Abstract
The era of genomics-based medicine promises to provide molecular tests that will permit precision medicine. However, in 2015, it is not clear what the terms genomics-based medicine, molecular tests, or precision medicine mean. In this report, we review the definitions of these terms and other important semantics relative to what it takes to get a tumor biomarker into standard clinical practice, and the potential clinical trial designs that are being considered to determine if tumor biomarker tests based on next-generation sequencing actually provide benefit to patients with cancer.
INTRODUCTION
Cancer is a disease in which the term “personalized medicine” is particularly relevant, given the often lethal nature of the condition and the egregious side effects of so many of the therapies. Therefore, there has been great interest in getting the right drug to the right patient at the right time in the right dose and schedule. However, with only a few exceptions, the treatment of cancer has been more of a “one size fits all” strategy, rather than an effort to individualize treatment approaches. This disconnect between goal and reality has been a function of several intersecting factors. First, several studies have shown that patients would, in general, prefer to be over- than under-treated, given the harsh realities of uncontrolled cancer. Second, and perhaps more importantly, the tools to carefully and accurately assess which patients should get what therapies have been lacking. Indeed, treatment selection for patients has been principally made using estimates of prognosis based on the anatomic findings of the cancer — codified in the TNM staging system in which size of the primary cancer (T), the presence or absence of metastases to surrounding regional lymph nodes (N), and the presence or absence of distant, detectable metastases (M) are the primary drivers. These measures are crude and relatively inaccurate for individual patient care.
Ideally, one would like to use molecular and biological features of the cancer to make important clinical decisions. Breast cancer represents perhaps the best example of doing so in all of oncology. Anti-estrogen or “endocrine” therapies are effective treatments in the metastatic, adjuvant, and preventive settings. However, these therapies only work against cancers that express the estrogen receptor (ER), and therefore measurement of ER is routinely performed on all breast cancer biopsy specimens (1). Likewise, anti−human epidermal growth factor 2 (HER2) therapies, such as trastuzumab, decrease breast cancer mortality in patients whose cancers over-express HER2, and thus HER2 analysis is also a routine component of pathologic workup of all breast cancers (2).
Cloning of the human genome raised great expectations that knowledge of inherited, germline single nucleotide polymorphisms and of tumor-associated somatic genomic abnormalities would lead to better understanding of cancer biology and oncogenesis, with accompanying advances in diagnosis and treatment. Indeed, the mutational spectrum of most cancers has now been defined by The Cancer Genome Atlas (TCGA) project, in which several hundred cases of many of the common human cancers have been fully sequenced, with the results made publically available (3). These findings have led to a plethora of different types of cancer-directed diagnostic tests, yet it is unclear whether they represent a step forward in patient care or simply an over-hyped fad.
WHAT DO WE MEAN? A SEMANTIC PRIMER, PART 1
Tumor Biomarker Tests
Much of the excitement about the explosion in “omics” is the identification of new biomarkers that might be useful for care of patients with cancer. However, it is important to recognize that the term “tumor biomarker” is relatively generic, referring to an identifiable biologic difference between the cancer and normal state. Indeed, there may be one or more assays for a given biomarker, and they may differ widely in accuracy and clinical applicability. As noted, demonstration of HER2 over-expression in breast cancer is a useful biomarker to guide whether patients should or should not receive anti-HER2 therapies, such as trastuzumab. Most (90%), but not all, HER2 over-expression is due to genetic amplification, whereas a small proportion is due to unregulated promoter activity. In addition, the recent results from the TCGA showed that 3% to 5% of breast cancers with normal copy number and expression of HER2 harbor an activating mutation (4). Therefore, assays have been developed to evaluate HER2 protein overexpression by enzyme-linked immunosorbent assay (ELISA), immunohistochemistry, and immunofluorescence; to evaluate RNA over-expression with Southern blots and reverse transcriptase polymerase chain reaction and to evaluate DNA amplification with Southern blots, dot blots, and more commonly using in situ hybridization assays with fluorochromes or chromogens (2). ELISAs have also been developed to quantitate circulating extracellular domain of HER2, and finally, DNA sequencing assays are now used to detect activating mutations for phase 2 clinical trials. Each of these tests measures HER2 abnormalities in very different ways, in different specimens, and for different uses. This example emphasizes the importance of distinguishing the assays and tests for a tumor biomarker from the general concept of the marker itself.
Omics
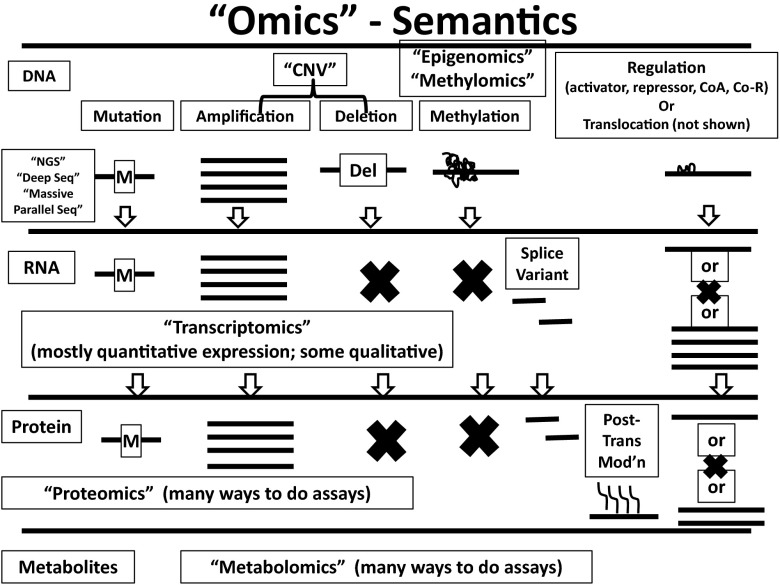
The recently popularized strategy of precision medicine is largely based on the concept of “omics,” which of course means many things to many people. High-throughput assay technology has permitted assessment of hundreds, thousands, and even millions of data-points into one database, which requires sophisticated bioinformatics analyses to make sense of it all. For clinicians, this circumstance can be overwhelming. Figure 1 represents a primer of the possible abnormalities in any given cancer and the semantics that have been used to describe their analyses. For example, the terms “next-generation sequencing (NGS),” “deep sequencing,” and “massive parallel sequencing” have all been used interchangeably. Likewise “transcriptomics” may refer to quantitative analysis of RNA expression of the entire genome, or of a selected few genes that appear to be of importance for a particular use in a specific cancer. Alternatively, or at times concurrently, the term may also apply to quantitative analyses of genetic or post-genetic alterations in the message, such as mutations, translocations, splice variations, etc. Similarly, the terms “proteomics” and “metabolomics” have come to mean all things to all people, with various efforts to do unstructured exploratory studies to find patterns of proteins associated with specific circumstances, or to select multiple candidate proteins for analysis in a single assay.
Fig. 1.
Omics semantics. The term “omics” can be applied to high throughput, large data analyses of changes in DNA, RNA, protein, or metabolites. Examples of the changes and terms used to describe them are provided. See text for explanation. Modified with permission from “Next-Generation Genomic Testing: An Understanding of Semantics Helps Guide Assay Use, Selection” in the 2014 Saturday ASCO Daily News (2014 ASCO Annual Meeting).
Omics-based Tests
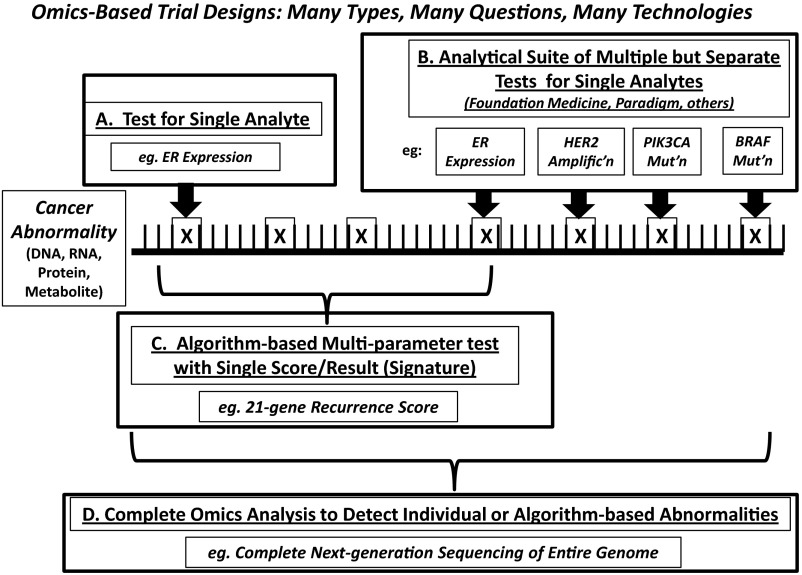
Assays for each of the omics abnormalities have been developed either for further investigation or even for marketing, and so one must be cognizant of these terms and their meaning. In fact, assays have been developed and marketed that purportedly represent omics-based tests, but in fact may not do so. Figure 2 displays the various possibilities. All clinicians are accustomed to an assay for a single analyte, with the example given above for measurement of ER in breast cancers being the paradigm (Figure 2A). Further, clinicians are accustomed to having groups of single analytes provided in a panel, such as is presented in a comprehensive blood count with white blood cells, hemoglobin, hematocrit, platelet count, and various red blood cell indices. In this case, the clinician can pick and choose which of these is of most importance for care of the patient. Again turning to breast cancer for an example, pathologists now routinely provide ER, progesterone receptor, and HER2 results on all breast cancer specimens, without the need for a specific order for each, under most circumstances (Figure 2B).
Fig. 2.
Types of biomarker tests for cancer. Various tumor tumor biomarker tests may be performed on human specimens. These include (A) tests for single analytes; (B) panel tests that include several different single analytes; (C) signature tests that consist of multiple parameters that are weighed in an algorithm to assign a specific score or category to the specimen; and (D) complete next-generation sequencing and “omicizing.” The horizontal line represents DNA, RNA, protein, or other molecule. The vertical lines represent “normal” components (base pairs, amino acids, etc.). The X's represent abnormalities (mutations, deletions, splice variants, post-translational modifications, etc.). See text for further explanation. Modified with permission from “Next-Generation Genomic Testing: An Understanding of Semantics Helps Guide Assay Use, Selection” in the 2014 Saturday ASCO Daily News (2014 ASCO Annual Meeting).
More recently, several companies have extended these kinds of panels to provide several assays for multiple different analytes that might be of interest for the disease in hand, but also provide results from other biomarkers that are not necessarily recommended by guidelines panels for that cancer (Figure 2B). Some manufacturers are even providing a multitude of candidate assays that might be of interest for that cancer or any other cancer, all in one panel, with the implication that if the biomarker is important for a specific use in one cancer it might be of value to direct therapy in a completely different type of malignancy.
Another approach has been to use omics-derived information to construct tumor biomarker tests that consist of multiple measurements combined into a single signature test with a range of scores, usually expressed as low to high (Figure 2C). In this case, cut-off values are chosen to place the patient into a category in which his/her treatment might differ, much the same as is done for a single analyte test. Again, turning to breast cancer as an example, the 21-gene recurrence score assay (OncotypeDx, Genomics Health Inc., Redwood, CA) is perhaps the paradigm of such a test. This assay incorporates an algorithm that weighs the relative expression of 16 different candidate genes (including ER and associated genes, HER2 and associated genes, and genes associated with proliferation and migration and metastases), normalized to 5 internal reference genes. It has now been highly recommended for use to identify patients with ER-positive, HER2-negative breast cancer and uninvolved axillary lymph nodes who, assuming they get proper adjuvant endocrine therapy, have such a favorable prognosis that adjuvant chemotherapy is not recommended (5).
Finally, several investigators have developed rapid and relatively inexpensive technologies that permit total DNA sequencing and transcriptomics so that the tumor (and accompanying germline DNA) is completely analyzed (Figure 2D) (6). In this case, the assay generates enormous amounts of data that must be distilled into actionable pieces of information. However, the field has not yet come to consensus about the definition of actionable, and these types of assays have remained isolated to a few academic centers.
HOW AND WHEN DO WE APPLY THESE TYPES OF ASSAYS IN CLINICAL MEDICINE? A SEMANTIC PRIMER, PART 2
The availability of omics-based tests has generated excitement that they might truly achieve the goal of personalized or precision medicine. However, as in all of medicine, hype often precedes reality. Moreover, sorting out when and how to use diagnostics has been much more complicated than therapeutics, and once again raises issues of semantics.
Analytical Validity, Clinical Validity, and Clinical Utility
Several guideline bodies and individual authors have long recognized the void of scientific rigor in which many diagnostics, especially tumor biomarker tests, have been studied. In 2009, the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) initiative proposed three semantic terms that have clarified the criteria for adopting new diagnostics into routine clinical practice (7).
Analytical validity refers to conduct of the test in the clinical laboratory, including pre-analytical issues such as handling of the tissue before it is received, and specific analytical issues regarding accuracy, reproducibility, and reliability of the assay. Clearly, a tumor biomarker test is of no value if it is not analytically validated. Clinical validity is achieved if the marker divides one population into two or more distinct subgroups that differ according to biology or, more commonly, clinical outcomes. However, although important, clinical validity does not imply that the assay should be used to guide patient care. Rather, clinical utility is essential to manage a patient's case with a tumor biomarker test. Clinical utility implies that high levels of evidence are available that show that use of the tumor biomarker test to direct patient care results in better outcomes, or equivalent outcomes with less cost or inconvenience, when compared to management in the absence of the marker data.
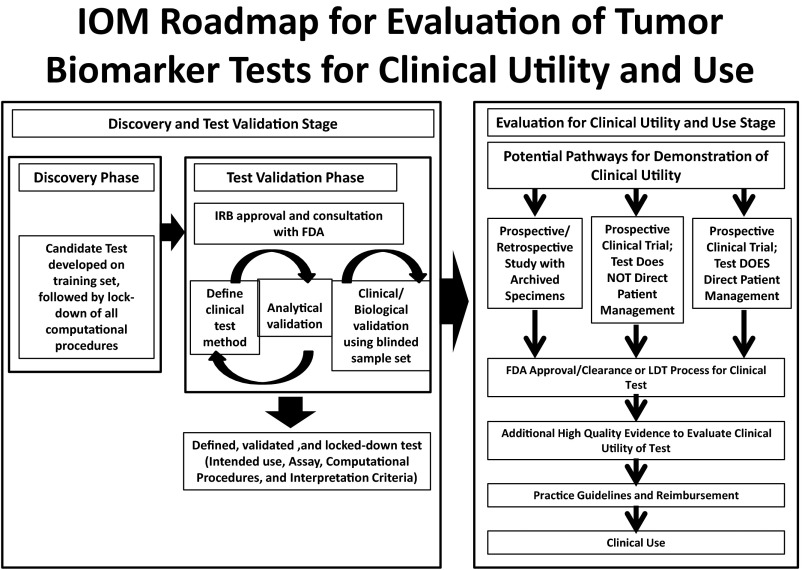
There are two avenues to generate high levels of evidence to show clinical utility. The ideal strategy is a prospective clinical trial in which the tumor biomarker is, in itself, the primary objective of the trial. There are several designs to conduct such trials (8,9), but they are expensive and time-consuming. Unlike new therapeutics, one might also conduct what have been called prospective retrospective clinical studies, using patient specimens that have been collected and archived from within previously conducted clinical trials that have addressed the potential use of the tumor biomarker test (10). These strategies have been incorporated into a roadmap for generation and validation of omics-based tests by a committee convened by the Institute of Medicine (Figure 3) (11).
Fig. 3.
A roadmap for generation of omics-based tests. The upper left panel represents discovery of new biomarkers that might be useful to care for patients. The upper right panel shows the necessary steps to prepare a highly analytically validated test for that marker with clinical validity. The lower panel depicts possible steps to generating high levels of evidence to support a claim of clinical utility for a given use and therefore clinical application of the biomarker test for patient care. Modified from Micheel (11) with permission.
TRIALS ENCOMPASSING OMICS-BASED TUMOR BIOMARKER TESTS: WHAT'S THE QUESTION?
Having established the background, we now turn to ongoing strategies to determine how to use omics-based tumor biomarkers in the clinic. Fundamentally, there are two categories of ongoing research: (1) What is good for the academic investigator and/or device manufacturer? and (2) What is good for the patient? One would hope that these two goals would coincide; sometimes they do and sometimes they do not. Studies that fall into the former include trials that use the test to test the drug, use the data to generate new tests, or to directly “test the test.” Studies that fall into the latter category are principally those that “test the strategy” of using the omics-based test(s) to determine if patient outcomes are improved compared to standard of care. Testing the test and testing the strategy may overlap considerably. However, all too often, companies, investigators, clinicians, and patients assume that because something is new, it must be better, and may forget that this assumption may not necessarily be true.
Testing the Drug
Classically, new therapeutic drugs are developed within sequential trials that first determine the safest dose and schedule (phase 1), secondly whether the drug has any activity (phase 2), and finally whether the drug provides additional and meaningful benefit compared to present standard of care (phase 3). Phase 2 trials represent “red light/green light” studies, and they are designed to provide a reasonably accurate estimate of whether the drug should proceed to larger and more costly phase 3 trials in as efficient a manner as possible. Traditionally, phase 2 trials are conducted within specific subgroups of patients, usually defined by tissue histology (for example, breast, lung, or colorectal cancers), and occasionally by biologic differences (such as endocrine therapy trials in only ER-positive breast cancers).
Omics-based tests are now being considered to make screening for phase 2 trials more efficient by interrogating several different markers and placing patients into available phase 2 trials that match these abnormalities. These trials, termed “basket,” or “bucket” designs, act a bit like an apple sorter by placing a patient into the trial that best fits his/her cancer. The first of these to be activated in North America is the Squamous Cell Lung Cancer Master Protocol (MAP trial). In this trial, cancer tissues from patients with this particular histopathological lung cancer diagnosis are profiled with a panel assay that provides broad biomarker profiling (Foundation Medicine, Inc., Cambridge, MA) (http://www.lung-map.org/). If their tumor contains the appropriate genetic changes, the patient is assigned to one of five associated basket trials, in which he/she is randomized to the investigational drug or standard of care. This trial combines both a test-the-drug as well as a test-the-test strategy, in that a secondary analysis is whether patients who are randomly assigned to investigational drug within the phase 2 baskets fare better than those who receive standard of care.
A second basket trial, now being planned by the National Cancer Institute (NCI), is termed the MATCH trial. While similar in design to the lung MAP trial, the MATCH trial will enroll patients regardless of their tumor histopathology, and the omics analyses will be performed in one of several Clinical Laboratory Improvement Amendments (CLIA) − approved laboratories that will contract with NCI for this purpose. At least 40 investigational drugs have been committed by several different pharmaceutical companies. This trial is expected to be open in late 2015.
Finally, fully-powered trials to test the strategy are being contemplated, although the exact trial design has not yet been determined. For example, the Lung MAP trial is already incorporating this concept, but only for investigational agents. Another approach is to assign patients to receive “matched” molecularly targeted drugs that are already approved, either for the cancer type they have or for a completely different cancer type. These trials will need to be large and relatively pragmatic, since many patients will not have actionable molecular matches, and furthermore, it is unknown if a genetic abnormality that predicts for benefit in one cancer type will actually do so in another.
CONCLUSION
In summary, we appear to be on the verge of enjoying the promise of precision medicine in the omics era, perhaps no more so than in oncology. However, the considerable hype has preceded the sparse known reality, and it is essential that the scientific method continue to be invoked before we apply the newer omics-based tests to management of patients with cancer. The issues of analytical validity and clinical utility must be rigorously evaluated. Nonetheless, these new technologies offer considerable promise for improved care of patients with cancer, at the least by permitting novel trial designs that get us the answers we need more quickly to translate these findings to standard clinical practice.
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Weiner, Iowa City: We are finding that most cancers don't have just one mutation but multiple mutations, and a given pair or set of mutations is relatively rare. How will you evaluate the efficacy of therapy in these very small subsets of patients where there are just not enough people to do a randomized phase 3 trial?
Hayes, Ann Arbor: The best example I have of this right now is breast cancers, which are the most common cancers in women, as all of you know. The HER2 gene is a real driver of about 20% of breast cancers based on overexpression as a function of amplification of the gene. We were told 20 years ago by a few investigators that an activating mutation in HER2 did not exist. But with The Cancer Genome Atlas it has turned out that 2% to 3% of all breast cancers that are not amplified and not overexpressed for HER2 do indeed have an activating mutation, and that mutation appears to be particularly sensitive to a tyrosine kinase inhibitor called neratinib. We are now trying to do a phase 2 trial. There are 10 centers, each of us have screened 100 patients. We now have about 8 patients on that trial because of the relative rarity of this. It's a real issue, and that's for a cancer that is pretty common, breast cancer. When we start talking about lymphomas and break those into 2% to 3%, I don't know how we are going to do it. It is going to take enormous cooperation. I think all around the world by cooperative groups. And I'm actually encouraged by both the Lung-MAP and by the NCI-MATCH trials. Everybody is willing to row in the same direction and sort of leave their egos at the doorstep to move forward. I am an oncologist. I am an eternal optimist that we can do this.
Agarwal, Birmingham: Given this big boom in personalized medicine, I believe the pharmaceutical industries are worried about how will they have drugs for each different mutation and keep up the pace, not only for cancer but several other diseases. Do you have a comment for that? The pharmaceutical industry is quite worried that they will need a lot of drugs for treating the various mutations, each specific mutation with a specific drug, and they don't have the time or the funding to be able to generate that many drugs.
Hayes, Ann Arbor: Those assays are like iPhones. We are getting down shorter and shorter in periods of time and less and less money to do the assays. The second issue is germ line inherited SNP and how they interact in the whole field of pharmacogenomics. Ten years ago I really thought we would all be getting our germ line DNA testing when we see our doctor, and then we would use those data to direct patient care. I have actually gotten very frustrated with the field, and I think that it's because the human genome is so complex. It's a reason we don't all die of the flu every year. There have been very few examples so far of effective germ line pharmacogenomics testing to direct care. I still think the field can move forward. I still think the field can be successful, but we are going to have to be much more clever than I assumed 10 years ago.
REFERENCES
- 1.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 3.The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumors. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bose R, Kavuri SM, Searleman AC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3:224–37. doi: 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 6.Roychowdhury S, Iyer MK, Robinson DR, et al. Personalized oncology through integrative high-throughput sequencing: a pilot study. Sci Transl Med. 2011;3:111ra21. doi: 10.1126/scitranslmed.3003161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teutsch SM, Bradley LA, Palomaki GE, et al. The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Initiative: methods of the EGAPP Working Group. Genet Med. 2009;11:3–14. doi: 10.1097/GIM.0b013e318184137c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sargent DJ, Conley BA, Allegra C, Collette L. Clinical trial designs for predictive marker validation in cancer treatment trials. J Clin Oncol. 2005;23:2020–7. doi: 10.1200/JCO.2005.01.112. [DOI] [PubMed] [Google Scholar]
- 9.Freidlin B, McShane LM, Korn EL. Randomized clinical trials with biomarkers: design issues. J Natl Cancer Inst. 2010;102:152–60. doi: 10.1093/jnci/djp477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101:1446–52. doi: 10.1093/jnci/djp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Micheel CM, Nass SJ, Omenn GS, editors. Evolution of Translational OMICs: Lessons Learned and the Path Forward. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]