 1° of the foveal center [18,19], so the substrate for the S-cone mediated rod red bias may be weakened, while the substrate for the L-M-cone mediated rod green bias remains strong. (Recall that for our equiluminant stimuli, the overall stimulation of L and M cones is equal for both yellow and blue stimuli.)
On the other hand, neither the present study nor a prior study [8] shows rod red bias to be more likely for larger foveal stimuli that would be expected to stimulate more S cones. Indeed, the two present instances of small but reliable rod red bias were both for the smaller 0.5° test stimulus, which would be expected to stimulate fewer S cones than would the 2° stimulus. Thus, the reasons for the weakened rod red bias for foveal stimuli remain unresolved.
Even though a rod red bias is seldom directly observed under the present foveal conditions, the generally smaller rod green bias at unique blue than at unique yellow, at the same luminance, may reveal the canceling effect of residual rod red bias at unique blue. Whether other factors (e.g., lower L/M ratio and higher S-cone stimulation at unique blue) also contribute to the different magnitudes of rod green bias is not known.
The present study makes clear that the rod green bias differs from the other two rod hue biases in its higher prevalence of influence on foveal stimuli. Prior studies have also noted the faster time course [15–17] and luminance dependence [11,12,3] of the rod green bias. These differences, as well as the specific spectral ranges and unique hues for which the rod hue biases occur, support the idea that the rod green bias is mediated by interaction of signals from rods and M and L cones, presumably in midget-ganglion-cell pathways, and the rod red bias and blue bias are mediated by interaction of signals from rods and S cones, possibly in small-bistratified-ganglion-cell pathways. Physiological studies [4,5] have shown that rod signals, like S-cone signals, drive ON responses in primate small-bistratified ganglion cells, which could provide a neural substrate for the present psychophysically observed rod red bias and rod blue bias. Although it seems likely that the rod green bias is mediated by midget-ganglion-cell pathways, the source of the differential weighting of rod influence that produces the green bias is not yet clear. Work in our lab [20] and elsewhere [21] indicates that the magnitude of rod green bias is independent of an observer’s L:M cone ratio, but another specific physiological substrate has not yet been demonstrated.
We have shown that even small foveal stimuli do not necessarily prevent rod hue biases. Indeed, most observers show a rod green bias for mesopic foveal stimuli, and individual observers can show additional idiosyncratic and unpredictable rod hue biases. This is a matter of practical concern for investigators studying foveal color vision at lower light levels with CRT or similar displays. It is not clear how high the light level must be to be sure of avoiding foveal rod hue biases but extrafoveal rod hue biases occur at luminances up to at least 2.6 cd/m2 for some observers [10].
Footnotes
OCIS codes: (330.1720) Color vision; (330.5020) Perception psychology; (330.5510) Psychophysics; (330.5310) Vision - photoreceptors.
References
- 1.Buck SL. Rod-cone interactions. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. MIT; 2004. pp. 863–878. [Google Scholar]
- 2.Buck SL. The interaction of rod and cone signals: pathways and psychophysics. In: Werner JS, Chalupa LM, editors. The New Visual Neurosciences. MIT; 2014. pp. 485–497. [Google Scholar]
- 3.Buck SL, Knight R, Fowler G, Hunt B. Rod influence on hue-scaling functions. Vis Res. 1998;38:3259–3263. doi: 10.1016/s0042-6989(97)00436-7. [DOI] [PubMed] [Google Scholar]
- 4.Field GD, Greschner M, Gauthier JL, Rangel C, Shlens J, Sher A, Marshak DW, Litke AM, Chichilnisky EJ. High-sensitivity rod photoreceptor input to the blue-yellow color opponent pathway in macaque retina. Nat Neurosci. 2009;12:1159–1164. doi: 10.1038/nn.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crook JD, Davenport CM, Peterson BB, Packer OS, Detwiler PB, Dacey DM. Parallel ON and OFF cone bipolar inputs establish spatially coextensive receptive field structure of blue-yellow ganglion cells in primate retina. J Neurosci. 2009;29:8372–8387. doi: 10.1523/JNEUROSCI.1218-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nerger JL, Volbrecht VJ, Ayde CJ. Unique hue judgments as a function of test size in the fovea and at 20-deg temporal eccentricity. J Opt Soc Am A. 1995;12:1225–1232. doi: 10.1364/josaa.12.001225. [DOI] [PubMed] [Google Scholar]
- 7.Thomas L, Buck SL. Foveal vs. extra-foveal contributions to rod hue biases. Vis Neurosci. 2006;23:539–542. doi: 10.1017/S0952523806233509. [DOI] [PubMed] [Google Scholar]
- 8.Buck SL, Thomas L, Hillyer N, Samuelson E. Do rods influence the hue of foveal stimuli? Vis Neurosci. 2006;23:519–523. doi: 10.1017/S0952523806233510. [DOI] [PubMed] [Google Scholar]
- 9.Buck SL, Cunningham C. Rod influence on desaturated color mixtures. J Vision. 2009;9(14):55. [Google Scholar]
- 10.Buck SL, Juve R, Wisner D, Concepcion A. Rod hue biases produced on CRT displays. J Opt Soc Am A. 2012;29:A36–A43. doi: 10.1364/JOSAA.29.000A36. [DOI] [PubMed] [Google Scholar]
- 11.Thomas L, Buck SL. Generality of rod hue biases with smaller, brighter, and photopically specified stimuli. Vis Neurosci. 2004;21:257–262. [PubMed] [Google Scholar]
- 12.Knight R, Buck SL. Rod influences on hue perception: Effect of background light level. Color Res Appl. 2001;26(S1):S60–S64. [Google Scholar]
- 13.Smith VC, Pokorny J. Spectral sensitivity of the foveal cone pigments between 400 and 500 nm. Vis Res. 1975;15:161–171. doi: 10.1016/0042-6989(75)90203-5. [DOI] [PubMed] [Google Scholar]
- 14.Smith VC, Pokorny J. Color matching and color discrimination. In: Shevell SK, editor. The Science of Color. 2. Elsevier; 2003. pp. 103–148. [Google Scholar]
- 15.Knight R, Buck SL. Time-dependent changes of rod influence on hue perception. Vis Res. 2002;42:1651–1662. doi: 10.1016/s0042-6989(02)00087-1. [DOI] [PubMed] [Google Scholar]
- 16.Buck SL, Knight R. Stimulus duration affects rod influence on hue perception. In: Mollon JD, Pokorny J, Knoblauch K, editors. Normal and Defective Colour Vision. Oxford University; 2003. pp. 177–184. [Google Scholar]
- 17.Buck SL, Thomas L, Connor C, Green K, Quintana T. Time-course of rod influences on hue perception. Vis Neurosci. 2008;25:517–520. doi: 10.1017/S0952523808080279. [DOI] [PubMed] [Google Scholar]
- 18.Williams DR, MacLeod DI, Hayhoe MM. Foveal tritanopia. Vis Res. 1981;21:1341–1356. doi: 10.1016/0042-6989(81)90241-8. [DOI] [PubMed] [Google Scholar]
- 19.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 20.Foote KG, Buck SL, Neitz J, Neitz M. Psychophysiscal consequences of L/M cone ratio. presented at the OSA Vision Meeting; Houston, Texas. October 4–6, 2013. [Google Scholar]
- 21.Cao D, Pokorny J, Smith VC. Matching rod percepts with cone stimuli. Vis Res. 2005;45:2119–2128. doi: 10.1016/j.visres.2005.01.034. [DOI] [PubMed] [Google Scholar]