Abstract
We discovered the prostaglandin transporter (PGT) and cloned the human cDNA and gene. PGT transports extracellular prostaglandins (PGs) into the cytoplasm for enzymatic inactivation. PGT knockout mice have elevated prostaglandin E2 (PGE2) and neonatal patent ductus arteriosus, which reflects PGT's control over PGE2 signaling at EP1/EP4 cell-surface receptors. Interestingly, rescued PGT knockout pups have a nearly normal phenotype, as do human PGT nulls. Given the benign phenotype of PGT genetic nulls, and because PGs are useful medicines, we have approached PGT as a drug target. Triazine library screening yielded a lead compound of inhibitory constant 50% (IC50) = 3.7 μM, which we developed into a better inhibitor of IC50 378 nM. Further structural improvements have yielded 26 rationally designed derivatives with IC50 < 100 nM. The therapeutic approach of increasing endogenous PGs by inhibiting PGT offers promise in diseases such as pulmonary hypertension and obesity.
THE HISTORY OF MANIPULATING ENDOGENOUS PROSTAGLANDIN LEVELS
The Ebers Papyrus from approximately 3,500 years ago, followed by Hippocrates, Celsus, Dioscorides, Pliny the Elder, and Galen 1,000 years later, all recommend that persons in pain — particularly women experiencing the pain of childbirth — as well as persons with fever ingest the bark or leaves of the willow tree (1). The willow is of the genus Salix, from which we derive the words salicylate and acetylsalicylic acid (aspirin) (1). Nonsteroidal anti-inflammatory drugs (NSAIDs), which are all derived from aspirin, block the synthesis of prostaglandins (PGs) (2). Thus, considering the trajectory starting with willow bark through aspirin to NSAIDs, humans have historically spent considerable time and effort reducing the synthesis of their endogenous PGs.
That said, topical PGs are used successfully in conditions such as glaucoma and obstetrics (3,4). When PGs of the E series are given systemically, they are generally well tolerated so long as the dose is not excessive (5,6). Analogues of prostaglandin I2 (PGI2, prostacyclin) are administered systemically in pulmonary artery hypertension (7). Unfortunately, the pharmacokinetic profile of most PGs renders them not useful for routine systemic clinical use (8,9).
MOLECULAR MECHANISMS OF PG INACTIVATION
Instead of administering exogenous PGs as medicines, our laboratory has focused on increasing the level of endogenous PGs by inhibiting their metabolism. Investigators had known since the 1970s that common prostaglandins (PGs) such as PGE2 and PGF2α are stable in blood (10–12). They are metabolized rapidly in tissues by a two-step process of carrier-mediated uptake across the plasma membrane followed by cytoplasmic enzymatic oxidation by 15-hydroxy prostaglandin dehydrogenase (HPGD) (13,14). Because PGs signal a diverse array of downstream physiological events (15), this two-step PG metabolism process must be broadly distributed and must function efficiently to keep PGs constrained to a local region, lest they diffuse from their site of action and signal promiscuously at a distance.
In 1995, our group reported discovering the rat PG transporter (PGT) (16) (subsequent gene names have been SLC21A1, SLCO2A1, and OATP2A1). PGT is a lactate/PG anion exchanger (17) that mediates the energetically active uptake into the cell of PGE2, PGF2α, PGD2, and PGI2, but not thromboxane (16). We subsequently cloned and characterized the mouse PGT cDNA (18) as well as the human PGT cDNA (19) and gene (20).
We showed that PGT is obligatory for PGE2 metabolism: reconstitution experiments using a cell line null for both PGT and HPGD revealed that neither alone is sufficient for PG metabolism (21). When we expressed green fluorescent protein — tagged PGT in a polarized Madin-Darby canine kidney (MDCK) cell monolayer, it was sorted to the apical membrane, thus recreating the pattern of PGT expression in the native renal collecting duct, where it serves to direct the release of newly synthesized PGE2 away from the apical, and toward the basolateral, compartment (22,23).
PGT CONTROLS PG SIGNALING
We performed a global knockout of the mouse PGT gene by flanking exon 1 with LoxP sites and crossing with a mouse transgenic for EIIA-Cre recombinase. Although the pups from PGT heterozygotes were born in a Mendelian genotypic ratio, several days later the PGT null pups were dead. Necropsy revealed that the PGT nulls had patent ductus arteriosus (24), which placed PGT squarely in the PG signaling pathway (25–27). Because systemic PGE2 levels were high in the PGT null mice (24), the most likely mechanism for the patent ductus is that persistently high post-natal PGE2 levels had opposed forces causing normal post-natal ductus contraction.
We have also examined directly the ability of PGT to compete for cell-surface PGE2 and thus control access of this ligand to its plasma membrane receptors. In a reconstituted system, PGT expression reduced PGE2 signaling through either EP1 or EP4 receptors (28).
PGT is also coordinately regulated with cyclooxygenase and/or various other components of the PG signaling systems (29–37).
Taken together, our studies and those of others indicate that PGT plays an essential role in controlling the metabolism of PGs in a broad variety of tissues and organs.
PG SIGNALING MODEL: RELEASE/REUPTAKE
Our present working model is that PG signaling is akin to synaptic signaling (22) (Figure 1). Both neurotransmitters and prostanoids are synthesized by inducible enzymes (38–41). Both systems are characterized by triggered release of ligand into the extracellular compartment (42,43). Both sets of G protein-coupled receptors (GPCRs) use similar molecular signaling and regulatory mechanisms (44–47). And both sets of ligands undergo reuptake by plasma membrane carriers that are located on the cell that released the signaling molecule (16,22,48–50).
Fig. 1.
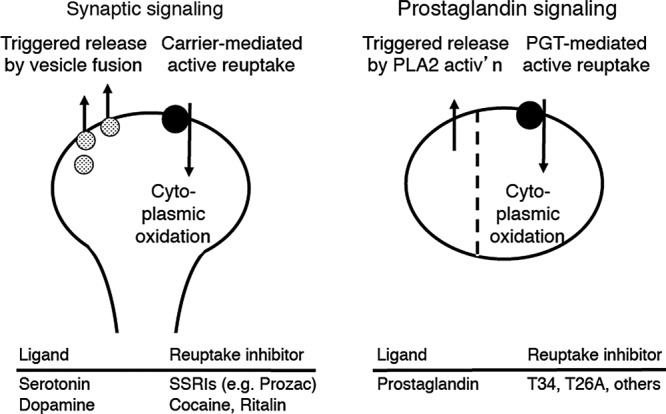
Model comparing neurotransmission with proposed eicosanoid signaling. In neurotransmission, an action potential triggers the release of neurotransmitter that has been compartmentalized in vesicles. The neurotransmitter undergoes reuptake by a carrier, followed by cytoplasmic oxidation or reuptake into vesicles. In prostaglandin signaling, an agonist induces triggered release via activation of cytoplasmic phopholipase A2 (cPLA2), causing delivery of archidonate to cyclooxygenase (Cox). We propose that prostaglandin signaling also involves PG reuptake via PGT and that, as in neurotransmission, PG reuptake is followed by cytoplasmic oxidation. The dotted vertical line implies compartmentalization between the PG synthesis/ release pathway on the one hand and the PG reuptake/oxidation pathway on the other. The nature of the compartmentalization remains to be determined. Adapted from Nomura et al (22) with permission.
In the neurotransmitter model system, there is good evidence that varying the rate of ligand reuptake varies the signaling (51–53). Indeed, these effects form the basis for treatment of psychiatric conditions with serotonin reuptake inhibitors (54,55). Accordingly, we asked whether small molecule inhibitors of PGT might be useful medicinally insofar as they would increase the levels of endogenous PGs.
PGT NULL MICE AND HUMANS
If we are to block PGT medicinally, it would be helpful to know phenotypic details of PGT null mice and humans, in other words, a “worst case scenario” of PGT inhibition.
Mice null at the PGT locus, once rescued through the perinatal period of ductus arteriosus closure, appear to grow normally without obvious pathology (24). Admittedly, more detailed phenotyping awaits improved methods of rescuing the null pups.
On the other hand, in the past 2 years there have been reported some 57 human subjects who have been null at the PGT locus from birth (56–64). These reported subjects, ranging in age from 7 to 88 years, have had no symptoms until around the time of puberty. Then, at an average age of 16 years, the males (but not the females) have developed thickened cephalic and facial skin, digital clubbing, and periosteal calcification. This condition has been termed variously “pachydermoperiostosis” or “hypertrophic osteoarthropathy.”
Systemic PGE2 levels are elevated, and five cases of 57 have been reported to have bone marrow fibrosis and anemia (61).
Despite the well-known role of PGs in pain signaling (65,66), and although approximately 40% of reported PGT null subjects do report pain, it is not generalized pain, rather the pain is confined to sites of periosteal calcification. Similarly, despite the well-known role of PGs in fever induction (67), subjects null for PGT do not have fever.
In the context of a large literature on the role of PGE2 in colon carcinogenesis (68–70), to date one PGT null subject has been reported to have colon carcinoma (56), a rate among the 57 reported subjects that appears to be equivalent to the rate of lifetime colon cancer development in the general population (71). That PGT gene expression is markedly turned off in colon cancers (72) suggests that further decreases in PGT, either from genetic mutations or from small molecule inhibitors, may not further influence the process of carcinogenesis. Clearly, however, further work in this area is needed.
Thus, at present it appears that the clinical consequences of systemically inhibiting PGT, either genetically or pharmacologically, are relatively minor. Based on these finding, we have pursued PGT as a prospective drug target.
DEVELOPMENT OF PGT INHIBITORS
To develop PGT-specific inhibitors with high affinity, we screened a library of 1842 triazine compounds using Madin-Darby canine kidney cells stably expressing rat PGT. In this screening, we found several effective PGT inhibitors. Among them, the most potent inhibitor had an IC50 of 3.7 μM. These inhibitors allowed us to isolate the efflux process of PGE2 and to show that PGT does not transport PGE2 outwardly under physiological conditions (73). The molecule with the highest affinity from the initial library screening was used in vivo to show that PGT regulates local cerebral blood flow via lactate-PG exchange (74).
We performed subsequent structural activity relationship (SAR) studies and developed a better inhibitor, T26A, with IC50 = 378 nM (75). In a proof of principle set of experiments, T26A injected into rats doubled the circulating [PGE2]; reduced circulating PGE2 metabolites by 50%; and slowed the metabolism of exogenously injected PGE2 (75). At the conclusion of our report on T26A, we wrote:
“In addition to being a powerful basic research tool for investigating the fundamental role of PGT in PG metabolism and signal termination, a potent PGT inhibitor such as T26A provides a starting point for the development of therapeutic agents targeting PGT. Because…PGT regulates the metabolism of PGs, a specific inhibitor of PGT could potentially be developed for clinical applications” (75).
Since then, our further SAR work has yielded more than two dozen rationally designed derivatives with IC50 < 100 nM (unpublished observations).
We are currently using these small molecular PGT inhibitors in preclinical studies of disease for which the literature suggests that PGs would be beneficial (eg, pulmonary artery hypertension and obesity).
Footnotes
This work was supported by the National Institutes of Health [5R01DK049688 to V. L. Schuster] and the American Heart Association [0830336N to Y. Chi].
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Zeidel, Boston: Is there only one of these transporters, or are there different types? Because you could imagine that if you knock this out all over the body there might be all kinds of adverse effects. But — like the Cox-2 which didn't turn out quite so well — the idea of being able to inhibit one kind or another might have a significant impact on the therapeutic efficacy of this strategy.
Schuster, New York: There are about five or six other transporters that have been shown to transport prostaglandins. Usually people just look at PGE2, but the affinities are quite low. This has an affinity of about 80 nanomolar, and those others have affinities up in the low micromolar range. And, if you do a head-to-head flux comparison, the other transporters really are not in the game. So this does seem to be the one. I would point out that the phenotype is pretty much the same whether you take out the transporter or you take out the downstream oxidase enzymes. It looks like there is pretty much one pathway for this inactivation.
Tweardy, Houston: At this point in time as you propose or are in the process of doing your IND-enabling toxicity studies in rats and, I presume, dogs, what is the duration of exposure you are going to use for those studies in anticipation of a phase 1?
Schuster, New York: There are a couple different ways to think about this as a drug strategy. One is an acute strategy; a one-time use. So we are engaged in some studies, for example, in prophylaxis of contrast-induced nephropathy. Or there are topical sorts of applications. And then long-term applications so we are exploring a number of these, and the question is, where is the efficacy maximized and where is the toxicity minimized?
REFERENCES
- 1.Jeffreys D. Aspirin: The Remarkable Story of a Wonder Drug. New York, NY: Bloomsbury. x; 2004. p. 335. [Google Scholar]
- 2.Vane JR. The fight against rheumatism: from willow bark to COX-1 sparing drugs. J Physiol Pharmacol. 2000;51:573–86. [PubMed] [Google Scholar]
- 3.Alm A. Prostaglandin derivates as ocular hypotensive agents. Prog Retin Eye Res. 1998;17:291–312. doi: 10.1016/s1350-9462(97)00003-7. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg AB, Greenberg MB, Darney PD. Misoprostol and pregnancy. N Engl J Med. 2001;344:38–47. doi: 10.1056/NEJM200101043440107. [DOI] [PubMed] [Google Scholar]
- 5.Heidrich H, Breddin HK, Rudofsky G, Scheffler P. [Cardiopulmonary effects of prostaglandin E1. Aspects of drug safety] Med Klin (Munich) 1992;87:123–30. [PubMed] [Google Scholar]
- 6.Cattral MS, Altraif I, Greig PD, Blendis L, Levy GA. Toxic effects of intravenous and oral prostaglandin E therapy in patients with liver disease. Am J Med. 1994;97:369–73. doi: 10.1016/0002-9343(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 7.Safdar Z. Treatment of pulmonary arterial hypertension: the role of prostacyclin and prostaglandin analogs. Respir Med. 2011;105:818–27. doi: 10.1016/j.rmed.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Schoenhard G, Oppermann J, Kohn FE. Metabolism and pharmacokinetic studies of misoprostol. Dig Dis Sci. 1985;30:126S–8S. doi: 10.1007/BF01309397. [DOI] [PubMed] [Google Scholar]
- 9.Tang OS, Schweer H, Seyberth HW, Lee SW, Ho PC. Pharmacokinetics of different routes of administration of misoprostol. Hum Reprod. 2002;17:332–6. doi: 10.1093/humrep/17.2.332. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira SH, Vane JR. Prostaglandins: their disappearance from and release into the circulation. Nature. 1967;216:868–73. doi: 10.1038/216868a0. [DOI] [PubMed] [Google Scholar]
- 11.Holmes SW, Horton EW, Stewart MJ. Observations on the extraction of prostaglandins from blood. Life Sci. 1968;7:349–54. doi: 10.1016/0024-3205(68)90032-5. [DOI] [PubMed] [Google Scholar]
- 12.Willman EA. The extraction of prostaglandin E1 from human plasma. Life Sci. 1971;10:1181–91. [PubMed] [Google Scholar]
- 13.Schuster VL. Molecular mechanisms of prostaglandin transport. Annu Rev Physiol. 1998;60:221–42. doi: 10.1146/annurev.physiol.60.1.221. [DOI] [PubMed] [Google Scholar]
- 14.Schuster VL. Prostaglandin transport. Prostaglandins Other Lipid Mediat. 2002;68–9:633–47. doi: 10.1016/s0090-6980(02)00061-8. [DOI] [PubMed] [Google Scholar]
- 15.Holmes TJ., Jr . Prostaglandins, leukotrienes, and essential fatty acids. In: Beale JMJ, Block JH, editors. Wilson and Gisvold's Organic Medicinal and Pharmaceutical Chemistry. 12th ed. New York, NY: Lippincott Williams & Wilkins; 2011. pp. 868–79. [Google Scholar]
- 16.Kanai N, Lu R, Satriano JA, Bao Y, Wolkoff AW, et al. Identification and characterization of a prostaglandin transporter. Science. 1995;268:866–9. doi: 10.1126/science.7754369. [DOI] [PubMed] [Google Scholar]
- 17.Chan BS, Endo S, Kanai N, Schuster VL. Identification of lactate as a driving force for prostanoid transport by prostaglandin transporter PGT. Am J Physiol Renal Physiol. 2002;282:F1097–1102. doi: 10.1152/ajprenal.00151.2001. [DOI] [PubMed] [Google Scholar]
- 18.Pucci ML, Bao Y, Chan B, Itoh S, Lu R, et al. Cloning of mouse prostaglandin transporter PGT cDNA: species-specific substrate affinities. Am J Physiol. 1999;277:R734–41. doi: 10.1152/ajpregu.1999.277.3.R734. [DOI] [PubMed] [Google Scholar]
- 19.Lu R, Kanai N, Bao Y, Schuster VL. Cloning, in vitro expression, and tissue distribution of a human prostaglandin transporter cDNA (hPGT) J Clin Invest. 1996;98:1142–9. doi: 10.1172/JCI118897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu R, Schuster VL. Molecular cloning of the gene for the human prostaglandin transporter hPGT: gene organization, promoter activity, and chromosomal localization. Biochem Biophys Res Commun. 1998;246:805–12. doi: 10.1006/bbrc.1998.8715. [DOI] [PubMed] [Google Scholar]
- 21.Nomura T, Lu R, Pucci ML, Schuster VL. The two-step model of prostaglandin signal termination: in vitro reconstitution with the prostaglandin transporter and prostaglandin 15 dehydrogenase. Mol Pharmacol. 2004;65:973–8. doi: 10.1124/mol.65.4.973. [DOI] [PubMed] [Google Scholar]
- 22.Nomura T, Chang HY, Lu R, Hankin J, Murphy RC, et al. Prostaglandin signaling in the renal collecting duct: release, reuptake, and oxidation in the same cell. J Biol Chem. 2005;280:28424–9. doi: 10.1074/jbc.M408286200. [DOI] [PubMed] [Google Scholar]
- 23.Endo S, Nomura T, Chan BS, Lu R, Pucci ML, et al. Expression of PGT in MDCK cell monolayers: polarized apical localization and induction of active PG transport. Am J Physiol Renal Physiol. 2002;282:F618–22. doi: 10.1152/ajprenal.00150.2001. [DOI] [PubMed] [Google Scholar]
- 24.Chang HY, Locker J, Lu R, Schuster VL. Failure of postnatal ductus arteriosus closure in prostaglandin transporter-deficient mice. Circulation. 2010;121:529–36. doi: 10.1161/CIRCULATIONAHA.109.862946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coggins KG, Latour A, Nguyen MS, Audoly L, Coffman TM, et al. Metabolism of PGE2 by prostaglandin dehydrogenase is essential for remodeling the ductus arteriosus. Nat Med. 2002;8:91–2. doi: 10.1038/nm0202-91. [DOI] [PubMed] [Google Scholar]
- 26.Loftin CD, Trivedi DB, Tiano HF, Clark JA, Lee CA, et al. Failure of ductus arteriosus closure and remodeling in neonatal mice deficient in cyclooxygenase-1 and cyclooxygenase-2. Proc Natl Acad Sci U S A. 2001;98:1059–64. doi: 10.1073/pnas.031573498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segi E, Sugimoto Y, Yamasaki A, Aze Y, Oida H, et al. Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem Biophys Res Commun. 1998;246:7–12. doi: 10.1006/bbrc.1998.8461. [DOI] [PubMed] [Google Scholar]
- 28.Chi Y, Suadicani SO, Schuster VL. Regulation of prostaglandin EP1 and EP4 receptor signaling by carrier-mediated ligand reuptake. Pharmacol Res Perspect. 2014;2:e00051. doi: 10.1002/prp2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chi Y, Pucci ML, Schuster VL. Dietary salt induces transcription of the prostaglandin transporter gene in renal collecting ducts. Am J Physiol Renal Physiol. 2008;295:F765–71. doi: 10.1152/ajprenal.00564.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banu SK, Lee J, Satterfield MC, Spencer TE, Bazer FW, et al. Molecular cloning and characterization of prostaglandin (PG) transporter in ovine endometrium: role for multiple cell signaling pathways in transport of PGF2alpha. Endocrinology. 2008;149:219–31. doi: 10.1210/en.2007-1087. [DOI] [PubMed] [Google Scholar]
- 31.Scafidi S, Douglas RM, Farahani R, Banasiak KJ, Haddad GG. Prostaglandin transporter expression in mouse brain during development and in response to hypoxia. Neuroscience. 2007;146:1150–7. doi: 10.1016/j.neuroscience.2007.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pucci ML, Endo S, Nomura T, Lu R, Khine C, et al. Coordinate control of prostaglandin E2 synthesis and uptake by hyperosmolarity in renal medullary interstitial cells. Am J Physiol Renal Physiol. 2006;290:F641–9. doi: 10.1152/ajprenal.00426.2004. [DOI] [PubMed] [Google Scholar]
- 33.Kang J, Chapdelaine P, Laberge PY, Fortier MA. Functional characterization of prostaglandin transporter and terminal prostaglandin synthases during decidualization of human endometrial stromal cells. Hum Reprod. 2006;21:592–9. doi: 10.1093/humrep/dei400. [DOI] [PubMed] [Google Scholar]
- 34.Kang J, Chapdelaine P, Parent J, Madore E, Laberge PY, et al. Expression of human prostaglandin transporter in the human endometrium across the menstrual cycle. J Clin Endocrinol Metab. 2005;90:2308–13. doi: 10.1210/jc.2004-1482. [DOI] [PubMed] [Google Scholar]
- 35.Ivanov AI, Romanovsky AA. Prostaglandin E2 as a mediator of fever: synthesis and catabolism. Front Biosci. 2004;9:1977–93. doi: 10.2741/1383. [DOI] [PubMed] [Google Scholar]
- 36.Banu SK, Arosh JA, Chapdelaine P, Fortier MA. Molecular cloning and spatio-temporal expression of the prostaglandin transporter: a basis for the action of prostaglandins in the bovine reproductive system. Proc Natl Acad Sci U S A. 2003;100:11747–52. doi: 10.1073/pnas.1833330100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Topper JN, Cai J, Stavrakis G, Anderson KR, Woolf EA, et al. Human prostaglandin transporter gene (hPGT) is regulated by fluid mechanical stimuli in cultured endothelial cells and expressed in vascular endothelium in vivo. Circulation. 1998;98:2396–403. doi: 10.1161/01.cir.98.22.2396. [DOI] [PubMed] [Google Scholar]
- 38.Saadat S, Sendtner M, Rohrer H. Ciliary neurotrophic factor induces cholinergic differentiation of rat sympathetic neurons in culture. J Cell Biol. 1989;108:1807–16. doi: 10.1083/jcb.108.5.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–82. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 40.Murakami M, Kudo I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog Lipid Res. 2004;43:3–35. doi: 10.1016/s0163-7827(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 41.Stichtenoth DO, Thoren S, Bian H, Peters-Golden M, Jakobsson PJ, et al. Microsomal prostaglandin E synthase is regulated by proinflammatory cytokines and glucocorticoids in primary rheumatoid synovial cells. J Immunol. 2001;167:469–74. doi: 10.4049/jimmunol.167.1.469. [DOI] [PubMed] [Google Scholar]
- 42.Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–30. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- 43.Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 2002;68–69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 44.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547–50. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 45.Lefkowitz RJ. G protein-coupled receptor kinases. Cell. 1993;74:409–12. doi: 10.1016/0092-8674(93)80042-d. [DOI] [PubMed] [Google Scholar]
- 46.Neuschafer-Rube F, Hermosilla R, Rehwald M, Ronnstrand L, Schulein R, et al. Identification of a Ser/Thr cluster in the C-terminal domain of the human prostaglandin receptor EP4 that is essential for agonist-induced beta-arrestin1 recruitment but differs from the apparent principal phosphorylation site. Biochem J. 2004;379:573–85. doi: 10.1042/BJ20031820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishigaki N, Negishi M, Ichikawa A. Two Gs-coupled prostaglandin E receptor subtypes, EP2 and EP4, differ in desensitization and sensitivity to the metabolic inactivation of the agonist. Mol Pharmacol. 1996;50:1031–7. [PubMed] [Google Scholar]
- 48.Bao Y, Pucci ML, Chan BS, Lu R, Ito S, et al. Prostaglandin transporter PGT is expressed in cell types that synthesize and release prostanoids. Am J Physiol Renal Physiol. 2002;282:F1103–10. doi: 10.1152/ajprenal.00152.2001. [DOI] [PubMed] [Google Scholar]
- 49.Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, et al. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- 50.Botolin S, McCabe LR. Inhibition of PPARgamma prevents type I diabetic bone marrow adiposity but not bone loss. J Cell Physiol. 2006;209:967–76. doi: 10.1002/jcp.20804. [DOI] [PubMed] [Google Scholar]
- 51.Jennings KA, Lesch KP, Sharp T, Cragg SJ. Non-linear relationship between 5-HT transporter gene expression and frequency sensitivity of 5-HT signals. J Neurochem. 2010;115:965–73. doi: 10.1111/j.1471-4159.2010.07001.x. [DOI] [PubMed] [Google Scholar]
- 52.Jennings KA, Licht CL, Bruce A, Lesch KP, Knudsen GM, et al. Genetic variation in 5-hydroxytryptamine transporter expression causes adaptive changes in 5-HT4 receptor levels. Int J Neuropsychopharm. 2011;15:1–9. doi: 10.1017/S1461145711001258. [DOI] [PubMed] [Google Scholar]
- 53.Vidal R, Valdizan EM, Mostany R, Pazos A, Castro E. Long-term treatment with fluoxetine induces desensitization of 5-HT4 receptor-dependent signalling and functionality in rat brain. J Neurochem. 2009;110:1120–7. doi: 10.1111/j.1471-4159.2009.06210.x. [DOI] [PubMed] [Google Scholar]
- 54.Sangkuhl K, Klein TE, Altman RB. Selective serotonin reuptake inhibitors pathway. Pharmacogen Genomics. 2009;19:907–9. doi: 10.1097/FPC.0b013e32833132cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mann JJ. The medical management of depression. N Engl J Med. 2005;353:1819–34. doi: 10.1056/NEJMra050730. [DOI] [PubMed] [Google Scholar]
- 56.Guda K, Fink SP, Milne GL, Molyneaux N, Ravi L, et al. Inactivating mutation in the prostaglandin transporter gene, SLCO2A1, associated with familial digital clubbing, colon neoplasia, and NSAID resistance. Cancer Prev Res (Phila) 2014;7:805–12. doi: 10.1158/1940-6207.CAPR-14-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Z, He JW, Fu WZ, Zhang CQ, Zhang ZL. Two novel mutations in the SLCO2A1 gene in a Chinese patient with primary hypertrophic osteoarthropathy. Gene. 2014;534:421–3. doi: 10.1016/j.gene.2013.10.051. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Z, He JW, Fu WZ, Zhang CQ, Zhang ZL. Mutations in the SLCO2A1 gene and primary hypertrophic osteoarthropathy: a clinical and biochemical characterization. J Clin Endocrinol Metab. 2013;98:E923–33. doi: 10.1210/jc.2012-3568. [DOI] [PubMed] [Google Scholar]
- 59.Cheng R, Li M, Guo Y, Yao Y, Gao C, et al. Three novel mutations in the SLCO2A1 gene in two Chinese families with primary hypertrophic osteoarthropathy. Eur J Dermatol. 2013;23:636–9. doi: 10.1684/ejd.2013.2154. [DOI] [PubMed] [Google Scholar]
- 60.Busch J, Frank V, Bachmann N, Otsuka A, Oji V, et al. Mutations in the prostaglandin transporter SLCO2A1 cause primary hypertrophic osteoarthropathy with digital clubbing. J Invest Dermatol. 2012;132:2473–6. doi: 10.1038/jid.2012.146. [DOI] [PubMed] [Google Scholar]
- 61.Diggle CP, Parry DA, Logan CV, Laissue P, Rivera C, et al. Prostaglandin transporter mutations cause pachydermoperiostosis with myelofibrosis. Human Mutation. 2012;33:1175–81. doi: 10.1002/humu.22111. [DOI] [PubMed] [Google Scholar]
- 62.Seifert W, Kuhnisch J, Tuysuz B, Specker C, Brouwers A, et al. (2012) Mutations in the prostaglandin transporter encoding gene SLCO2A1 cause primary hypertrophic osteoarthropathy and isolated digital clubbing. Human Mutation. 2012;33:660–4. doi: 10.1002/humu.22042. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Z, Xia W, He J, Ke Y, Yue H, et al. Exome sequencing identifies SLCO2A1 mutations as a cause of primary hypertrophic osteoarthropathy. Am J Hum Gen. 2012;90:125–32. doi: 10.1016/j.ajhg.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sasaki T, Niizeki H, Shimizu A, Shiohama A, Hirakiyama A, et al. Identification of mutations in the prostaglandin transporter gene SLCO2A1 and its phenotype-genotype correlation in Japanese patients with pachydermoperiostosis. J Dermatol Sci. 2012;68:36–44. doi: 10.1016/j.jdermsci.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 65.Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci U S A. 2003;100:9044–9. doi: 10.1073/pnas.1332766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bley KR, Hunter JC, Eglen RM, Smith JA. The role of IP prostanoid receptors in inflammatory pain. Trends Pharmacol Sci. 1998;19:141–7. doi: 10.1016/s0165-6147(98)01185-7. [DOI] [PubMed] [Google Scholar]
- 67.Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, et al. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature. 1998;395:281–4. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- 68.Holla VR, Mann JR, Shi Q, DuBois RN. Prostaglandin E2 regulates the nuclear receptor NR4A2 in colorectal cancer. J Biol Chem. 2006;281:2676–82. doi: 10.1074/jbc.M507752200. [DOI] [PubMed] [Google Scholar]
- 69.Wang D, Buchanan FG, Wang H, Dey SK, DuBois RN. Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res. 2005;65:1822–9. doi: 10.1158/0008-5472.CAN-04-3671. [DOI] [PubMed] [Google Scholar]
- 70.Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276:18075–81. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- 71.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–17. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 72.Holla VR, Backlund MG, Yang P, Newman RA, DuBois RN. Regulation of prostaglandin transporters in colorectal neoplasia. Cancer Prev Res (Phila) 2008;1:93–9. doi: 10.1158/1940-6207.CAPR-07-0009. [DOI] [PubMed] [Google Scholar]
- 73.Chi Y, Khersonsky SM, Chang YT, Schuster VL. Identification of a new class of prostaglandin transporter inhibitors and characterization of their biological effects on prostaglandin E2 transport. J Pharmacol Exp Ther. 2006;316:1346–50. doi: 10.1124/jpet.105.091975. [DOI] [PubMed] [Google Scholar]
- 74.Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–9. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chi Y, Min J, Jasmin JF, Lisanti MP, Chang YT, et al. Development of a high-affinity inhibitor of the prostaglandin transporter. J Pharmacol Exp Ther. 2011;339:633–41. doi: 10.1124/jpet.111.181354. [DOI] [PMC free article] [PubMed] [Google Scholar]


