Abstract
BACKGROUND
Soy milk (SM) and its fermented products are identified as rich sources of bioactive compounds helping to manage and to reduce the risk of chronic disease. This study aimed to compare the effects of SM and probiotic SM (PSM) consumption on serum low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) in diabetic Wistar rats.
METHODS
Probiotic SM was prepared by fermentation of the plain SM with a native strain of Lactobacillus plantarum. 20 streptozotocin-nicotinamide-induced diabetic Wistar rats were divided into two groups based on the type of administered SM (SM group and PSM group). The animals were fed with 1 ml/day of either soy or PSM for 21 days. The serum lipoprotein levels were analyzed at baseline and the end of the intervention period.
RESULTS
HDL-C increased significantly in PSM group. Furthermore, this group showed more percent of change in increased HDL-C in compression with SM group (P < 0.050). Regarding LDL-C level, rats fed with SM was not significantly different from the PSM group (P < 0.050); though, this biomarker was reduced in both group.
CONCLUSION
Probiotic SM could modulate blood lipoprotein levels. Thus, it may be considered in managing diabetes complications and atherosclerotic risks.
Keywords: Lactobacillus, Probiotic, Low Density Lipoprotein Cholesterol, High Density Lipoprotein Cholesterol, Soy Milk
Introduction
Diabetes is a chronic metabolic disease with growing prevalence and incidence throughout the world.1 Apart from impaired glucose homeostasis, its complications usually occurred as a range of abnormalities in lipoprotein metabolism which results in dyslipidemia. Diabetic dyslipidemia is most commonly characterized by elevated levels of triglycerides, low levels of high-density lipoprotein cholesterol (HDL-C), and postprandial lipidemia. In addition, the low-density lipoprotein cholesterol (LDL-C) particles in diabetic dyslipidemia tend to convert a smaller and denser type which raises their atherogenicity.1 The increasing rate of population suffered from diabetes (from 382 million to 592 million in 2035),2 aging population, lack of sufficient physical activity, disobedience principles of appropriate nutrition,2-4 and an economic burden on the health care system5 highlights the necessity of doing more research on prevention and treatment of this disease.
Some bioactive ingredients in soy milk (SM) showed a protective effect against diabetes.6,7 Their intake through SM consumption was usually resulted in regulation of blood glucose and insulin levels and rising insulin sensitivity.8,9 On the other hand, SM fermentation increases the bioavailability of its isoflavones, as the beneficial effects of ultimate fermented product get higher than those of unfermented.10 Furthermore, soy proteins cooperation with soy isoflavones have demonstrated a positive role in reducing some atherogenic lipid and lipoproteins serum levels.11 In addition, fermentation of SM probability associated with regulation of lipid metabolism genes expression.12 From another point of view, fermented products with probiotic bacteria could decrement complications associated with diabetes.13-15 Probiotics are living microorganisms which modulate the specific function of an organism by activation of specific molecular pathways.16 Among various species of these microorganisms, Lactobacillus and Bifidobacterium genera are commonly used in the preparation of probiotic products.17 Lactobacillus plantarum A7 (KC 355240, LA7) is a native strain with proven probiotic properties.18-20 This strain of bacteria was found to be capable of decreasing serum total cholesterol, LDL-C and triglyceride in mice.19 The present study designed to evaluate the effects of probiotic SM (PSM) fermented by LA7 serum HDL-C and LDL-C in streptozotocin (STZ)-nicotinamide (NA)-induced diabetic rats.
Materials and Methods
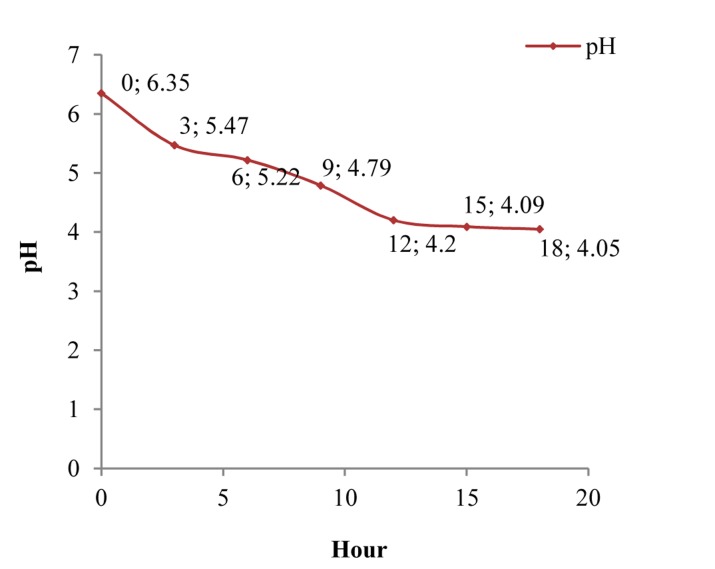
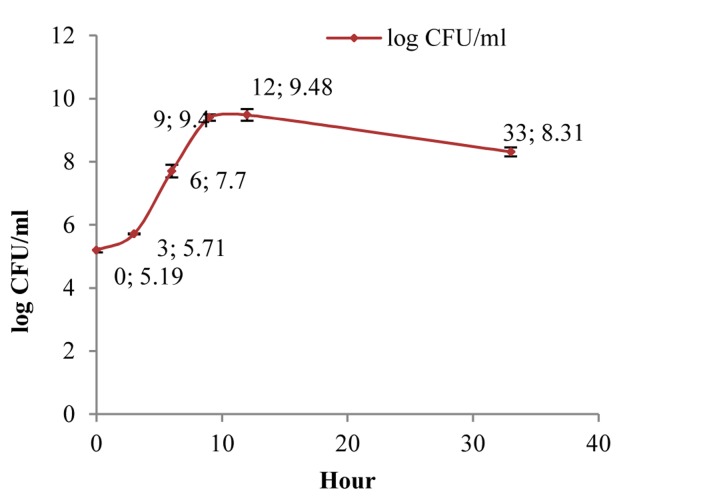
In the present study, pasteurized plain SM was bought from Shir Soya Isfahan Company in Iran. Its composition and energy are shown in table 1. A strain of L. plantarum A7 (KC 355240, LA7) which was obtained from microbial collection of food microbiology laboratory in School of Nutrition and Food Science, Isfahan University of Medical Sciences, was used for preparation PSM. Overnight cultures were provided with 1% inoculums in de‑Mans-Rogosa broth (MRS) (Merck‑Germany) and aerobically incubation at 37 °C for 24 h (Irankhodsaz-Iran). Active bacteria were obtained through 2 successive overnight cultures. Bacterial pellet was achieved by three steps centrifugation at 6000, 8000, 10,000 rpm each took 15 min (Sequrita-B. Braun, Hamburg, Germany) following by washing with normal saline. Then suspension of bacterial prepared with 0.5 optical density at 620 (λ) wavelength determined by spectrophotometer (Jenway-UK). A flask containing SM (200 ml) was added with 1% (v/v) of the active culture, and the cultured samples were undergone fermentation at 37°C in an aerobic condition. Base of our study on pH and the growth of LA7 in SM revealed that cell numbers of LA7 can pick to 109 colony form unit (CFU)/ml in the presence 9 h fermentation that followed in the preparation of PSM samples in the presented study (Figures 1 and 2).
Table 1.
Nutrient composition and energy content of soy milk used in this study*
| Content | Mean value |
|---|---|
| Fat (g/100g) | 1.20 |
| Protein (g/100g) | 2.10 |
| Carbohydrate (g/100g) | 1.50 |
| Energy (mJ/kg) | 1.06 |
Declared by producer: Shir Soya Isfahan Company
Figure 1.
Variation curve of pH in fermentation time
Figure 2.
Growth curve of LA7 in fermentation time CFU: Colony form unit
16-week-old male Wistar rats weighing about 185 ± 25 g which grew in the animal house of Isfahan School of Pharmacy and Pharmaceutical Sciences were housed in cages and subjected to a 12 h light/dark cycle with a maintained relative humidity of 45-55%, and temperature at 22 ± 2 °C. The animals were given free access standard pellet rat diet (Table 2) (Behparvar Company, Tehran, Iran) and water.
Table 2.
The chemical composition and energy content of the standard pellet rat diet*
| Contents | Value** |
|---|---|
| Protein (g/100 g) | 19.50-20.5 |
| Fat (g/100 g) | 3.50-4.5 |
| Fiber (g/100 g) | 4.00-4.5 |
| Ashe (g/100 g) | Maximum 10 |
| Calcium (g/100 g) | 0.95-1.0 |
| Phosphorus (g/100 g) | 0.65-0.7 |
| Salt (g/100 g) | 0.50-0.5 |
| Moisture (g/100 g) | Maximum 10 |
| Lysine (g/100 g) | 1.15 |
| Methionine (g/100 g) | 0.33 |
| Threonine (g/100 g) | 0.72 |
| Tryptophan (g/100 g) | 0.25 |
| Energy (mJ/kg) | 16.16-17.0 |
Declared by producer: Behparvar Company, Tehran, Iran;
Data presented in ranges show minimum and maximum values
Diabetes was induced in overnight fasted rats by administering a single intraperitoneal injection of freshly prepared STZ (Sigma-Aldrich Company, Germany) 50 mg/kg b.w. followed 15 min later by 100 mg/kg of NA (Sigma-Aldrich Company, Germany) in 0.1 M citrate buffer (pH = 4.5).21-24 Diabetes was confirmed in the STZ-NA-treated rats by measuring fasting blood glucose levels after one week of induction. Rats with fasting blood glucose of more than 200 mg/dl were considered as diabetics.24 Then they were randomly divided into two separate groups of SM and (PSM) PSM groups of 10 rats in each.
The study was carried out according to the guidelines of Research Ethics Committee for animal experiments set forth by Isfahan University of Medical Sciences.
During the study period, the SM and PSM groups of rats were gavaged 1 ml/day by plain SM and PSM samples, respectively. Blood samples were collected by microcapillary tube from orbital sinus plexus under light ether anesthesia25 after overnight starvation at 1st day and 21st day of the study. For analysis of blood lipoprotein, serum was obtained by centrifugation at 3000 rpm for 20 min and stored in a freezer at −80 °C until analysis. HDL-C and LDL-C concentrations were determined by using commercial kit (Biosystems, Spain) on A15-Autoanalyzer set (Biosystems, Barcelona, Spain).
The data were expressed as mean ± standard error. Values were evaluated by IBM SPSS for Windows (version 20, SPSS Inc., Chicago, IL, USA). The percent change for each variable was also obtained by the formula (E-B)/B × 100, where E is the end-of-treatment values and B is the baseline values. The values of HDL-C and LDL-C in the two groups were compared using independent sample t-tests. The changes in the level of variables between the beginning and end of the intervention were compared by paired sample t-test. Differences with P < 0.050 were considered to be statistically significant.
Results
Serum HDL-C levels of PSM group were significantly increased in comparison with the relevant levels in SM group at the end of the intervention period (P = 0.013) (Table 3). Furthermore, at the end of the study, PSM group demonstrated a significant increase in serum HDL-C levels compared to the values in the baseline (P = 0.044) (Table 3). Moreover, in this group, more percent change in serum HDL-C level was calculated than that of SM group (16.8 vs. 9.036%) (Table 3).
Table 3.
Serum levels of low-density lipoprotein cholesterol (LDL-C) and High-density lipoprotein cholesterol (HDL-C) in the study groups
| Variable | Soy milk | Probiotic soy milk | P* |
|---|---|---|---|
| HDL-C (mg/dl) | |||
| Baseline | 30.71 ± 3.33 | 32.66 ± 2.44 | 0.608 |
| After intervention | 32.14 ± 1.89 | 39.48 ± 2.14 | 0.013 |
| Percent change | 9.36 ± 7.70 | 16.80 ± 5.10 | 0.491 |
| P** | 0.693 | 0.044 | |
| LDL-C (mg/dl) | |||
| Baseline | 9.33 ± 1.65 | 15.33 ± 3.96 | 0.143 |
| After intervention | 7.10 ± 0.63 | 10.01 ± 4.48 | 0.407 |
| Percent change | 3.30 ± 20.77 | -13.31 ± 19 | 0.688 |
| P** | 0.159 | 0.376 |
Values are presented as mean ± standard error;
P values refer to mean comparisons between groups (independent t-test);
P values refer to variation from day 0 to day 21 within groups (paired t-test);
LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol
LDL-C levels in both groups of rats were relatively decreased through the study period. This reduction was not sufficient to meet the statistical difference neither between the groups (P = 0.407) nor among each of the SM group (P = 0.159) and PSM group (P = 0.376). However, LDL-C percent change in PSM group were more than SM group (13.31 vs. 3.3%, P = 0.688) (Table 3).
Discussion
In this study, although serum LDL-C concentration in both treated groups of rats was not significantly reduced, but in PSM group, administration of fermented product led to more change in serum LDL-C concentration. PSM group, also, developed more percent change in increased HDL-C level than SM group.
The results of present study were in accordance with the results of those studies that claimed on SM inhibition effects against diabetes progression and its complications exacerbation through glycemic control.26-28
According to some previous studies, SM isoflavones enhance lipoprotein clearance and HDL-C biogenesis through the regulation of gene expression related to nuclear receptors of peroxisome proliferator-activated receptor and can improve glucose and lipid metabolism.12,29-32 Furthermore, they regulate beta-cell secretory pattern partially by activating the signal transduction pathway, CAMP/protein kinase.33 In addition, soy peptides may lead to inducing the expression of LDL-C receptors in liver and decreased the levels of serum cholesterol.34
Fermentation process led to structural and functional changes in soy bean which provided the production of a diverse mix of peptides and amino acids with advantageous physiological effects.35-37 Furthermore, fermentation converts glucoside isoflavones to aglycone one that has higher physiological activity.38 Inhibitory activity against the enzyme glucosidase and alpha-amylase in fermented SM is more than SM.8
Xie et al. Showed that supplementation of L. plantarum 9-41-A and Lactobacillus fermentum M1-16 in rats under high cholesterol diet a 6 week feeding period impose beneficial effects in triglycerides, total cholesterol, and LDL-C which are associated with positive impacts on intestinal Lactobacillus and Bifidobacterium bacteria population as well as more body weight lost.39 In that study, L. plantarum 9-41-A was more effective than L. fermentum M1-16 in above-mentioned effects, since; authors proposed that L. plantarum may be involved in lipid metabolism.
Results of this study are consistent with the results of Rossi et al. In that intake of the soy product fermented by Enterococcus faecium and Lactobacillus jugurti cause a 17.8% increase in the level of the HDL-C fraction in hypercholesterolemic rabbits during 30 day.35 Though, in the present study less percent change in HDL-C enhancement, about 7.8% was resulted that might be due to shorter study period than the above-mentioned study.
Wang et al. demonstrated that 4 weeks consumption of 2 ml of fermented SM by L. plantarum P-8 in experimental hyperlipidemic rats caused significant changes in the serum LDL-C and HDL-C comparing the control group having only physiological slain but not in the group of rat that was fed with plain SM. In that study, a notable decrease in LDL-C serum and an increment of HDL-C were observed in the rats treated with fermented soy compared with control group. These changes only relatively were occurred in the plain soy group. Furthermore, fermented SM enhanced total bile acids and lipid levels in fecal hyperlipidemic rats.40 In comparison to our results, the trends in LDL-C reduction and HDL-C increase are comparable to the latter study, however, in the present study, HDL-C enhancement by fermented SM was definitely shown to be more effective than the unfermented relevant product.
It can be concluded that PSM could be more effective in increasing HDL-C level and decreasing LDL-C level than SM.
Development of human studies on PSM is suggested in the future that could improve the finding of this study. Furthermore, formulation of PSM for human consumption considering the society’s attitudes toward drinkable SM can be considered in future studies.
A noticeable difference in serum LDL-C levels (9.33 vs. 15.33) between compared groups in the baseline could be regarded as a limitation of this study which may interfere with the obtained results on serum LDL-C. Characterization of serum LDL-C and HDL-C in the tested rats before their grouping would have rectified such a source of error. Nevertheless, this may restrain the sampling randomization.
Conclusion
Probiotic SM by LA7 could provide higher HDL-C levels and lower LDL-C levels in comparison with plain SM. It seems PSM may be more effective in controlling diabetes and reduction its complications associated with atherosclerosis.
Acknowledgments
The authors would like to thank the staff of Food Security Research Center and The Research Center Of Agricultural Science And Natural Resources, Isfahan, for their valuable assistance in this project. This study was extracted from MSc. dissertation which was approved by School of Nutrition and Food Sciences, Isfahan University of Medical Sciences (code 392322).
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2014;37(1):81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 2.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–49. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 4.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P, Zhang X, Brown J, Vistisen D, Sicree R, Shaw J, et al. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(3):293–301. doi: 10.1016/j.diabres.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Venter CS. Health benefits of soy beans and soy products: a review. Journal of Family Ecologyand Consumer Sciences. 1999;27(1):24–33. [Google Scholar]
- 7.Liu ZM, Chen YM, Ho SC. Effects of soy intake on glycemic control: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2011;93(5):1092–101. doi: 10.3945/ajcn.110.007187. [DOI] [PubMed] [Google Scholar]
- 8.Ju HE, Han JS. Hypoglycemic effect of fermented soymilk added with bokbunja (Rubus coreanus Miquel) in diabetic mice. Food Science and Biotechnology. 2010;19(4):1041–6. [Google Scholar]
- 9.Ribnicky DM, Roopchand DE, Poulev A, Kuhn P, Oren A, Cefalu WT, et al. Artemisia dracunculus L. polyphenols complexed to soy protein show enhanced bioavailability and hypoglycemic activity in C57BL/6 mice. Nutrition. 2014;30(7-8 Suppl):S4–10. doi: 10.1016/j.nut.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhan S, Ho SC. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am J Clin Nutr. 2005;81(2):397–408. doi: 10.1093/ajcn.81.2.397. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Yan L, Wang J, Zhang Q, Zhou Q, Sun T, et al. Fermentation characteristics of six probiotic strains in soymilk. Annals of Microbiology. 2012;62(4):1473–83. [Google Scholar]
- 12.Kim Y, Yoon S, Lee SB, Han HW, Oh H, Lee WJ, et al. Fermentation of soy milk via Lactobacillus plantarum improves dysregulated lipid metabolism in rats on a high cholesterol diet. PLoS One. 2014;9(2):e88231. doi: 10.1371/journal.pone.0088231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marazza JA, LeBlanc JG, de Giori GS, Garro MS. Soymilk fermented with Lactobacillus rhamnosus CRL981 ameliorates hyperglycemia, lipid profiles and increases antioxidant enzyme activities in diabetic mice. Journal of Functional Foods. 2013;5(4):1848–53. [Google Scholar]
- 14.Giacco R, De Giulio B, Vitale M, Cozzolino R. Functional Foods: Can Food Technology Help in the Prevention and Treatment of Diabetes? Food and Nutrition Sciences. 2013;4:827–37. [Google Scholar]
- 15.Ademiluyi AO, Oboh G, Boligon AA, Athayde ML. Effect of fermented soybean condiment supplemented diet on α-amylase and α-glucosidase activities in Streptozotocin-induced diabetic rats. Journal of Functional Foods. 2014;9:1–9. [Google Scholar]
- 16.Bomba A, Brandeburovل A, Ricanyovل J, Strojn L, Chmelلrovل A, Szabadosovل V. The role of probiotics and natural bioactive compounds in modulation of the common molecular pathways in pathogenesis of atherosclerosis and cancer. Biologia. 2012;67(1):1–13. [Google Scholar]
- 17.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 18.Mirlohi M, Soleimanian-Zad S, Dokhani S, Sheikh-Zeinodin M, Abghary A. Investigation of Acid and Bile Tolerance of Native Lactobacilli Isolated from Fecal Samples and Commercial Probiotics by Growth and Survival Studies. Iranian Journal of Biotechnology. 2009;7(4):233–40. [Google Scholar]
- 19.Fazeli H, Moshtaghian J, Mirlohi M, Shirzadi M. Reduction in serum lipid parameters by incorporation of a native strain of Lactobacillus Plantarum A7 in Mice. Iran J Diabetes Lipid Disord. 2010;9:1–7. [Google Scholar]
- 20.Mirlohi M, Soleimanian-Zad S, Dokhani Sh, Sheikh-Zeinodin M. Microbial and physiochemical changes in yoghurts containing different Lactobacillus delbrueckii subsp. bulgaricus strains in association with Lactobacillus plantarum as an adjunct culture. International Journal of Dairy Technology. 2014;67(2):246–54. [Google Scholar]
- 21.Tahara A, Matsuyama-Yokono A, Nakano R, Someya Y, Shibasaki M. Hypoglycaemic effects of antidiabetic drugs in streptozotocin-nicotinamide-induced mildly diabetic and streptozotocin-induced severely diabetic rats. Basic Clin Pharmacol Toxicol. 2008;103(6):560–8. doi: 10.1111/j.1742-7843.2008.00321.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen T, Kagan L, Mager DE. Population pharmacodynamic modeling of exenatide after 2-week treatment in STZ/NA diabetic rats. J Pharm Sci. 2013;102(10):3844–51. doi: 10.1002/jps.23682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JH, Lee DE, Choi SH, Cha JH, Bak EJ, Yoo YJ. Diabetic characteristics and alveolar bone loss in streptozotocin- and streptozotocin-nicotinamide-treated rats with periodontitis. J Periodontal Res. 2014;49(6):792–800. doi: 10.1111/jre.12165. [DOI] [PubMed] [Google Scholar]
- 24.Petchi RR, Vijaya C, Parasuraman S. Antidiabetic activity of polyherbal formulation in streptozotocin - nicotinamide induced diabetic wistar rats. J Tradit Complement Med. 2014;4(2):108–17. doi: 10.4103/2225-4110.126174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minaiyan M, Ghannadi A, Movahedian A, Ramezanlou P, Osooli FS. Effect of the hydroalcoholic extract and juice of Prunus divaricata fruit on blood glucose and serum lipids of normal and streptozotocin-induced diabetic rats. Res Pharm Sci. 2014;9(6):421–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Lu MP, Wang R, Song X, Chibbar R, Wang X, Wu L, et al. Dietary soy isoflavones increase insulin secretion and prevent the development of diabetic cataracts in streptozotocin-induced diabetic rats. Nutr Res. 2008;28(7):464–71. doi: 10.1016/j.nutres.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Nordentoft I, Jeppesen PB, Hong J, Abudula R, Hermansen K. Increased insulin sensitivity and changes in the expression profile of key insulin regulatory genes and beta cell transcription factors in diabetic KKAy-mice after feeding with a soy bean protein rich diet high in isoflavone content. J Agric Food Chem. 2008;56(12):4377–85. doi: 10.1021/jf800504r. [DOI] [PubMed] [Google Scholar]
- 28.Noriega-Lopez L, Tovar AR, Gonzalez-Granillo M, Hernandez-Pando R, Escalante B, Santillan-Doherty P, et al. Pancreatic insulin secretion in rats fed a soy protein high fat diet depends on the interaction between the amino acid pattern and isoflavones. J Biol Chem. 2007;282(28):20657–66. doi: 10.1074/jbc.M701045200. [DOI] [PubMed] [Google Scholar]
- 29.Mezei O, Banz WJ, Steger RW, Peluso MR, Winters TA, Shay N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J Nutr. 2003;133(5):1238–43. doi: 10.1093/jn/133.5.1238. [DOI] [PubMed] [Google Scholar]
- 30.Ricketts ML, Moore DD, Banz WJ, Mezei O, Shay NF. Molecular mechanisms of action of the soy isoflavones includes activation of promiscuous nuclear receptors. A review. J Nutr Biochem. 2005;16(6):321–30. doi: 10.1016/j.jnutbio.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Potter SM. Overview of proposed mechanisms for the hypocholesterolemic effect of soy. J Nutr. 1995;125(3 Suppl):606S–11S. doi: 10.1093/jn/125.3_Suppl.606S. [DOI] [PubMed] [Google Scholar]
- 32.Torres N, Torre-Villalvazo I, Tovar AR. Regulation of lipid metabolism by soy protein and its implication in diseases mediated by lipid disorders. J Nutr Biochem. 2006;17(6):365–73. doi: 10.1016/j.jnutbio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Veloso RV, Latorraca MQ, Arantes VC, Reis MA, Ferreira F, Boschero AC, et al. Soybean diet improves insulin secretion through activation of cAMP/PKA pathway in rats. J Nutr Biochem. 2008;19(11):778–84. doi: 10.1016/j.jnutbio.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Cho SJ, Juillerat MA, Lee CH. Cholesterol lowering mechanism of soybean protein hydrolysate. J Agric Food Chem. 2007;55(26):10599–604. doi: 10.1021/jf071903f. [DOI] [PubMed] [Google Scholar]
- 35.Rossi EA, Vendramini RC, Carlos IZ, Ueiji IS, Squinzari MM, Silva Junior SI, et al. Effects of a novel fermented soy product on the serum lipids of hypercholesterolemic rabbits. Arq Bras Cardiol. 2000;74(3):209–16. doi: 10.1590/s0066-782x2000000300003. [DOI] [PubMed] [Google Scholar]
- 36.Kwon DY, Daily JW, Kim HJ, Park S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr Res. 2010;30(1):1–13. doi: 10.1016/j.nutres.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Wang JC, Zhang WY, Zhong Z, Wei AB, Bao QH, Zhang Y, et al. Transcriptome analysis of probiotic Lactobacillus casei Zhang during fermentation in soymilk. J Ind Microbiol Biotechnol. 2012;39(1):191–206. doi: 10.1007/s10295-011-1015-7. [DOI] [PubMed] [Google Scholar]
- 38.Kawakami Y, Tsurugasaki W, Nakamura S, Osada K. Comparison of regulative functions between dietary soy isoflavones aglycone and glucoside on lipid metabolism in rats fed cholesterol. J Nutr Biochem. 2005;16(4):205–12. doi: 10.1016/j.jnutbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Xie N, Cui Y, Yin YN, Zhao X, Yang JW, Wang ZG, et al. Effects of two Lactobacillus strains on lipid metabolism and intestinal microflora in rats fed a high-cholesterol diet. BMC Complement Altern Med. 2011;11:53. doi: 10.1186/1472-6882-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Bao Y, Zhang Y, Zhang J, Yao G, Wang S, et al. Effect of Soymilk Fermented with Lactobacillus plantarum P-8 on Lipid Metabolism and Fecal Microbiota in Experimental Hyperlipidemic Rats. Food Biophysics. 2012;8(1):43–9. [Google Scholar]