Abstract
While growing in the tsetse fly, Trypanosoma brucei expresses a major surface glycoprotein, the procyclic acidic repetitive protein (PARP). The parp genes are transcribed by an alpha-amanitin-resistant RNA polymerase. We have determined the sequence requirements for parp promoter activity. Studies of RNA produced from input DNA in transiently transfected trypanosomes indicate that the RNA is correctly processed by trans-splicing and polyadenylation. Deletion analyses show that 330 bp are sufficient for full promoter and splicing activity and that the promoter structure is complex, involving at least three elements whose mutual spacing is important. Mutagenesis pin-pointed two sequences vital for promoter activity; neither bears any resemblance to known prokaryotic or eukaryotic promoter elements.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian N. Trans splicing of nuclear pre-mRNAs. Cell. 1990 Jun 29;61(7):1157–1160. doi: 10.1016/0092-8674(90)90674-4. [DOI] [PubMed] [Google Scholar]
- Anderson S. J., Chou H. S., Loh D. Y. A conserved sequence in the T-cell receptor beta-chain promoter region. Proc Natl Acad Sci U S A. 1988 May;85(10):3551–3554. doi: 10.1073/pnas.85.10.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellofatto V., Cross G. A. Expression of a bacterial gene in a trypanosomatid protozoan. Science. 1989 Jun 9;244(4909):1167–1169. doi: 10.1126/science.2499047. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Tjian R. Transcription factors that activate the Ultrabithorax promoter in developmentally staged extracts. Cell. 1988 Jun 3;53(5):699–711. doi: 10.1016/0092-8674(88)90088-8. [DOI] [PubMed] [Google Scholar]
- Bucher P., Trifonov E. N. Compilation and analysis of eukaryotic POL II promoter sequences. Nucleic Acids Res. 1986 Dec 22;14(24):10009–10026. doi: 10.1093/nar/14.24.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
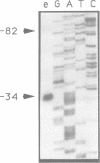
- Clayton C. E., Fueri J. P., Itzhaki J. E., Bellofatto V., Sherman D. R., Wisdom G. S., Vijayasarathy S., Mowatt M. R. Transcription of the procyclic acidic repetitive protein genes of Trypanosoma brucei. Mol Cell Biol. 1990 Jun;10(6):3036–3047. doi: 10.1128/mcb.10.6.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquelet H., Steinert M., Pays E. Ultraviolet irradiation inhibits RNA decay and modifies ribosomal RNA processing in Trypanosoma brucei. Mol Biochem Parasitol. 1991 Jan;44(1):33–42. doi: 10.1016/0166-6851(91)90218-u. [DOI] [PubMed] [Google Scholar]
- Dorn P. L., Aman R. A., Boothroyd J. C. Inhibition of protein synthesis results in super-induction of procyclin (PARP) RNA levels. Mol Biochem Parasitol. 1991 Jan;44(1):133–139. doi: 10.1016/0166-6851(91)90229-y. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983 Nov;35(1):79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- Evers R., Hammer A., Köck J., Jess W., Borst P., Mémet S., Cornelissen A. W. Trypanosoma brucei contains two RNA polymerase II largest subunit genes with an altered C-terminal domain. Cell. 1989 Feb 24;56(4):585–597. doi: 10.1016/0092-8674(89)90581-3. [DOI] [PubMed] [Google Scholar]
- Garvin A. M., Pawar S., Marth J. D., Perlmutter R. M. Structure of the murine lck gene and its rearrangement in a murine lymphoma cell line. Mol Cell Biol. 1988 Aug;8(8):3058–3064. doi: 10.1128/mcb.8.8.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Gottesdiener K., Chung H. M., Brown S. D., Lee M. G., Van der Ploeg L. H. Characterization of VSG gene expression site promoters and promoter-associated DNA rearrangement events. Mol Cell Biol. 1991 May;11(5):2467–2480. doi: 10.1128/mcb.11.5.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondal E. J., Evers R., Kosubek K., Cornelissen A. W. Characterization of the RNA polymerases of Trypanosoma brucei: trypanosomal mRNAs are composed of transcripts derived from both RNA polymerase II and III. EMBO J. 1989 Nov;8(11):3383–3389. doi: 10.1002/j.1460-2075.1989.tb08502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies D., Tebabi P., Pays E. Transient activity assays of the Trypanosoma brucei variant surface glycoprotein gene promoter: control of gene expression at the posttranscriptional level. Mol Cell Biol. 1991 Jan;11(1):338–343. doi: 10.1128/mcb.11.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kownin P., Bateman E., Paule M. R. Effects of single-base substitutions within the Acanthamoeba castellanii rRNA promoter on transcription and on binding of transcription initiation factor and RNA polymerase I. Mol Cell Biol. 1988 Feb;8(2):747–753. doi: 10.1128/mcb.8.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kownin P., Iida C. T., Brown-Shimer S., Paule M. R. The ribosomal RNA promoter of Acanthamoeba castellanii determined by transcription in a cell-free system. Nucleic Acids Res. 1985 Sep 11;13(17):6237–6248. doi: 10.1093/nar/13.17.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- König E., Delius H., Carrington M., Williams R. O., Roditi I. Duplication and transcription of procyclin genes in Trypanosoma brucei. Nucleic Acids Res. 1989 Nov 11;17(21):8727–8739. doi: 10.1093/nar/17.21.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laban A., Wirth D. F. Transfection of Leishmania enriettii and expression of chloramphenicol acetyltransferase gene. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9119–9123. doi: 10.1073/pnas.86.23.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Van der Ploeg L. H. Homologous recombination and stable transfection in the parasitic protozoan Trypanosoma brucei. Science. 1990 Dec 14;250(4987):1583–1587. doi: 10.1126/science.2177225. [DOI] [PubMed] [Google Scholar]
- Lewin B. Commitment and activation at pol II promoters: a tail of protein-protein interactions. Cell. 1990 Jun 29;61(7):1161–1164. doi: 10.1016/0092-8674(90)90675-5. [DOI] [PubMed] [Google Scholar]
- Locker J., Buzard G. A dictionary of transcription control sequences. DNA Seq. 1990;1(1):3–11. doi: 10.3109/10425179009041342. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Mowatt M. R., Clayton C. E. Developmental regulation of a novel repetitive protein of Trypanosoma brucei. Mol Cell Biol. 1987 Aug;7(8):2838–2844. doi: 10.1128/mcb.7.8.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. M., Long G. L. A general method of site-specific mutagenesis using a modification of the Thermus aquaticus polymerase chain reaction. Anal Biochem. 1989 Jul;180(1):147–151. doi: 10.1016/0003-2697(89)90103-6. [DOI] [PubMed] [Google Scholar]
- Pays E., Coquelet H., Tebabi P., Pays A., Jefferies D., Steinert M., Koenig E., Williams R. O., Roditi I. Trypanosoma brucei: constitutive activity of the VSG and procyclin gene promoters. EMBO J. 1990 Oct;9(10):3145–3151. doi: 10.1002/j.1460-2075.1990.tb07512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins K. K., Dailey G. M., Tjian R. In vitro analysis of the Antennapedia P2 promoter: identification of a new Drosophila transcription factor. Genes Dev. 1988 Dec;2(12A):1615–1626. doi: 10.1101/gad.2.12a.1615. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rudenko G., Bishop D., Gottesdiener K., Van der Ploeg L. H. Alpha-amanitin resistant transcription of protein coding genes in insect and bloodstream form Trypanosoma brucei. EMBO J. 1989 Dec 20;8(13):4259–4263. doi: 10.1002/j.1460-2075.1989.tb08611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko G., Le Blancq S., Smith J., Lee M. G., Rattray A., Van der Ploeg L. H. Procyclic acidic repetitive protein (PARP) genes located in an unusually small alpha-amanitin-resistant transcription unit: PARP promoter activity assayed by transient DNA transfection of Trypanosoma brucei. Mol Cell Biol. 1990 Jul;10(7):3492–3504. doi: 10.1128/mcb.10.7.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A., Patil N., Chao M. A constitutive promoter directs expression of the nerve growth factor receptor gene. Mol Cell Biol. 1988 Aug;8(8):3160–3167. doi: 10.1128/mcb.8.8.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman D. R., Geliebter J., Cross G. A. Rapid and simple amplification of a specific RNA sequence by the polymerase chain reaction. Trends Genet. 1989 May;5(5):137–137. doi: 10.1016/0168-9525(89)90052-8. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Levin J. R., Ingles C. J., Agabian N. In trypanosomes the homolog of the largest subunit of RNA polymerase II is encoded by two genes and has a highly unusual C-terminal domain structure. Cell. 1989 Mar 10;56(5):815–827. doi: 10.1016/0092-8674(89)90686-7. [DOI] [PubMed] [Google Scholar]
- Soeller W. C., Poole S. J., Kornberg T. In vitro transcription of the Drosophila engrailed gene. Genes Dev. 1988 Jan;2(1):68–81. doi: 10.1101/gad.2.1.68. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Elwood H. J., Gunderson J. H. Evolutionary diversity of eukaryotic small-subunit rRNA genes. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1383–1387. doi: 10.1073/pnas.83.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H., Elwood H. J., Alonso R. A., Peattie D. A. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989 Jan 6;243(4887):75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Mougey E. B. News from the nucleolus: rRNA gene expression. Trends Biochem Sci. 1991 Feb;16(2):58–62. doi: 10.1016/0968-0004(91)90025-q. [DOI] [PubMed] [Google Scholar]
- Struhl K. Molecular mechanisms of transcriptional regulation in yeast. Annu Rev Biochem. 1989;58:1051–1077. doi: 10.1146/annurev.bi.58.070189.005155. [DOI] [PubMed] [Google Scholar]
- Stuart K. RNA editing in mitochondrial mRNA of trypanosomatids. Trends Biochem Sci. 1991 Feb;16(2):68–72. doi: 10.1016/0968-0004(91)90027-s. [DOI] [PubMed] [Google Scholar]
- Vijayasarathy S., Ernest I., Itzhaki J. E., Sherman D., Mowatt M. R., Michels P. A., Clayton C. E. The genes encoding fructose bisphosphate aldolase in Trypanosoma brucei are interspersed with unrelated genes. Nucleic Acids Res. 1990 May 25;18(10):2967–2975. doi: 10.1093/nar/18.10.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B. Transcription elements and factors of RNA polymerase B promoters of higher eukaryotes. CRC Crit Rev Biochem. 1988;23(2):77–120. doi: 10.3109/10409238809088317. [DOI] [PubMed] [Google Scholar]
- White T. C., Rudenko G., Borst P. Three small RNAs within the 10 kb trypanosome rRNA transcription unit are analogous to domain VII of other eukaryotic 28S rRNAs. Nucleic Acids Res. 1986 Dec 9;14(23):9471–9489. doi: 10.1093/nar/14.23.9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingender E. Transcription regulating proteins and their recognition sequences. Crit Rev Eukaryot Gene Expr. 1990;1(1):11–48. [PubMed] [Google Scholar]
- Wobbe C. R., Struhl K. Yeast and human TATA-binding proteins have nearly identical DNA sequence requirements for transcription in vitro. Mol Cell Biol. 1990 Aug;10(8):3859–3867. doi: 10.1128/mcb.10.8.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomerdijk J. C., Ouellette M., ten Asbroek A. L., Kieft R., Bommer A. M., Clayton C. E., Borst P. The promoter for a variant surface glycoprotein gene expression site in Trypanosoma brucei. EMBO J. 1990 Sep;9(9):2791–2801. doi: 10.1002/j.1460-2075.1990.tb07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]