Abstract
BACKGROUND
Obesity is a low-grade chronic inflammation. This epidemic is growing in different age groups including adolescents. It is accompanied with a decrease in the age for incidence of obesity-related disorders. Chemerin, as a chemokine and stimulator of anti-inflammatory adiponectin, links immune system, adipose tissue and inflammation. It may be useful in predicting obesity in the hit phase of life. This study aims to assess serum chemerin and adiponectin in relation to the inflammation and obesity indices.
METHODS
This case-control study was conducted on 82 adolescent girls, aged12-18 years. They were categorized based on the percentiles of the body mass index (BMI).Serum chemerin, adiponectin, high-sensitive C-reactive protein (Hs-CRP), body fat mass and its percent, waist circumference (WC) ,hip circumference (HC) were measured; BMI and waist-to-hip ratio (WHR)were calculated. Data were analyzed by independent Student’s t-test and Pearson correlation; path analysis was conducted, as well.
RESULTS
We found a negative significant association between chemerin and adiponectin levels in both obese and non-obese groups(r =-0.387, P = 0.014 vs. r = 0.362, respectively, P = 0.018). Serum chemerin was higher in obese than in non-obese adolescents (441.83 ± 47.79 vs. 409.30 ± 66.12 µg /l, respectively, P = 0.012), whereas mean adiponectin level was lower in obese participants than in the other group (4.79 ± 0.94 versus 5.2 ± 0.53µg/ml, respectively, P = 0.016). Chemerin concentrations had significant positive correlation with Hs-CRP levels, BMI, WC, HC, WHR, body fat mass and its percent (P < 0.05).
CONCLUSION
Chemerin concentrations were associated with and adiponectin levels in obese girl adolescents, negatively. Hs-CRP, BMI, WC, HC, WHR, body fat mass and its’ percent were in positive relation with chemerin levels, and inverse association with serum adiponectin concentrations. Our findings suggest that chemerin can be considered as an early marker of the inflammatory process in obesity.
Keywords: Chemerin, Adiponectin, Obesity, Inflammation, Adolescents
Introduction
Obesity is a growing health problem which is accompanied with increasing the rate of morbidity and mortality.1 During the recent decades, the prevalence of obesity in childhood and adolescent is doubled.2 The probability of being obese adults is higher in obese adolescents and this risk is much higher in girls.3 Unfortunately, the incidence of obesity-based diseases is increasing sharply in recent years and its’ control need to urgent programming among adolescents.4
Obesity is an excess of white adipose tissue (WAT) which is known by chronic inflammation of adipose tissue.5 The pathophysiologic mechanisms that link the inflammation of obesity and obesity-related disorders to the innate and adaptive immune system may be based on the multifunctional roles of adipose mass. Adipose tissue is a fat supply6 and endocrine organ. It can be effective in energy balance and macronutrient metabolism.6,7
One of its’ pro-inflammatory adipokine is chemerin which has autocrine and paracrine roles in enhancing the adipocyte differention1,7 and lipolysis of WAT.1,7 It reflects chemokine roles in leukocytes direction and the recruitment of antigen presenting cells1,8 such as macrophage1,9,10 dendritic cells (DC),9,10 and natural killer cells.9,10 Furthermore, chemerin is an adiponectin secretion stimulator and adiponectin gene expression is reduced in chemerin knockdown cells.7
Adiponectin is characterized as an anti-inflammatory11 and anti-atherogenic adipokine.12 It can suppress adipocytes differention12 and it’s effect is mediated by increasing cyclic adenosine monophosphate (cAMP) accumulation,13 while chemerin effects in managing cell metabolism, mediated by stimulating calcium release and inhibiting cAMP accumulation supply.14,15
The controversial observation about the functions of mediated second massagers attracted us to study serum levels of these adipokines in obese adolescents. Hence, the main goal of this study is assessing chemerin levels in relation to adiponectin concentrations and general and central obesity and inflammation index in girl adolescents for the first time.
Materials and Methods
This is a case-control study which was conducted on 82 healthy non-athlete and non-smoker girl adolescents sampled from Sadigheh-Tahereh Hospital, Isfahan, Iran.16 They were between 12 and 18 years old. Detailed medical and family history was checked. Participants had been matched based on their mentioned variables, individually. Their exclusion criteria were determined as having any history of chronic, inflammatory, infective, metabolic or endocrine diseases and taking any drugs or supplements. The informed written consent was achieved from the parents of all of the adolescents and participants who accepted to participate.
The survey was performed from August 2010 to December 2010. The participants were divided by CDC percentiles of body mass index (BMI) according to the age and sex.2,17 The obese group is defined based on BMI percentile > 95th and the normal-weight group is described as having BMI percentile 5-85th, they were matched according to age and sex.18
The height was assessed using calibrated Seca meter to the nearest 0.1 cm at maximal respiration (SECA, Hamburg, Germany).2 Their weight was measured by Seca scale closest to 0.1 kg in barefoot and light dress, after emptying urinary and GI apparatuses (Seca Model 770, Hamburg, Germany). BMI was calculated as dividing weight by the square of height (kg/m2).19
The waist circumstance (WC)2 was determined at the point midway between the superior border of the iliac crest and lower border of the rib cage using non-elastic tape nearest to 0.5 cm at the normal expiration, and their hip circumstance (HC) was measured to the nearest 0.5 cm at the widest part of the hip at the levels of the greatest trochanter by a non-stretchable tape meter.2 The waist to hip ratio (WHR) was computed from dividing waist by HC.17
The body fat percent was measured by bioelectrical impedance analysis (BioScan 916 Maltron, Rayleigh, UK).20 The participants puberty were assessed according to the criteria of Tanner stage by general physician.17,21 All of the measurements were performed by informed expert.
The blood sample was taken from the antecubital venous after 10 h of overnight fast between 8 and 10 AM. Blood sample were centrifuged for 15 min at 4500 g within 30 min of sampling and frozen at −70 °C.22
Serum chemerin levels were determined by an enzyme-linked immunosorbent assay (ELISA) (BioVendor Research and Diagnostic Products, Inc., Modrice Czech Republic). The detection limit of the assay was 0.13 μg/l, and the intra-assay and inter-assay coefficient of variation (CV) were 7 and 6.9%, respectively.23-25
Serum adiponectin was measured by ELISA method, Orgenium Laboratories, Inc. (AviBion Human Adiponectin (Acrp30) ELISA Kit, Helsinki, Finland) the intra-and inter assay CVs are ≤ 10 and ≤ 12% respectively.19 High sensitive C-reactive protein (Hs-CRP) was measured by ELISA (DRG-Diagnostica, Marburg, Germany); the intra- and inter assay CVs are 5.1 and 14.3%, respectively.26
Data were analyzed using SPSS software (version 15.0, SPSS Inc., Chicago, IL, USA) and AMOS software (version 16, ADC, Chicago, IL). Distribution of parameters were checked for normality using Kolmogrov-Smirnov test. All variables were compared using independent Student’s t-test between two groups. Pearson correlation tests were used to analysis the bivariate associations of variables. Path analysis was performed using weighted least squares procedures to explore whether Hs-CRP mediated the relation between adiposity and adipokines. Variables without normal distribution had been transformed, statistically. Standardized path coefficients and t-values are provided. The final model was completely saturated, with degrees of freedom equal to zero. Descriptive fit indices, comparative fit index, root mean square error of approximation and goodness-of-fit for the models were acceptable. The threshold for analysis assess was set at P < 0.05.
Results
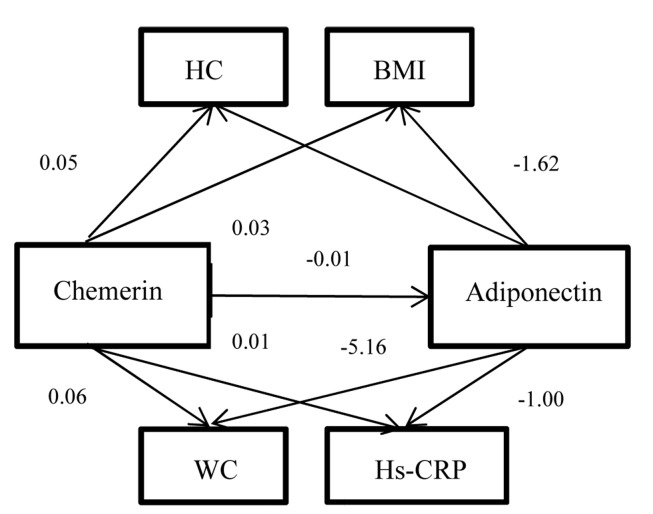
A sample consisted of 40 obese and 42 normal-weight girl adolescents were studied. Demographic and clinical characteristics of the population are presented in table 1. Participants were categorized in post-pubertal stage (Stage V). There was not significant difference between demographic characteristics of participants (data not shown). There was a negative association between chemerin and adiponectin levels in both groups (Pearson correlation, r = -0.387, P = 0.014 vs. r = -0.362, P = 0.018, respectively). Chemerin levels were higher in obese group than normal-weight adolescents. There was a positive association between chemerin levels and BMI in all of the adolescents, significantly. The same association was seen between chemerin concentrations and WC (Pearson correlation, r = +0.451, P = 0.004 vs. r = 0.317, P = 0.041, respectively), HC (Pearson correlation, r = +0.338, P = 0.036 vs. r = +0.349, P = 0.027, respectively), WHR (Pearson correlation, r = +0.419, P = 0.007 vs. r = +0.325, P = 0.036, respectively), body fat mass (Pearson correlation, r = +0.366, P = 0.020 vs. r = +0.352, P = 0.028, respectively) and its’ percent (Pearson correlation, r = +0. 416, P = 0.008 vs. r = +0.423, P = 0.005). We found a positive correlation between serum chemerin and Hs-CRP levels in obese adolescents (Pearson correlation, r = +0.325, P = 0.047). There adiponectin levels were higher in normal-weight girls than obese group (P = 0.018). Adiponectin concentrations was inversely correlated with BMI (Pearson correlation, r = -0.369, P = 0.019 vs. r = -0.421, P = 0.018, respectively), WC (Pearson correlation, r = -0.427, P = 0.007 vs. r = -0.423, P = 0.005, respectively), HC (Pearson correlation, r = -0.390, P = 0.014 vs. r = -0.333, P = 0.036, respectively), WHR (Pearson correlation, r = -0.361, P = 0.022 vs. r = -0.312, P = 0.044, respectively), body fat mass (Pearson correlation, r = -0.528, P < 0.0001 vs. r = -0.346, P = 0.031, respectively) and its’ percent (Pearson correlation, r = -0.386, P = 0.014 vs. r = -0.340, P = 0.028, respectively) in obese and normal-weight girls (P < 0.05).We observed a negative association between adiponectin and Hs-CRP concentrations in obese adolescents (Pearson correlation, r = -0.361, P = 0.026). Estimated value for the standardized regression weights of the relation between chemerin and adiponectin levels with general, central obesity indices and Hs-CRP are shown in table 2. Path analytical diagram between chemerin, adiponectin and obesity indices is represented in figure 1.
Table 1.
Anthropometric, demographic and biochemical data from the study girl adolescents (n = 82)
| Variables | Groups |
P | |
|---|---|---|---|
| Obese adolescents (n = 40) | Non-obese adolescents (n = 42) | ||
| Age (year) | 13.90 ± 1.80 | 14.63 ± 2.22 | 0.1070 |
| Weight (kg) | 75.70 ± 7.55 | 49.20 ± 3.10 | < 0.0001* |
| BMI (kg/m2) | 29.50 ± 2.22 | 19.00 ± 0.77 | < 0.0001* |
| Body fat mass (kg) | 27.20 ± 4.03 | 11.80 ± 3.20 | < 0.0001* |
| Body fat (%) | 37.80 ± 2.94 | 23.80 ± 4.86 | < 0.0001* |
| WC (cm) | 95.30 ± 6.85 | 73.90 ± 5.03 | < 0.0001* |
| HC (cm) | 110.00 ± 5.30 | 92.50 ± 4.20 | < 0.0001* |
| WHR (cm/cm) | 0.85 ± 0.04 | 0.79 ± 0.46 | < 0.0001* |
| Hs-CRP (mg/dl) | 3.80 ± 2.42 | 0.78 ± 0.68 | < 0.0001* |
| Adiponectin (آµg/ml) | 4.79 ± 0.94 | 5.20 ± 0.53 | 0.0160* |
| Chemerin (آµg/l) | 441.83 ± 47.49 | 409.30 ± 66.12 | 0.0120* |
Statistically significant differences between obese and normal-weight participants (P < 0.05 is significant);
The comparisons were done using independent Student’s t-test; Results are presented as mean ± standard deviation; BMI: Body mass index; WC: Waist circumstance; HC: Hip circumstance; WHR: Waist to hip ratio; Hs-CRP: High sensitive C-reactive protein
Table 2.
Estimated value for the standardized regression weights of the relation between chemerin and adiponectin levels with general, central obesity indices and Hs-CRP
| Variables | BMI (kg/m2) | HC (cm) | WC (cm) | Hs-CRP (mg/dl) | P |
|---|---|---|---|---|---|
| Chemerin (μg/l) | 0.03 | 0.05 | 0.06 | 0.01 | < 0.05 |
| Adiponectin (μg/dl) | -1.62 | -3.66 | -5.16 | -1.00 | < 0.05 |
BMI: Body mass index; HC: Hip circumference; WC: Waist circumference; Hs-CRP: High sensitive C-reactive protein
Figure 1.
Path analytical diagram between chemerin, adiponectin and obesity indices HC: Hip circumference; BMI: Body mass index; WC: Waist circumference; Hs-CRP: High sensitive C-reactive protein
Discussion
To our knowledge, it is the first study that found a negative relation between chemerin and adiponectin levels in girl adolescents. A negative association was seen between chemerin and adiponectin mRNA expression in epicardial adipose tissue of patients.3 Similar negative association was observed, peviously. However, Weigert et al. found non-significant association between these two adipokines.27 Goralski et al. showed that chemerin knockdown gene decreased adiponectin expression,7 and chemerin is introduced as a stimulator of adiponectin gene expression. Chemerin and its receptor secretion and function changes during adipogenesis, and its’ gene transcription increases and chemokine like receptor 1 expression decreases during 3T3-L1 cells differention, and it could be compared with leptin resistance in obese subjects.6 Higher concentrations of chemerin may be accompany with the desensitization of its receptor or obstruction of chemerin signaling during adipocytes differention.7 The indirect association between chemerin and adiponectin concentrations may be based on a compensatory increase of chemerin levels. General obesity increases serum chemerin levels and chemerin gene expression is higher in obese subjects in comparison with leans and this observation were similar to the previous studies.6,23,24,28 Similar to the previous findings, we seen positive association between chemerin levels and BMI,3,22,27,29 central obesity indices including WC,3,29-31 HC,31 body fat mass24,32 and its’ percent.6,28,31 Adolescents with higher central fat area had higher serum chemerin concentrations.24,27,29,32 Serum Hs-CRP and chemerin levels were correlated positively and it is based on that low-grade inflammation of obesity,5 which associated with increasing acute-phase proteins and pro-inflammatory cytokines levels5 and increasing inflammatory markers release as the immune cells reflection.5,12 The macrophage act as an antigen presenting cell and the existence of chemerin receptors on immature DCs shows the chemerin roles in the recruitment and migration of macrophage and premature DCs which can be known as a link between immune system functions and excessive adipose tissue.33 Interleukin 6 production which can increase after infiltration of macrophages to adipocytes.5,12 Induces CRP secretion, indirectly or there may be possibility that WAT act as the source of these factors.12 Direct relation between BMI and CRP was seen, previously, too.22,27,30-32,34 Our data indicated that girls with higher BMI percentile according to the age and sex had lower adiponectin levels like other studies.11,16-17,35-38 This finding is as the same as negative correlation between adiponectin levels and BMI.11,17,35,37-38 The negative correlation between serum adiponectin and WC was similar to Bottner et al. observation,17 however, Vikram et al.35 and Snehalatha et al. surveys39 reflected no significant association that can be raised from not paying attention to the pubertal stages of subjects or different species of participants. There was a negative relationship between adiponectin levels and HC, like observation of Bottner et al. assessment.17 Body fat mass and its percent showed a negative association with serum adiponectin concentration. Adolescents with higher body fat showed a lower adiponectin levels. This relation is as the same as findings of Vikram et al.,35 Panagopoulou et al.,11 Bauche et al.37 and Snehalatha et al. studies.39
There was a negative association between serum adiponectin and CRP levels. This observation confirmed by a negative relation between adiponectin expression and CRP levels, and it can reflect the adipose tissue roles in the circulation source of CRP.5 Adiponectin suppresses macrophage phagocytosis process5,12 and it can affect on its cytokine expression. Several studies confirmed our finding,40-42 while Wagner et al. study showed that CRP levels are lower in children group and it can be the base of non-significant association between CRP and adiponectin.43 There were some limitations in the present study including the nature of cross-sectional study and small size of participants, and hence future study on the larger number of the participants and in prospective study are recommended.
Conclusion
In conclusion, our study shows that serum chemerin concentrations were associated with general and abdominal obesity indices, adiponectin and Hs-CRP levels in obese adolescents. It may be based on a potent pathophysiologic link between adipokines and inflammation. These findings suggest that chemerin may be an mediated marker which reflect central and general obesity in response to the immunity status. Further research is suggested to confirm these observations and to assess serum chemerin levels in younger age and in various chemerin and adiponectin isoforms.
Acknowledgments
We wish to thank all the participants and their parents to participate in all study process, patiently.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Takahashi M, Takahashi Y, Takahashi K, Zolotaryov FN, Hong KS, Kitazawa R, et al. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. FEBS Lett. 2008;582(5):573–8. doi: 10.1016/j.febslet.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 2.Kelishadi R, Ardalan G, Gheiratmand R, Majdzadeh R, Hosseini M, Gouya MM, et al. Thinness, overweight and obesity in a national sample of Iranian children and adolescents: CASPIAN Study. Child Care Health Dev. 2008;34(1):44–54. doi: 10.1111/j.1365-2214.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 3.Gao X, Mi S, Zhang F, Gong F, Lai Y, Gao F, et al. Association of chemerin mRNA expression in human epicardial adipose tissue with coronary atherosclerosis. Cardiovasc Diabetol. 2011;10:87. doi: 10.1186/1475-2840-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badman MK, Flier JS. The adipocyte as an active participant in energy balance and metabolism. Gastroenterology. 2007;132(6):2103–15. doi: 10.1053/j.gastro.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 5.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17(1):4–12. [PubMed] [Google Scholar]
- 6.Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148(10):4687–94. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 7.Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282(38):28175–88. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 8.Allen SJ, Zabel BA, Kirkpatrick J, Butcher EC, Nietlispach D, Handel TM. NMR assignment of human chemerin, a novel chemoattractant. Biomol NMR Assign. 2007;1(2):171–3. doi: 10.1007/s12104-007-9047-7. [DOI] [PubMed] [Google Scholar]
- 9.Xiang D, Zhang J, Chen Y, Guo Y, Schalow A, Zhang Z, et al. Expressions and purification of a mature form of recombinant human Chemerin in Escherichia coli. Protein Expr Purif. 2010;69(2):153–8. doi: 10.1016/j.pep.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Berg V, Sveinbjornsson B, Bendiksen S, Brox J, Meknas K, Figenschau Y. Human articular chondrocytes express ChemR23 and chemerin; ChemR23 promotes inflammatory signalling upon binding the ligand chemerin(21-157). Arthritis Res Ther. 2010;12(6):R228. doi: 10.1186/ar3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panagopoulou P, Galli-Tsinopoulou A, Fleva A, Pavlitou-Tsiontsi E, Vavatsi-Christaki N, Nousia-Arvanitakis S. Adiponectin and insulin resistance in childhood obesity. J Pediatr Gastroenterol Nutr. 2008;47(3):356–62. doi: 10.1097/MPG.0b013e31817fcb67. [DOI] [PubMed] [Google Scholar]
- 12.Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand. 2005;184(4):285–93. doi: 10.1111/j.1365-201X.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- 13.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102(11):1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 14.Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le PE, Migeotte I, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198(7):977–85. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roh SG, Song SH, Choi KC, Katoh K, Wittamer V, Parmentier M, et al. Chemerin a new adipokine that modulates adipogenesis via its own receptor. Biochem Biophys Res Commun. 2007;362(4):1013–8. doi: 10.1016/j.bbrc.2007.08.104. [DOI] [PubMed] [Google Scholar]
- 16.Weiss R, Dufour S, Groszmann A, Petersen K, Dziura J, Taksali SE, et al. Low adiponectin levels in adolescent obesity: a marker of increased intramyocellular lipid accumulation. J Clin Endocrinol Metab. 2003;88(5):2014–8. doi: 10.1210/jc.2002-021711. [DOI] [PubMed] [Google Scholar]
- 17.Bottner A, Kratzsch J, Muller G, Kapellen TM, Bluher S, Keller E, et al. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab. 2004;89(8):4053–61. doi: 10.1210/jc.2004-0303. [DOI] [PubMed] [Google Scholar]
- 18.Doost Mohammadian A, Dorosty A, Mahmoodi M, Yeganeh H. The survey of the nutritional factors related to weight of girl adolescents. Iranian journal of nutrition and food science. 2009:51–6. [Google Scholar]
- 19.Ozkan B, Doneray H, Keskin H. The effect of vitamin D treatment on serum adiponectin levels in children with vitamin D deficiency rickets. J Clin Res Pediatr Endocrinol. 2009;1(6):262–5. doi: 10.4274/jcrpe.v1i6.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Husain R, Yen Tan S. Proceedings of the 4th Asia-Oceania Conference on Obesity; Seoul, South Korea.: 2007. Body composition of healthy Malaysian adults using bioimpedance analysis. [Google Scholar]
- 21.Punthakee Z, Delvin EE, O'loughlin J, Paradis G, Levy E, Platt RW, et al. Adiponectin, adiposity, and insulin resistance in children and adolescents. J Clin Endocrinol Metab. 2006;91(6):2119–25. doi: 10.1210/jc.2005-2346. [DOI] [PubMed] [Google Scholar]
- 22.Lehrke M, Becker A, Greif M, Stark R, Laubender RP, von ZF, et al. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur J Endocrinol. 2009;161(2):339–44. doi: 10.1530/EJE-09-0380. [DOI] [PubMed] [Google Scholar]
- 23.Stejskal D, Karpisek M, Hanulova Z, Svestak M. Chemerin is an independent marker of the metabolic syndrome in a Caucasian population--a pilot study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008;152(2):217–21. doi: 10.5507/bp.2008.033. [DOI] [PubMed] [Google Scholar]
- 24.Sell H, Laurencikiene J, Taube A, Eckardt K, Cramer A, Horrighs A, et al. Chemerin is a novel adipocyte-derived factor inducing insulin resistance in primary human skeletal muscle cells. Diabetes. 2009;58(12):2731–40. doi: 10.2337/db09-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopp HP, Krzyzanowska K, Mohlig M, Spranger J, Pfeiffer AF, Schernthaner G. Effects of marked weight loss on plasma levels of adiponectin, markers of chronic subclinical inflammation and insulin resistance in morbidly obese women. Int J Obes (Lond) 2005;29(7):766–71. doi: 10.1038/sj.ijo.0802983. [DOI] [PubMed] [Google Scholar]
- 26.Kelishadi R, Sharifi M, Khosravi A, Adeli K. Relationship between C-reactive protein and atherosclerotic risk factors and oxidative stress markers among young persons 10-18 years old. Clin Chem. 2007;53(3):456–64. doi: 10.1373/clinchem.2006.073668. [DOI] [PubMed] [Google Scholar]
- 27.Weigert J, Neumeier M, Wanninger J, Filarsky M, Bauer S, Wiest R, et al. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin Endocrinol (Oxf) 2010;72(3):342–8. doi: 10.1111/j.1365-2265.2009.03664.x. [DOI] [PubMed] [Google Scholar]
- 28.Bozaoglu K, Segal D, Shields KA, Cummings N, Curran JE, Comuzzie AG, et al. Chemerin is associated with metabolic syndrome phenotypes in a Mexican-American population. J Clin Endocrinol Metab. 2009;94(8):3085–8. doi: 10.1210/jc.2008-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin HY, Lee DC, Chu SH, Jeon JY, Lee MK, Im JA, et al. Chemerin levels are positively correlated with abdominal visceral fat accumulation. Clin Endocrinol (Oxf) 2012;77(1):47–50. doi: 10.1111/j.1365-2265.2011.04217.x. [DOI] [PubMed] [Google Scholar]
- 30.Hah YJ, Kim NK, Kim MK, Kim HS, Hur SH, Yoon HJ, et al. Relationship between Chemerin Levels and Cardiometabolic Parameters and Degree of Coronary Stenosis in Korean Patients with Coronary Artery Disease. Diabetes Metab J. 2011;35(3):248–54. doi: 10.4093/dmj.2011.35.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tonjes A, Fasshauer M, Kratzsch J, Stumvoll M, Bluher M. Adipokine pattern in subjects with impaired fasting glucose and impaired glucose tolerance in comparison to normal glucose tolerance and diabetes. PLoS One. 2010;5(11):e13911. doi: 10.1371/journal.pone.0013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ress C, Tschoner A, Engl J, Klaus A, Tilg H, Ebenbichler CF, et al. Effect of bariatric surgery on circulating chemerin levels. Eur J Clin Invest. 2010;40(3):277–80. doi: 10.1111/j.1365-2362.2010.02255.x. [DOI] [PubMed] [Google Scholar]
- 33.Zabel BA, Allen SJ, Kulig P, Allen JA, Cichy J, Handel TM, et al. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J Biol Chem. 2005;280(41):34661–6. doi: 10.1074/jbc.M504868200. [DOI] [PubMed] [Google Scholar]
- 34.Dong B, Ji W, Zhang Y. Elevated serum chemerin levels are associated with the presence of coronary artery disease in patients with metabolic syndrome. Intern Med. 2011;50(10):1093–7. doi: 10.2169/internalmedicine.50.5025. [DOI] [PubMed] [Google Scholar]
- 35.Vikram NK, Misra A, Pandey RM, Dwivedi M, Luthra K. Adiponectin, insulin resistance, and C-reactive protein in postpubertal Asian Indian adolescents. Metabolism. 2004;53(10):1336–41. doi: 10.1016/j.metabol.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Silha JV, Krsek M, Skrha JV, Sucharda P, Nyomba BL, Murphy LJ. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: correlations with insulin resistance. Eur J Endocrinol. 2003;149(4):331–5. doi: 10.1530/eje.0.1490331. [DOI] [PubMed] [Google Scholar]
- 37.Bauche IB, El Mkadem SA, Pottier AM, Senou M, Many MC, Rezsohazy R, et al. Overexpression of adiponectin targeted to adipose tissue in transgenic mice: impaired adipocyte differentiation. Endocrinology. 2007;148(4):1539–49. doi: 10.1210/en.2006-0838. [DOI] [PubMed] [Google Scholar]
- 38.Valle M, Martos R, Gascon F, Canete R, Zafra MA, Morales R. Low-grade systemic inflammation, hypoadiponectinemia and a high concentration of leptin are present in very young obese children, and correlate with metabolic syndrome. Diabetes Metab. 2005;31(1):55–62. doi: 10.1016/s1262-3636(07)70167-2. [DOI] [PubMed] [Google Scholar]
- 39.Snehalatha C, Yamuna A, Ramachandran A. Plasma adiponectin does not correlate with insulin resistance and cardiometabolic variables in nondiabetic Asian Indian teenagers. Diabetes Care. 2008;31(12):2374–9. doi: 10.2337/dc08-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsushita K, Yatsuya H, Tamakoshi K, Wada K, Otsuka R, Takefuji S, et al. Comparison of circulating adiponectin and proinflammatory markers regarding their association with metabolic syndrome in Japanese men. Arterioscler Thromb Vasc Biol. 2006;26(4):871–6. doi: 10.1161/01.ATV.0000208363.85388.8f. [DOI] [PubMed] [Google Scholar]
- 41.Gilardini L, McTernan PG, Girola A, da Silva NF, Alberti L, Kumar S, et al. Adiponectin is a candidate marker of metabolic syndrome in obese children and adolescents. Atherosclerosis. 2006;189(2):401–7. doi: 10.1016/j.atherosclerosis.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 42.Winer JC, Zern TL, Taksali SE, Dziura J, Cali AM, Wollschlager M, et al. Adiponectin in childhood and adolescent obesity and its association with inflammatory markers and components of the metabolic syndrome. J Clin Endocrinol Metab. 2006;91(11):4415–23. doi: 10.1210/jc.2006-0733. [DOI] [PubMed] [Google Scholar]
- 43.Wagner A, Simon C, Oujaa M, Platat C, Schweitzer B, Arveiler D. Adiponectin is associated with lipid profile and insulin sensitivity in French adolescents. Diabetes Metab. 2008;34(5):465–71. doi: 10.1016/j.diabet.2008.02.006. [DOI] [PubMed] [Google Scholar]