Abstract
Background:
Laminin-332 (LM-332, formerly termed laminin-5) is a heterotrimeric glycoprotein that promotes cellular adhesion and migration. The heterotrimer consists of an α3, a β3, and a γ2 chain. The aim of this investigation was to clarify the clinicopathologic significance of laminin-332β3 (LNβ3) chain expression and determine its influence on survival in pancreatic ductal adenocarcinoma.
Materials and Methods:
Quantitative real-time polymerase chain reaction was used to validate and detect the expression of LNβ3 mRNA in 37 pancreatic carcinoma tissue specimens and non-neoplastic pancreatic tissue samples. In addition, the protein expression of LNβ3 was detected by immunohistochemistry methods in 96 pancreatic carcinoma specimens and 90 non-neoplastic pancreatic tissues. We analyzed the association between immunohistochemically detected LNβ3 expression in pancreatic ductal adenocarcinoma and clinicopathologic characteristics. Survival curves were completed using the Kaplan-Meier method and compared using log-rank analysis.
Results:
Quantitative real-time polymerase chain reaction indicated that the relative value of LNβ3 mRNA was 1.427±1.554 and 1.423±1.439 by 2−Δ(ΔCt) in pancreatic carcinoma and non-neoplastic pancreatic tissues, respectively, values that were not statistically associated (P=0.991). Immunostaining for LNβ3 was expressed in all patients with pancreatic ductal adenocarcinoma. LNβ3 expression was related to differentiation (P=0.000) and advanced stage (P=0.034). Tumors with low expression of LNβ3 had a survival advantage compared with tumors that had high expression of LNβ3 (P=0.016). Multivariate analysis indicated that location is an independent predictor of overall survival, whereas other clinicopathologic characteristics such as tumor size, duodenal invasion, differentiation, extent of invasion, hepatic metastasis, and expression of LNβ3 were not.
Conclusion:
Our results suggest that LNβ3 expression may play a key role in the progression and prognosis of pancreatic ductal adenocarcinoma.
Key Words: pancreatic ductal adenocarcinoma, laminin-332 (LM-332), laminin β3 (LNβ3)
Pancreatic ductal adenocarcinoma is one of the most deadly cancers of the gastrointestinal tract. Although diagnosis and treatment methods continue to improve, this cancer is almost invariably fatal. Surgical resection and chemotherapy are the only possible cure for patients with pancreatic carcinoma. Therefore, it is necessary to determine the biological characteristics of the carcinoma and identify better therapy targets to improve the prognosis.
Tumor invasion and metastasis may initiate from invading of surrounding tissue and then break through the basement membrane (BM) by tumor cells. Laminins are structural molecules of the extracellular matrix (ECM) that separate epithelial cells from the underlying stromal tissues and play an important role in cell adhesion, growth, migration, proliferation, and differentiation. Laminins are heterotrimeric glycoproteins composed of 3 different chains (α, β, and γ), encoded in humans by 5α, 4β, and 3γ genes.1 At present, 15 different laminin isoforms have been identified in mammals.2 Laminin-332/laminin-5 (LM-332) consists of α3, β3, and γ2 chains, which are encoded by the LAMA3, LAMB3, and LAMC2, genes respectively.
At present, the association between the laminin-332 β3 chain and pancreatic cancer has not been studied. We investigated both mRNA and protein expression of LNβ3. The purpose of this study was to determine the impact of their expression relative to other typical clinicopathologic variables on outcomes in patients undergoing resection of pancreatic ductal adenocarcinoma.
MATERIALS AND METHODS
Between February 2010 and March 2013, tissue specimens (neoplastic and matched non-neoplastic pancreatic tissues) were collected from 37 patients with pancreatic ductal adenocarcinoma at the Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, College of Medicine, Zhejiang University. Pathologic analyses confirmed the diagnosis of each patient. The tissues were immediately frozen in liquid nitrogen and stored at −80°C until use. The formalin-fixed, paraffin-embedded sections of 96 pancreatic ductal adenocarcinomas were used for immunohistochemistry. Of 96 cases, 90 tissue samples were confirmed by a single pathologist to contain the tumor with adjacent normal pancreatic ductal tissue, whereas 6 blocks of tissue contained the tumor with abnormal adjacent pancreatic ductal tissue. We reviewed the clinical information of 96 of these randomly selected patients after approval by our institutional review board. Data on age, sex, tumor location, size, bile duct/duodenal invasion, differentiation, lymph node invasion, perineural invasion, vascular invasion, pancreatic capsule invasion, portal vein invasion, margin status, degree of invasion, hepatic metastasis, and TNM staging were collected.
Quantitative Real-Time Polymerase Chain Reaction (QRT-PCR)
Total RNA was extracted from pancreatic cancer tissues and adjacent tissues using the TRIzol reagent (Invitrogen, CA), and cDNA was synthesized from the total RNA (2 μg) using iScript cDNA Synthesis (Bio-Rad laboratories). Real-time PCR was performed with an ABIPRISM 7900 Sequence Detection System (Applied Biosystems) using an iTaq universal SYBR Green supermix (Bio-Rad laboratories). Amplification reactions included 1 μL of cDNA template, 0.3 μL each of the forward and reverse primers (10 μM), 0.2 μL of 50× ROX Reference Dye II (Takara), and 5 μL of 2×SYBR Premix DimerEraserin, for a total volume of 10 μL. The primers for the 2 genes were as follows: LAMB3, (forward) 5′-GGCAGATGATTAGGGCAGCCGAGGAA-3′ and (reverse) 5′-CGGACCTGCTGGATTAGGAGCCGTGT-3′; β-actin, (forward) 5′-CTTAGTTGCGTTACACCCTTTC-3′ and (reverse) 5′-GAGTTAAAAGCAGCCCTGGT-3′. Amplification of the transcripts involved an initial denaturation at 95°C for 30 seconds, followed by 40 cycles at 95°C for 5 seconds, 55°C for 30 seconds, and 72°C for 34 seconds. The comparative threshold cycle method was used for relative quantification. β-actin was used as an internal control for normalization. All real-time PCRs were performed in triplicate. Data were calculated using the 2−Δ(ΔCt) method.3
Immunohistochemistry
Formalin-fixed, paraffin-embedded tumor tissue samples from 96 patients were sectioned (4-μm thick), mounted on poly-l-lysine-coated glass slides, and allowed to dry overnight at 65°C. Briefly, slides were deparaffinized in xylene, transferred through 3 changes of 95% ethanol, and then transferred into water. For antigen retrieval (β3), the slides were boiled in a pressure cooker containing Tri-EDTA (pH 9.0) for 20 minutes then cooled for 20 minutes at room temperature. Endogenous peroxidase activity was blocked in 1.5% methanol hydrogen peroxide for 8 minutes at room temperature. Following incubation, the slides were washed 3 times in PBS for 2 minutes each. Then, the slides were incubated with the primary antibody Lamininβ-3 (Santa Cruz, sc-133178) in 1:100 dilution overnight at 4°C. After washing 3 times in PBS for 2 minutes each, the bound primary antibody was detected using a ready-to-use secondary antibody kit (Dako, K5007) for 30 minutes at room temperature, and the chromogenic substrate 3,3-diaminobenzidine tetrahydro-chloride. The specimens were then counterstained with hematoxylin, mounted and examined by light microscopy.
Tumor cell percentage was scored as follows: 0=≤5% of tumor cells positive; 1=6 to 25% of tumor cells positive; 2=26 to 50% of tumor cells positive; and 3=≥51% of tumor cells positive. Scoring criteria for staining intensity were as follows: 0=no staining; 1=weak staining (light yellow); 2=moderate staining (yellow brown); and 3=strong staining (brown). The staining index was calculated as the product of the percentage of positive tumor cells and the staining intensity score.
Using this method of estimation, we evaluated expression of LNβ3 in the tumor and adjacent normal pancreatic ductal tissue by determining the staining index with scores of 0, 1, 2, 3, 4, 6, or 9. In statistical analysis, an optimal cutoff value was assessed as follows: a staining index score of >6 was used to indicate tumors with high LNβ3 expression, and a staining index score of ≤6 was used to define low LNβ3 expression.
Statistical Analysis
All analyses were performed using SPSS version 21.0 software. The χ2 2-tailed test or Fisher test was used to assess the associations between immunohistochemical expression and clinicopathologic characteristics. P <0.05 were considered statistically significant. A P-value between 0.05 and 0.20 was considered a trend toward an association. The survival time was considered calculated from the date of resection until the date of death from any cause. Dates of death were obtained from follow-up telephone calls or patient hospital records. Survival curves were completed using the Kaplan-Meier method and compared by log-rank analysis. Multivariate analysis was performed by the Cox proportional hazard method.
RESULTS
mRNA Expression of LNβ3 in the Primary Pancreatic Adenocarcinoma and Corresponding Non-Neoplastic Pancreatic Tissues
In this study, we randomly selected 37 pairs of primary pancreatic adenocarcinoma and corresponding adjacent non-neoplastic pancreatic tissues for transcriptional mRNA level of LNβ3 by real-time PCR. LNβ3mRNA level in 15 pancreatic cancers were higher than corresponding adjacent tissues, however, another 22 pairs of tissue showed the opposite results which meant LNβ3 mRNA may frequently be downregulated in pancreatic carcinoma. The relative value of LNβ3 mRNA was 1.427±1.554 in pancreatic carcinoma and 1.423±1.439 by 2−Δ(ΔCt) in non-neoplastic pancreatic tissues in this 37 cases. The difference of expression levels of LNβ3 mRNA between carcinoma and non-neoplastic pancreatic tissues was not statistically significant (P=0.991).
Overexpression of LNβ3 in Pancreatic Ductal Adenocarcinoma
Expression of LNβ3 was detected in the tumors of all 96 patients, although their expression intensity varied. Strong (high expression) staining for LNβ3 was observed in 83 (86.5%) of 96 cases (Table 1).
TABLE 1.
Expression of LNβ3 in Tissue
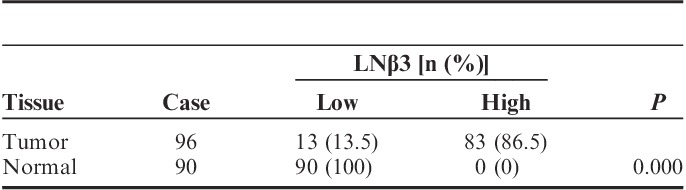
Normal pancreatic ducts stained negative, weakly positive, or moderately positive for LNβ3 (Table 1). Staining for LNβ3 was not observed in the normal pancreatic tissue (Fig. 1A). Expression of LNβ3 of 6 adjacent normal pancreatic ductal tissues was not observed (Table 1).
FIGURE 1.
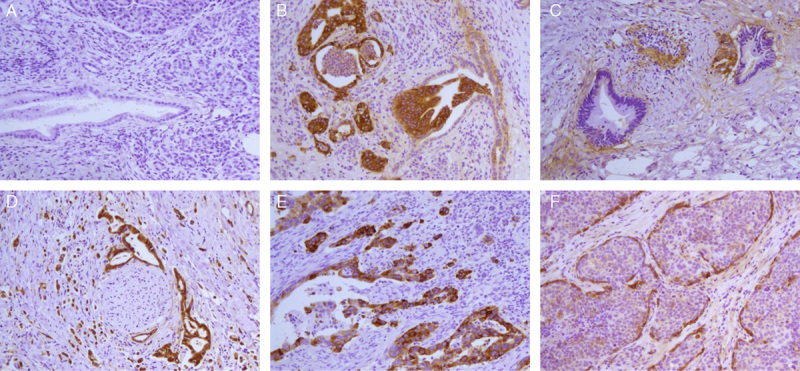
Immunohistochemistry of LNβ3 in pancreatic ductal adenocarcinoma. A, Pancreatic tissue is negative for staining (×200). B, Residual duct invasion is observed in moderately differentiated adenocarcinoma. LNβ3 is strongly expressed in tumor cells but not in normal epithelium. Weak expression is observed in the stroma and heterotype epithelium (×200). C, Well-differentiated adenocarcinoma is negative for staining, but marked expression of LNβ3 is detected in cancer cells contacting the stroma at the edge of clustered cancer cells. A very small focal section of moderately differentiated adenocarcinoma stained strongly (×200). D, Extensive perineural invasion is observed in a poorly differentiated adenocarcinoma. Strong (high expression) LNβ3 staining is shown in tumor cells (×200). E, Poorly differentiated pancreatic ductal adenocarcinoma positive for staining. Tumor cell reproduction was seen in the outer margin. Expression of LNβ3 is strong (×200). F, Adenocarcinoma is accompanied by squamous metaplasia. Strong (high expression) staining of LNβ3 is seen in cancer cells contacting the stroma at the edge the cluster of cancer cells. Cells in the center of the cluster stained weakly (×200).
Figures 1B to F show expression results of immunohistochemistry for LNβ3 in pancreatic ductal adenocarcinoma. In carcinoma tissues, LNβ3 expression, when present, was observed predominantly in the cytoplasm of cancer cells. Strong staining for LNβ3 is often observed in poorly differentiated tumors and weak expression in heterotype epitheliums and well-differentiated tumors (Figs. 1B, C). Extensive perineural invasion is found easily in pancreatic cancer with high expression of LNβ3 (Fig. 1D). The cytoplasmic staining was more intense in the stain pattern of LNβ3 known as invasion front or dividing cancer cells (Fig. 1E). In the domain of squamous metaplasia, the cytoplasmic immunoreactivity was predominantly expressed in cancer cells at the edge of groups of cancerous cells, with only weak staining of the cells in the center of the group (Fig. 1F). It is obvious that the expression of LNβ3 was increased with regression of differentiation in our study. In some cases, the mesenchymal expression for LNβ3 in the ECM manifested a more irregular and curly and diffuse staining pattern, particularly in the invasive areas (not shown in Fig. 1).
Overall, LNβ3 of pancreatic adenocarcinoma tissues was significantly overexpressed compared with that in normal pancreatic tissue (P=0.000; Table 1).
Association of LNβ3 Expression With Clinicopathologic Characteristics
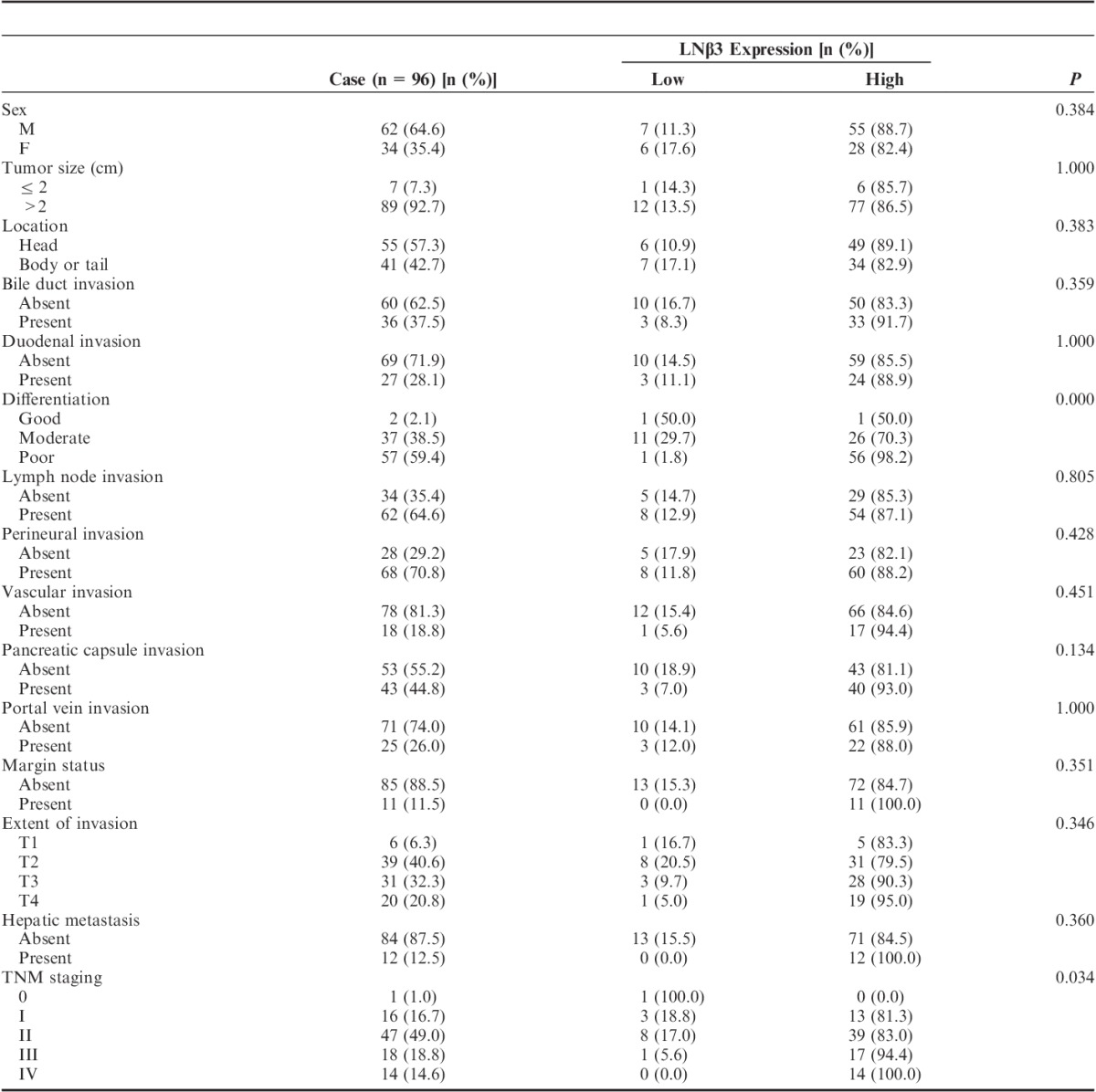
Table 2 summarizes the clinical data, including age, sex, tumor size, tumor location, bile duct/duodenal invasion, differentiation, lymph node invasion, perineural invasion, vascular invasion, pancreatic capsule invasion, portal vein invasion, margin status, extent of invasion, hepatic metastasis, and TNM staging, along with the staining intensity of LNβ3 in the 96 patients with pancreatic ductal carcinoma. LNβ3 expression was significantly correlated with differentiation and advanced-stage neoplasia (P<0.05). The cases with positive LNβ3 staining were more likely to have pancreatic capsule invasion than were those that stained negative (P=0.134).
TABLE 2.
Clinicopathologic Characteristics Based on Expression of Laminin β3
Survival
Univariate analysis: The median survival time of patients who exhibited low expression of LNβ3 was 19.200 months, whereas the median survival time of patients who exhibited high expression of LNβ3 was 8.984 months. The 1-year survival rate of cases with low expression was 68%, whereas that of cases with high expression of LNβ3 was 28%. The correlation between low expression for LNβ3 and high expression for LNβ3 was significant (Gehan test, u=9.011; P=0.003; Table 3). Tumors with low expression of LNβ3 conferred a survival advantage compared with tumors with high expression of LNβ3 (P=0.016; Fig. 2).
TABLE 3.
The Survival of Cases With LNβ3 Expression
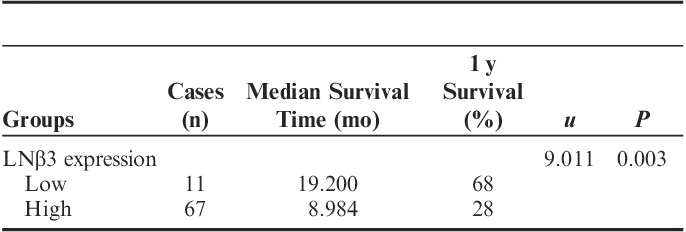
FIGURE 2.

Kaplan-Meier overall survival curve for patients with pancreatic adenocarcinoma based on LNβ3 expression.
Multivariate analysis: The independent prognostic factors most likely to impact survival were entered into the multivariate analysis model. Location was a significant predictor of survival, whereas other histopathologic factors such as tumor size, duodenal invasion, differentiation, extent of invasion, hepatic metastasis, and expression of LNβ3 were not predictive (Table 4).
TABLE 4.
Potential Predictors of Overall Survival in 96 Patients With Pancreatic Cancer who Underwent Surgical Resection

DISCUSSION
At present, there are some reports about LNβ3 expression in human cancer cells. In these literature, expression of LNβ3 has been reported to associated with LNγ2 in hepatocellular carcinoma, squamous cell carcinoma of the tongue, colorectal carcinoma, biliary cancer, and gastric cancer.4–7 To our knowledge, LNβ3 had not been reported in pancreatic adenocarcinoma previously, further analysis was required to explore the mechanism of expression.
Previous study revealed that expression of LNβ3 genes was lost in several cell lines and tumors. It was lost in 4 of 5 (80%) bladder cancer cell lines and 6 of 8 (75%) bladder tumors.8 In gastric cancer tissues, the LNβ3 gene was transcriptionally silenced by methylation of the promoter CpG islands in several cell lines.7 In our experiments, although LNβ3 mRNA was expressed in pancreatic adenocarcinoma on expression profiles of LM-332-encoding genes by QRT-PCR, part of tumors had lost expression. It needs further research to whether the expression deletion of LNβ3 genes is associated with the promoter methylation in pancreatic adenocarcinoma.
Immunohistochemistry revealed that expression of LNβ3 was predominantly in cytoplasm of pancreatic cancer cells in these included specimens. Strong staining of LNβ3 predominantly in the cytoplasm of cancer cells was observed in 83 (86.5%) of 96 patients with pancreatic adenocarcinoma that was correlated with differentiation (low differentiated type) and advanced stage. Tumors with low expression of LNβ3 had a survival advantage compared with tumors with high expression of LNβ3. It also showed that expression of LNβ3 was associated with the extent of invasion and advanced stage in gastric cancer.7 Overexpression of LNβ3 could stimulate invasion, migration, and tumorigenicity.
Of interest, in pancreatic adenosquamous carcinoma, strong staining for LNβ3 was predominantly seen in the cancer cells at the edge of the tumor nests, with the cells in the center of the tumor nests staining weaker. It was further confirmed that LNβ3 expression was correlated with differentiation of pancreatic adenocarcinoma and squamous cell carcinomas.
In addition, we observed that LNβ3 staining was often more intense at the invasive front and in reproducing pancreatic cancer cells. Meanwhile, immunohistochemistry staining of LNβ3 and LNγ2 was mainly observed in the cytoplasm of biliary tract cancer cells. The invasive front-dominant pattern of expression for LNβ3 was observed in 23 (38%) patients with biliary tract cancer.6
In some cases, the mesenchymal expression for LNβ3 was not continuous along tumor BMs but irregular and curly and diffuse in the ECM, particularly in the invasive areas. The staining pattern or absence of a BM seen in carcinoma may be due to decreased synthesis of BM components induced by proteolytic degradation. In contrast, cancer cells may send out signals that induce invasion by initiating synthesis of gene products in stromal cells that facilitate cancer cell migration and invasion.9 This suggests that interactions of pancreatic adenocarcinoma cells with the ECM at the invasive margin could cause the accumulation of LNβ3 there, facilitating local metastasis.
These results imply that the expression of LNβ3 in the cytoplasm of pancreatic adenocarcinoma cells indicates an aggressive phenotype of carcinoma cells, resulting in rapid progression of pancreatic adenocarcinoma.
Although only a few studies have examined LNβ3 expression in human cancer, the mechanism of interaction between LNβ3 expression and cancer invasion has been researched and discussed. The laminin EGF-like domain of the β3 short arm is a binding site for type VII collagen, which decreases the cell adhesion activity of LM-332 and plays an important role in signal transduction.10 In squamous cell carcinoma, the interaction between LNβ3 and type VII collagen could activate the PI3K pathway, which is important in carcinogenesis, invasion, and protection from apoptosis.11 These findings indicate a dramatic selectivity of expression and function for LNβ3 in pancreatic adenocarcinoma tumorigenesis and provide evidence supporting its usefulness as a target for anticancer therapy.
In conclusion, overexpression of LNβ3 indicates cancer cell invasion and is therefore correlated with disease progression and poor patient survival rate. The demonstration of the mechanism of LNβ3 in the invasion and metastasis of cancer cells in pancreatic ductal adenocarcinoma may elucidate a new molecular target for therapies used in these patients.
Footnotes
J.C. and W.W. contributed equally.
The authors declare no conflict of interest.
REFERENCES
- 1.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. [DOI] [PubMed] [Google Scholar]
- 2.Aumailley M, Bruckner-Tuderman L, Carter WG, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. [DOI] [PubMed] [Google Scholar]
- 3.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 4.Giannelli G, Fransvea E, Bergamini C, et al. Laminin-5 chains are expressed differentially in metastatic and nonmetastatic hepatocellular carcinoma. Clin Cancer Res. 2003;910 pt 13684–3691. [PubMed] [Google Scholar]
- 5.Akimoto S, Nakanishi Y, Sakamoto M, et al. Laminin 5 beta3 and gamma2 chains are frequently coexpressed in cancer cells. Pathol Int. 2004;54:688–692. [DOI] [PubMed] [Google Scholar]
- 6.Oka T, Yamamoto H, Sasaki S, et al. Overexpression of beta3/gamma2 chains of laminin-5 and MMP7 in biliary cancer. World J Gastroenterol. 2009;15:3865–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.li M, Yamamoto H, Taniquchi H, et al. Co-expression of laminin β3 and γ2 chains and epigenetic inactivation of laminin α3 chain in gastric cancer. Int J Oncol. 2011;39:593–599. [DOI] [PubMed] [Google Scholar]
- 8.Sathyanarayana UG, Maruyama R, Padar A, et al. Molecular Detection of noninvasive and invasive bladder tumor tissues and exfoliated cells by aberrant promoter methylation of laminin-5 encoding genes. Cancer Res. 2004;64:1425–1430. [DOI] [PubMed] [Google Scholar]
- 9.Dano K, Behrendt N, Brunner N, et al. The urokinase receptor: protein structure and role in plasminogen activation and cancer invasion. Fibrinolysis. 1994;8:189–203. [Google Scholar]
- 10.Nakashima Y, Kariya Y, Yasuda C, et al. Regulation of cell adhesion and type VII collagen binding by the beta3 chain short arm of laminin-5: effect of its proteolytic cleavage. J Biochem. 2005;138:539–552. [DOI] [PubMed] [Google Scholar]
- 11.Waterman EA, Sakai N, Nguyen NT, et al. A laminin collagen complex drives human epidermal carcinogenesis through phosphoinositol-3-kinase activation. Cancer Res. 2007;67:4264–4270. [DOI] [PubMed] [Google Scholar]


