Supplemental Digital Content is available in the text.
Key Word: thyroid indeterminate nodules, Galectin-3, HBME-1, p27, triple immunostaining, digital image quantitation
Abstract
Indeterminate thyroid nodules form a heterogenous group of lesions that constitute 5% to 30% of thyroid cytology diagnoses. We introduce a triple immunostaining protocol for subtyping. Galectin-3, HBME-1, and p27 triple immunostaining, performed on destained cytology slides and formalin-fixed paraffin-embedded tissue, was developed and applied to 51 patients retrospectively with preoperative cytologic diagnoses of follicular lesion of undetermined significance (n=40), atypia of undetermined significance (n=6), and suspicious for follicular neoplasm (n=5). The malignant rate in this series was 43.1% (22/51). A hierarchal evaluation algorithm was generated based on digital image quantitation of triple-stained histologic sections, and applied to both cytology and histology specimens. Fifty of 51 cytology cases have triple staining validated by internal controls. In cytology specimens, the individual sensitivities and specificities of p27, Galectin3, and HBME1 for cancer with 95% confidence interval are: 86.2% (0.674, 0.955)/66.7% (0.431, 0.845); 77.3% (0.542, 0.913)/72.4% (0.525, 0.866); and 72.7% (0.496, 0.884)/93.1% (0.758, 0.988), respectively. Sensitivity is increased to 95.5% (0.751, 0.998), but specificity is decreased to 69.0% (0.490, 0.840), if Galectin3 and HBME1 are both used in combination as markers for malignancy. However, the level of specificity is increased to 86.2% (0.674, 0.955) and sensitivity remains high 100% (0.808, 1) if in addition, using the Galectin3/HBME1:p27 ratio (ratio ≥2 indicating malignancy) for 2 or 3 markers positive cases. Thus, the triple staining method on cytology slides and histology sections shows a similar sensitivity/specificity/positive predictive value/negative predictive value of 100.0%/86.2%/84.0%/100.0% and 95.5%/86.2%/84.0%/96.2%, respectively (P=0.92). Overall, p27 is the most frequent single positive marker (19/50, 38% in cytology), consistent with benign nature of most indeterminate thyroid nodules. Galectin-3 and HBME-1 colocalization (positive in the same cell) was demonstrated in thyroid cancer in 45.5% (10/22) of histology sections, but in none of the normal thyroid tissues and benign thyroid lesions. This supports the notion that synchronous activation of Galectin-3 and HBME-1 occurs in thyroid malignancy and is highly specific for malignancy. We have demonstrated the performance and pattern of triple immunostaining for subtyping indeterminate thyroid nodules. Further studies and validation in different larger populations are warranted.
BACKGROUND
Fine needle aspiration (FNA) is widely considered to be the most accurate method for evaluation of a thyroid nodule.1 On the basis of FNA cytology results and according to Bethesda categorization, approximately 60% of the thyroid nodules are benign, <10% of the nodules are malignant,2,3 with the remainders being follicular lesions of undetermined significance (FLUS)/atypia of undetermined significance (AUS), suspicious for follicular neoplasm (SFN), and suspicious for malignancy (SFM) except nondiagnostic samples. With malignant risk of <3% for benign nodule, 60% to 75% for SFM, and 97% to 99% for malignant,2,3 patients will be followed clinically (for benign) or undergo surgery (for SFM or malignant). Most clinicians consider FLUS/AUS and SFN as “indeterminate.” The “indeterminate” category includes lesions with cytologic and architectural features neither definitively benign nor malignant. The rate of “indeterminate” diagnoses range from 3.0% to 27.2% for FLUS/AUS4–9 and 2.3% to 16.3% for SFN.4–8,10,11 The rate of malignancy, diagnosed postoperatively, ranges from 5% to 48% for FLUS/AUS4–7 and 12% to 67% for SFN.4–8,10,11
This wide variability in diagnostic rates results from multiple factors including patient population, sampling issues (sparse or bloody specimens, technical artifacts, etc.), and interpretative subjectivity. The widely variable rate of malignancy among indeterminate diagnoses, especially FLUS/AUS, leads to difficulties in clinical decision making and patient anxiety.
Numerous strategies have been investigated to make indeterminate diagnoses more definitive. Recognizing that many of the equivocal (FLUS/AUS/SFN) FNA cases are the result of inadequate number of cells or poorly visualized cells, Bethesda System for Reporting Thyroid Cytopathology has recommended adequacy evaluation.2 Expert consultation and group consensus reviews have also been reported to minimize the diagnosis of FLUS.12 Repeat FNA was definitive in 65% of FLUS/AUS,13 and 48.8% of nodules were reclassified as benign.14
Molecular tests have been vigorously investigated for the usefulness in thyroid diagnosis. BRAF, RAS mutations, or RET/PTC rearrangement occur in 70% of papillary thyroid carcinomas (PTCs). Similarly, 70% of follicular carcinomas (FC) harbor RAS mutations or PAX8/PPARγ translocations.15 Besides the diagnostic usage, BRAF mutation is also associated with advanced stage, higher risk of recurrence, and decreased response to radioiodine therapy, thereby providing valuable additional clinical information.15
Various RNA-based or miRNA-based tests have also shown promise as adjunctive studies for indeterminate or suspicious thyroid cytology. The commercial gene expression classifier Afirma, which compares mRNA from the FNA specimen to 167 gene expression fingerprints, has recently been validated for FLUS/AUS and SFN, with negative predictive value (NPV) of 95%, positive predictive value (PPV) of 38%, sensitivity of 90%, and specificity of 53%.16
Together, DNA-based and RNA-based molecular tests have improved classification of cytologically indeterminate thyroid nodules. Still, scant specimens are important factors in “indeterminate” cytology diagnosis. Thyroid aspiration may yield limited diagnostic material, insufficient for additional testing. Molecular changes in DNA and RNA might be reflected in their corresponding gene products, which may be detected by immunochemistry; therefore, immunochemistry may also be helpful in diagnosis. We reviewed the performance of 3 immunomarkers, Galectin-3, HBME1, and p27, in both cytology and histology specimens.
Galectin-3 is a β-galactoside-binding lectin. It crosslinks glycoproteins at the cell surface forming a lattice that inhibits endocytosis of EGFR, thereby enhancing its function. Cytoplasmic location of Galectin-3 is related to antiapoptotic function, induced by abnormal p53 expression. Therefore, the cytoplasm/membrane location in thyroid follicular, rather than nuclear presentation, of Galectin-3 is for malignancy.17 Galectin-3 is also expressed in the nuclei of macrophages, neutrophils, endothelial cells, and some stromal cells, providing an internal staining control.18 Galectin-3 is the most extensively investigated marker for classifying indeterminate thyroid nodules, with a reported accuracy of 82.96% in 27 studies using preoperative FNA material.19 A meta-analysis of 39 studies showed sensitivity of 82% and specificity of 81% for malignancy using Galectin-3 immunochemistry.20
HBME-1 is a monoclonal antibody targeting an unknown antigen of mesothelial microvilli. HBME-1 is abnormally expressed in thyroid cancer, showing cytoplasmic location with membrane accentuation, and is usually negative in normal follicular cells.21 It is the second most extensively investigated immunochemical marker in thyroid cancer. It has a sensitivity of 78.3% and specificity of 85.4% in 10 studies using preoperative FNA specimens.19 Similar sensitivity of 77% and specificity of 83% have been reported in meta-analysis of 21 immunohistochemical studies on tissue paraffin sections.20
p27 is a cyclin-dependent kinase inhibitor at the G0/G1 to G2 cell cycle checkpoint. It is expressed in normal thyroid cells, which have a long life span.22 p27 has been shown to be downregulated in malignantly transformed cells, but not in benign adenoma cells.22,23 RET/PTC fusion protein and BRAF mutation contributes to this downregulation.24,25 Together with Ki-67, p27 can help subcategorize thyroid cancer into prognostically relevant groups with low p27KIP1 expression (P=0.03) and high proliferative rate (P=0.02) associated with poor survival.26 Loss of p27 protein expression coupled with BRAF mutation is associated with lymph node metastasis in subcentimeter thyroid carcinomas.27 We speculate that loss of p27 is specific for thyroid malignancy.
Some studies support the combined use of Galectin-3 and HBME-1 to increase sensitivity in indeterminate thyroid nodules to 90.9% to 97.3%.28–30 However, the specificity of this combination is variable, ranging from 75.8% to 91.2%.28–30 The relationship of p27 to malignancy and prognosis suggests that detection for loss of p27 expression would add specificity to Galectin-3 or HBME-1 positivity for thyroid malignant diagnosis.
Another confounding factor in ancillary studies using cytology materials is that the lesions of indeterminate thyroid nodules could be patchy in distribution and may not be present on every pass or every cytology slide. To overcome these limitations, we developed a triple immunostaining technique, which is applicable to both cytology slides and paraffin sections, using a panel of markers consisting of p27, Galectin-3, and HBME-1.
MATERIALS AND METHODS
Cytologic and Histologic Specimens
From January 2007 to August 2012, 1240 thyroid FNA specimens (including outside consultations) were followed by surgery in University of Chicago Medical Center. Following The Bethesda System for Reporting Thyroid Cytopathology, the diagnoses of FLUS/AUS and SFN constituted 15%/5.4% and 3%, respectively, of all those cases. For histology, PTC was diagnosed based on the presence of papillary architecture and classic nuclear features of PTC. The follicular variant of papillary thyroid carcinoma (FVPTC) grows in a predominantly infiltrative follicular pattern compared with PTC. FC was distinguished from follicular adenoma (FA) based on the presence or absence of transcapsular and/or vascular invasion. Fifty-one consecutive cases were retrieved meeting the following criteria: at least 100 lesional follicular cells on 1 single slide (smear-based or liquid-based preparation); surgical follow-up and histology correlation available; and cytomorphology of lesional cells in the cytology specimen compatible with that in histology specimen. Original cytologic and histologic diagnoses were reassessed and concurred by 2 senior pathologists (R.M.D. and T.K.) and 1 junior pathologist (L.Z.) using the Bethesda and histology criterion aforementioned. Histology diagnoses are used as gold standard for this study. Consensus reassessment led to change of 2 histology diagnosis: (1) FA to FVPTC in case #30 due to the presence of nuclear pseudoinclusion, nuclear groove, and nuclear overlapping; and (2) FVPTC to FA in case #38 due to insufficient nuclear features and absence of capsule invasion. The cytologic diagnoses for the selected cases were: FLUS (n=40), AUS (n=6), and SFN (n=5). The histologic diagnoses were: benign, non-neoplastic (n=16, including 13 cases of colloid nodule, 2 cases of chronic lymphocytic thyroiditis, and 1 case of Graves disease), FA (n=13), FC [n=4, minimal invasive (transcapsular) invasion 3, vascular invasion 1], PTC (n=12), and FVPTC (n=6). The study was approved by the institutional review board of University of Chicago Medical Center.
Triple Immunostaining
Body fluids with benign mesothelial cells and macrophages were used as cytologic controls (mesothelial cells are positive for HBME-1 and p27, negative for Galectin-3; macrophages are positive for Galectin-3, negative for HBME-1). Control slides were either air dried or prefixed in Cytolyte and stained by Diff Quik or Papanicolaou methods, respectively. These slides were then destained in basic alcohol solution. A case of PTC with tumor cells positive for HBME-1 and Galectin-3 and adjacent normal thyroid cells positive for p27 was used as the histologic control.
This triple staining uses red, blue, and brown chromogens. The color assignment and staining location are: p27, red/nuclear; Galectin3, brown/cytoplasmic and membrane; and HBME1, blue/cytoplasmic with membrane accentuation.
There were 51 cytology slides (liquid-based preparations=22, smears=29), either Papanicolaou (n=26) or Diff Quik (n=25) stained. The destained cytology slides and corresponding deparaffinized formalin-fixed paraffin-embedded histology slides were postfixed in picric acid formalin fixative (Picri, Newcomer supply Cat#13381) for 10 minutes, followed by antigen retrieval in steamer for 20 minutes, endogenous peroxidase inactivation for 5 minutes, and permeation for 20 minutes. The slides were incubated in Galectin-3 (NCL-Gal, 1:50; Novocastra) and p27 (04-240, 1:25; EMD Millipore) cocktail antibody solution for 1 hour at room temperature. Following TBS wash, slides were incubated with MACH2 double stain 2 (MRCT525H; Biocare Medical) for 30 minutes at room temperature. The antigen-antibody binding was detected by DAB (K3468; Dako) system for Galectin-3 and Wrap Red (WR806H; Biocare Medical) for p27, respectively. The slides were further denatured (DNS001H; Biocare) to ensure that the second staining protocol would not cross react with the first staining protocol. After that, slides were treated with anti-HBME-1 antibody (M3505, 1:15; Dako) for 1 hour except 1 control slide, and antigen-antibody reaction was visualized by Bond Polymer Refine red detection (DS9390; Leica) and Ferangi blue (FB813; Biocare). The HBME-1 antibody skipped control slide should be negative of blue stain following Bond polymer and Ferangi blue visualization. After air drying, slides were coverslipped using xylene substitute mounting medium.
Digital Image Analysis
The original archival cytology slides, triple-stained cytology slides, triple-stained histology slides, and corresponding consecutive hematoxylin and eosin (HE) slides were scanned into digital files using the ImageScope (Aperio). One annotation layer was created in the triple-stained histology image and 1 synchronized layer in its corresponding HE image. The 2 layers have the same size (area) and same location. Thyroid colloid and vascular stroma were carefully excluded using the negative pen. Aperio colocalization algorithm was used in triple-stained images to generate parameters including area analyzed, total positive triple-stained area, intensity, and percentage area positive for each color with differential of 2 or 3 colors locating at the same site. Nuclear algorithm was used in HE-stained mate image to detect area analyzed, nuclear size, and number of nuclei in the annotation layer. For each image captured, at least 3 different foci are analyzed for lesion and normal control (if available) respectively, with each focus containing >100 nuclei and measuring 0.1 to 1.0 mm2 in area.
Calculation and Statistics
Labeling index (percentage of positively stained cells) is calculated using 2 equations based on nuclear versus cytoplasmic/membrane location of signals. Equation A is used for p27 nuclear labeling: A=[p27 positive area (%) × total positive triple-stained area (mm2)]/[average nuclear size (mm2) × total number of nuclei on matched HE slide]. Equation B is used for HBME-1 and Galectin-3 labeling: B=[HBME-1 or Galectin-3 positive area (%) × total positive triple-stained area (mm2)]/[HE total analyzed area−(average nuclear size × nuclear number on matched HE slide)].
Receiver operating characteristic curve (ROC) was generated by placing sensitivity (true positive fraction or rate) on the y axis and 1−specificity (false positive rate or fraction) on the x axis, and drawing a curve along the dots using online software (http://www.rad.jhmi.edu/jeng/javarad/roc). Each point on the ROC plot represents a sensitivity/specificity pair corresponding to a particular cutoff. Quantitative measures of accuracy, for example, the area under the ROC curve (AUC) was also calculated by the software. The sensitivity, specificity, PPV, NPV, and corresponding 95% confidence interval were calculated using online software (http://vassarstats.net/index.htm). The Yates χ2 test and P-value were used to evaluate disparity of staining performance between cytology and histology groups.
RESULTS
Representative triple Galectin-3/HBME-1/p27 immunocytochemical stains and immunohistochemical stains are illustrated in Figure 1 (see online supplementary Fig. 1, Supplemental Digital Content 1, http://links.lww.com/AIMM/A59, for more pictures and quantification of staining). Positive HBME-1 and Galectin-3 stains are shown in case #16 (AUS in cytology and diagnosis of PTC in histology), and the cells that are simultaneously positive for both markers are better visualized in cytology staining. Case #4 (FLUS in cytology and colloid nodule in histology) shows positive HBME-1 and p27 staining in the same cells, again better demonstrated in cytology preparation. Case #9 (SFN in cytology and FA in histology) is positive for p27 only. HBME-1 and Galectin-3 positive individually or in combination (case #16) indicates malignancy. p27 positive only (case #9) supports diagnosis of benign lesion. An algorithm is required for Galectin-3/HBME-1 and p27 dual-positive or triple-positive cases.
FIGURE 1.
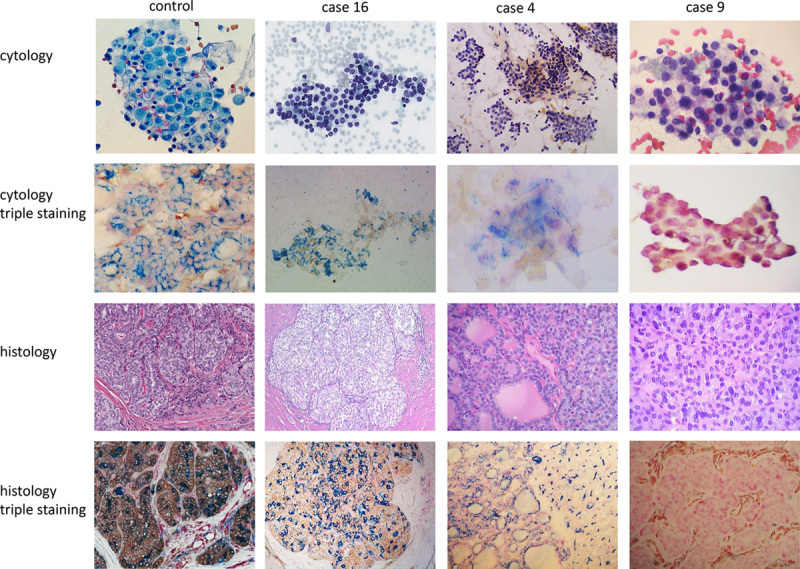
Illustration of triple-stained cases. Case #16 showed very subtle nuclear clearing and slightly overcrowded nuclei on cytology (Diff Quik). This patient has been aspirated 3 times with 2 AUS and 1 FLUS cytology diagnoses. Triple staining on cytology revealed coexpression of Galectin-3 and HBME-1 in the same cell with no p27 labeling. The histology diagnosis is PTC. Case #4 showed mixed macrofollicles and microfollicles with very scant colloid on cytology (Diff Quik). The cytology diagnosis is FLUS. Triple staining on cytology demonstrated colabeling of p27 and HBME-1 in the same cell. Histology diagnosis is multiple nodular goiter. Control staining from liquid-based preparation and 1 PTC formalin-fixed paraffin-embedded section is shown in the first column. Case #9 showed microfollicles with very rare trabecular architecture on cytology (Pap stain). The cytology diagnosis is FLUS and histology diagnosis is follicular adenoma. Triple staining highlights the nuclei of benign follicular cells in both cytology and histology. The vascular stromal cells are positive for Galectin-3 on histology. AUS indicates atypia of undetermined significance; FLUS, follicular lesion of undetermined significance; PTC, papillary thyroid carcinoma.
Because thyroid aspiration targets nodules and the normal thyroid cells are underrepresented in the cytologic specimen, we decided to use histologic sections, which have normal thyroid tissue adjacent to the thyroid lesions, as normal controls. We then quantitated a total of 158 histologic digital images from the 51 patients, including 79 HE stains and 79 paired triple stains for protein marker cutoff value determination. The results are summarized in Figures 2 and 3 and supplementary Figure 2 (Supplemental Digital Content 2, http://links.lww.com/AIMM/A60). The difference in average staining intensity of each marker among different histologic diagnosis groups is much smaller compared with difference of labeling index (supplementary Fig. 2, Supplemental Digital Content 2, http://links.lww.com/AIMM/A60). We therefore used labeling index in our next step data analysis, which can either be judged semiquantitatively under light microscopy or evaluated more quantitatively by digital imaging.
FIGURE 2.
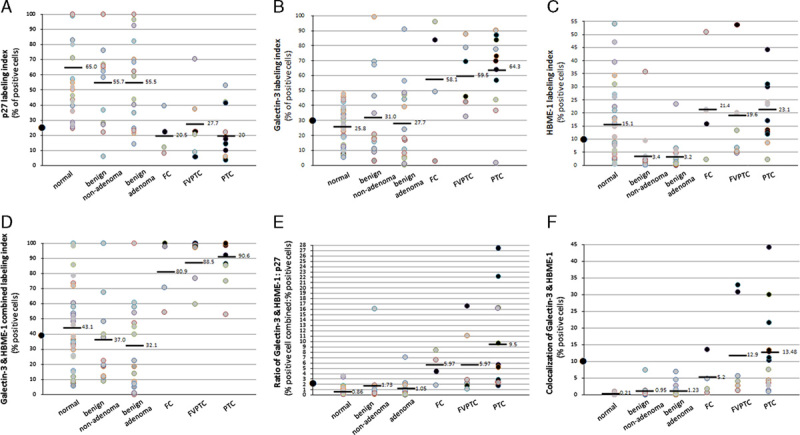
A–F, Quantitation of p27/Galectin-3/HBME-1 triple staining on histology sections, indication for combined use of markers and suggested cutoff value. The histologic distribution of quantitated cases: normal thyroid (normal tissue adjacent to benign thyroid lesions, n=28), benign nonadenoma (n=14, including nodular goiter, chronic lymphocytic thyroiditis, Graves disease), benign adenoma (n=15), follicular carcinoma (FC) (n=4), follicular variant papillary thyroid carcinoma (FVPTC) (n=6), and papillary thyroid carcinoma (PTC) (n=12). The cutoff value is 25% for p27, 30% for Galectin-3, 10% for HBME-1, 40% for Galectin-3 and HBME-1 combination, 10% for Galectin-3 and HBME-1 colocalization, and 2 for the ratio of Galectin-3/HBME-1:p27, as marked by solid dots on y-axis. The solid black dots in the scatter plots indicate the 10 cases that were positive for colocalization of Galectin-3 and HBME-1. The grey colored dots refers to the rest cases.
FIGURE 3.
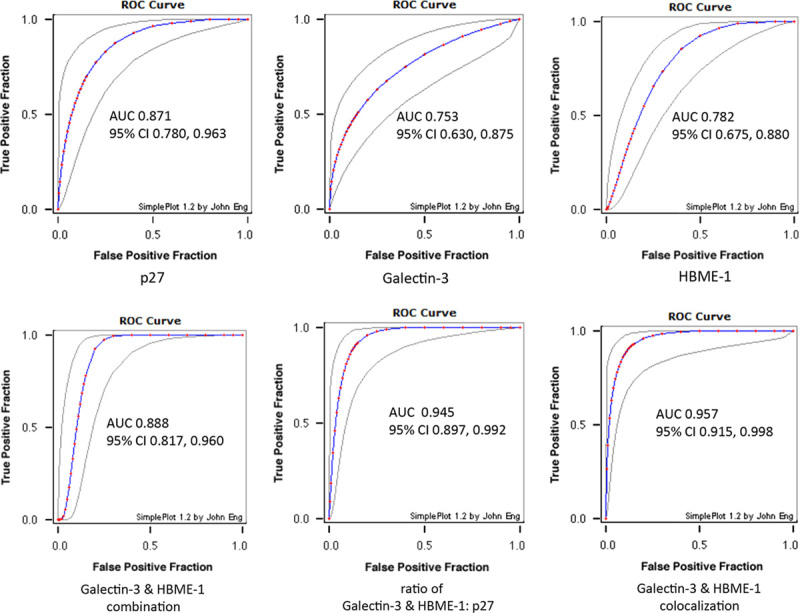
Receiver operating curve (ROC) for individual marker and markers combination. Estimated binormal ROC curves, with lower and upper bounds of the asymmetric 95% confidence interval for true-positive fraction at a variety of false-positive fraction are demonstrated. Combined use of immunomarkers (lower panel) show improved area under curve (AUC) compared with individual marker (upper panel).
The cutoff value for each marker was chosen based on histology sections. The average labeling index for normal/benign and malignant thyroid lesions (Fig. 2, upper panel) suggests a threshold. The cutoff labeling index was 25% for p27 (the average labeling index for malignancy), 30% for Galectin-3, and 10% for HBME-1 (the respective average labeling index for benign). When Galectin-3 and HBME-1 signals are added together, a new threshold of 40% is suggested, which is the average labeling index for normal and benign (Fig. 2D). The cutoff value for the ratio of HBME-1 and Galectin-3 combination (HG) to p27 (symbol as HG:p27) is 2, which is the average value for normal and benign thyroid lesions (Fig. 2E). Another parameter is the colocalization of Galectin-3 and HBME-1, defined by expression of the 2 proteins in the same cell (eg, case #16 in Fig. 1), and can be quantitated by Aperio software. The cutoff value for colocalization is 10%, which is the average value for normal and benign thyroid lesions (Fig. 2F).
AUC is used as a quantitative measure of accuracy of each marker. AUC<0.75 was not clinically useful and AUC=1 is perfect. Each individual marker p27, Galectin-3, and HBME-1 has AUC of 0.871, 0.753, and 0.782, respectively, in triple staining (Fig. 3, upper panel). When Galectin-3 and HBME-1 signals were added together, the AUC increased from 0.753 (for Galectin-3) and 0.782 (for HBME-1) to 0.888 (for combination) (Fig. 3, lower left). When the HG:p27 ratio was evaluated, the AUC increased to 0.945 (Fig. 3, lower mid). Another equally good parameter is the colocalization of Galectin-3 and HBME-1, defined by positive Galectin-3 and HBME-1 signals within the same cell. The AUC of Galectin-3/HBME-1 colocalization for malignancy prediction is 0.957 (Fig. 3, lower right).
The quantitative analysis on histology staining point to an algorithm: (1) p27 positive only=benign; (2) Galectin-3 positive only, or HBME-1 positive only, or Gal and HBME both positive, or colocalization=malignant; (3) p27 positive plus Galectin-3 or HBME-1 or Gal and HBME both positive: benign if HG:p27<2; malignant if HG:p27≥2.
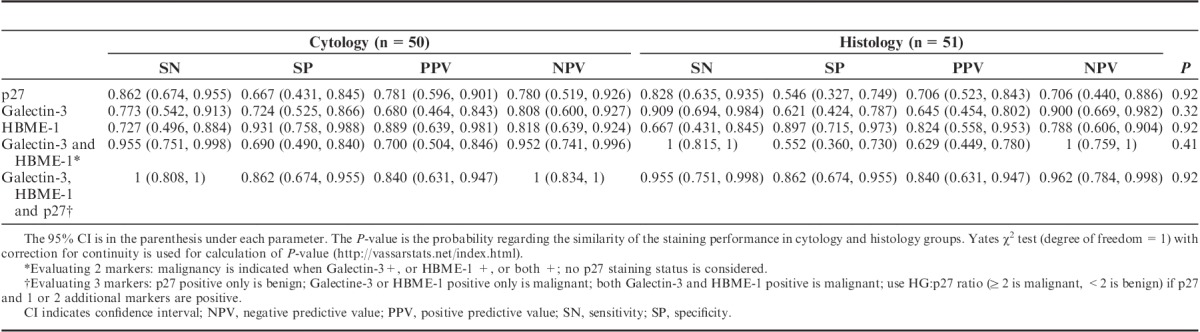
We then applied these cutoffs and a hierarchal analysis algorithm to cytology specimens. One cytology stain that failed internal control (no staining at all: no p27 staining in normal cells or no Galectin-3 staining in inflammatory cells) was considered invalidate and removed from the statistical analysis. A total of 50 cytology cases and 51 histology cases with validated staining were included for statistical analysis. Two cases had discrepancy in staining pattern between cytology and histology. These 3 cases are illustrated in Table 1. The staining performance for cytology and histology slides as shown by sensitivity, specificity, PPV, and NPV are demonstrated in Table 2. When 3 markers are evaluated (last row in Table 2), the triple staining performance in cytology and histology slides is very similar (P=0.92).
TABLE 1.
Discrepancy of Immunostains Between Cytology and Histology
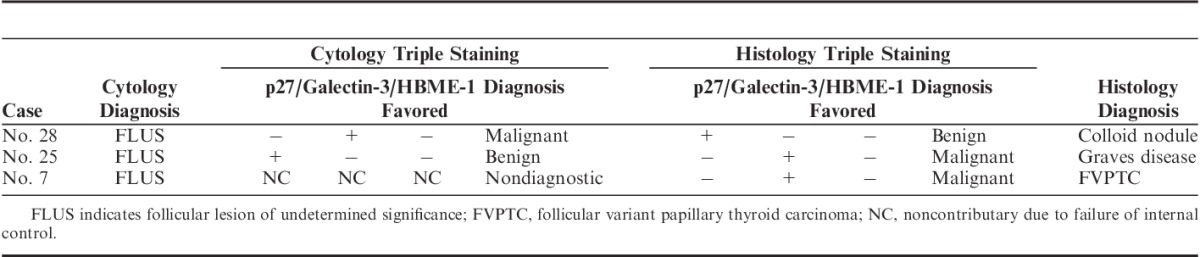
TABLE 2.
Performance Comparison of Hierarchical Algorithms for Triple Staining: SN, SP, PPV, and NPV with 95% CI
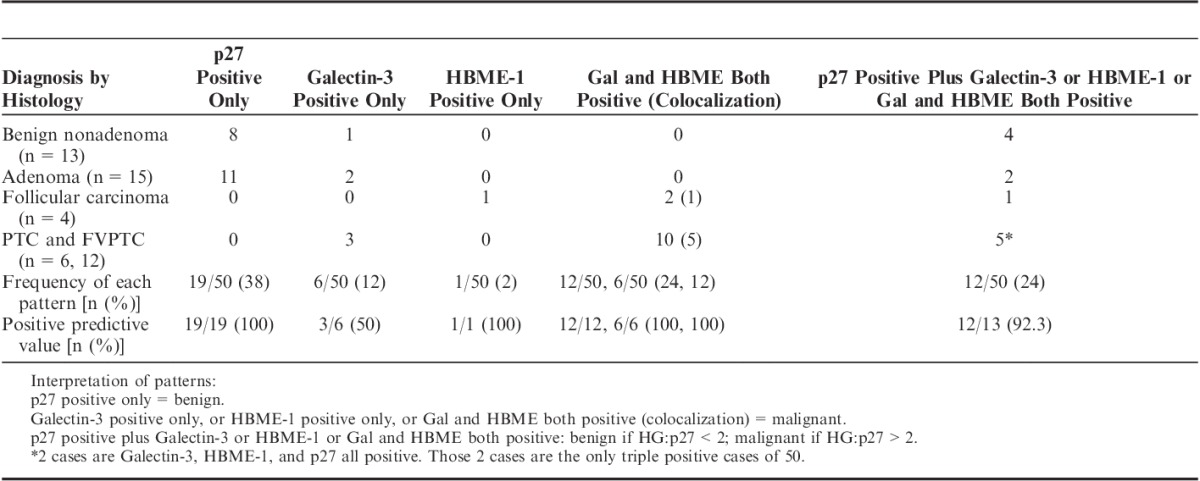
The staining patterns in cytology (Table 3) reveal that: (1) p27 is the most frequently positive single marker, consistent with benign nature of most indeterminate thyroid nodules. (2) Simultaneous positivity for benign (p27) and malignant (Galectin-3 and HBME-1) markers has occurred in 24% (12/50) of cases, and equally distributed between benign (n=6) and malignant (n=6) histologic diagnosis. Using HG:p27 cutoff value of 2 correctly subclassified 92.3% (12/13) of these gray zone cases. (3) Galectin-3 positive alone is less specific with PPV of 50% (3/6). (4) Combined Galectin-3 and HBME-1 positivity with p27 loss (n=12) or without p27 loss (n=2) had a PPV of 100% in our study, and 50% (6/12 and 1/2) of combined Galectin-3 and HBME-1 positivity has Galectin-3 and HBME-1 expressed in the same cell (colocalization) visible in triple staining in cytology preparation and quantitated by digital image in histology slides. The colocalization directly indicates that Galectin-3 and HBME-1 can be simultaneously activated in transformed follicular cells of thyroid.
TABLE 3.
Illustration of Staining Patterns of p27/Galectin-3/HBME-1 Triple Immunocytochemistry in 50 Indeterminate Thyroid Nodules Correlated to Histology Diagnosis: Frequency of Presentation and Positive Predictive Value
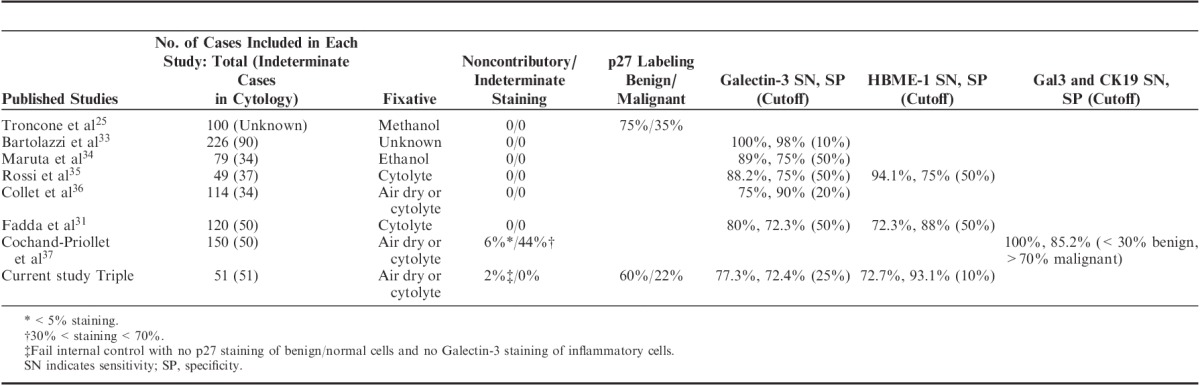
Lastly, we also compared the performance of p27, Galectin-3, and HBME-1 in triple staining and in reported single/sequential immunocytochemical staining (Table 4).
TABLE 4.
Comparison of the Performance of p27, Galectin-3, and HBME-1 in Triple Staining and Single/Sequential Immunocytochemical Staining
DISCUSSION
This is the first triple immunochemical staining reported using thyroid cytologic preparations and histology tissue. Cytology specimens fixed by air drying, methanol, ethanol, and cytolyte have all been used successfully in immunocytochemical assays.22,29,31–35 The sensitivity and specificity of Galectin-3 and HBME-1 in single assays varies from 72% to 95% to 100% (Table 4). Possible explanations for this wide variation include different percentages of cytologically indeterminate cases in various studies, different cutoff values, and different staining protocols. Nonetheless, the performance of Galectin-3 and HBME-1 in triple staining was within the range of single cytochemical staining (Table 4). Compared with single staining, the labeling index for p27 in our triple staining was 15% lower for both benign and malignant lesions22; however, the difference between benign and malignant labeling remains the same (40% for single staining, 38% for triple staining). This indicates a “systemic variation” between the 2 methods as a result of different protocol used. This difference can be compensated by using different cutoffs for p27, thus the performance of p27 in differentiating benign (nontumor and adenoma) from malignant in the 2 assays would be the same.
Of note, seperate staining of Galectin-3 and CK19 on 2 cytology slides has achieved a sensitivity of 100% and specificity of 85.2% at the cost of 44% indeterminate staining, if cutoffs of <30% combined staining favor benign and >70% combined staining favor malignant are used35 (Table 4). CK19 strongly and diffusely stains classic PTC, less so the FVPTC, and does not label FC.36 As for its specificity, it is usually negative in FA or hyperplastic nodule, but stains nontumorous thyroid parenchyma in FVPTC.36
On the basis of the immunostaining patterns in cytology, p27 positivity alone with no Galecin-3 and HBME-1 stain is (100%) diagnostic of benign lesions, and Galectin-3 and HBME-1 dual positivity (Galectin-3-positive cells >30%, and HBME-1-positive cells >10% in 1 specimen) is 100% specific for malignancy. In our cytology series, Galectin-3, HBME1 single or dual positivity accounts for 86% (18/21) of malignancies, in comparison with that of 88% and 86.5% in Galectin-3/HBME-1 sequential immunocytochemical staining29,33 (Table 3). Of those 18 cases, 2 cases in our series with histology diagnosis of PTC showed triple positivity for Galectin-3/HBME-1/p27. Those 2 cases both had Galectin-3/HBME-1:p27 ratio >2, immunophenotype favoring malignant. This further suggests that Galectin-3/HBME-1 dual positivity is specific for malignancy irrespective of p27 status.
Colocalization (≥2 protein markers positive in 1 cell) analysis on corresponding histology stains of these 2 triple-positive cases revealed that Galectin-3/HBME-1/p27 all 3 proteins were simultaneously present in 33% of cells in 1 case (supplementary Fig. 1, Supplemental Digital Content 1, http://links.lww.com/AIMM/A59, case #31), and 1.7% of cells in the other. This implies that follicular cells do not need to lose p27 expression in order to be transformed; Galectin-3 and HBME-1 coactivation might be able to overcome the negative regulation function of p27.
Galectin-3 and HBME-1 colocalization (expression in the same cell and present in >10% of cells) was demonstrated by triple staining in thyroid cancer in 28.6% (6/21) of cytology preparations (Table 3), and 45.5% (10/22) of histology sections, but in none of the 28 normal thyroid tissues and 29 benign thyroid lesions (Fig. 2F). This supports the notion that synchronous activation of Galectin-3 and HBME-1 occurs in thyroid malignancy.
Two cytohistologic discrepancy cases have been noticed (Table 1). The false-positive Galectin-3 staining in cytology preparation in case #28 might be due to macrophage staining. The false-positive Galectin-3 staining in histology section in case #25 is related to epithelioid granuloma staining in Graves disease (supplementary Fig. 2, Supplemental Digital Content 2, http://links.lww.com/AIMM/A60). Galectin-3, although sensitive, was least specific for thyroid malignancy in our series. Galectin-3 positivity has been observed in adenoma, colloid nodule (Table 3), as well as in neutrophils, macrophages, and vascular stroma in our study and other published series.17 We have also observed Galectin-3 nuclear and cytoplasmic positivity in ultimobranchial body and epithelioid granuloma (supplementary Fig. 3, Supplemental Digital Content 3, http://links.lww.com/AIMM/A61). Although nuclear stain is observed in benign cells and only cytoplasmic and membrane stain is considered malignant for Galectin-3, the subcellular compartment localization can be difficult to assess especially in cytology slides. Therefore, immunostaining result should not be interpreted in isolation (although this is a design of this study for objectivity). Careful correlation to morphology is the solution to prevent overinterpretation of those cells as positive staining and this should be added to the algorithm of immunostaining analysis. Marking the macrophages or scanning the cytology slides before immunostaining for later correlation is recommended.
Lastly, it would be a further save of staining time and cost if a same detection system were applied for HBME-1 and Galectin-3 antibody, for example, HBME-1 and Galectin-3 were assigned the same single color. This simplified protocol would be easily applied to automatic immunostainer and be a step forward toward practice use.
Also of note, the FLUS/AUS rate as well as malignancy rate presented here is at the high end of similar studies.4–11,37 This is because most cases with “indeterminate” cytology diagnosis were consult ones and patients were referred to the thyroid surgical center here for treatment. This selection bias can be controlled in a larger prospective study in different hospitals.
Also need to mention, this is a pilot study and the reference cutoff is generated from quantitation of histology specimens and applied to cytology preparations from the same patient population. This may lead to overestimation of the predictive abilities of the test. The performance of triple staining, although promising, needs to be validated in a different larger population.
Our study showed potential of triple immunochemical staining to be used as an ancillary test to clarify cytologic diagnoses of indeterminate thyroid nodules. We also demonstrated the diagnostic value of dual positive/colocalization of Galectin-3 and HBME-1 for thyroid malignancy. The prognostic and biological significance of synchronous activation of Galectin-3 and HBME-1 needs to be further studied.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Terri Shihong Li MD, Xing Wang for immunostaining technical support; Lei-Ann Arceneaux for digital imaging scanning; Drs Ward Reeves, Jeffrey Mueller, and Tatjana Antic for help in case collection; Drs Jack Yang, David N. Lewin, and Mary S. Richardson for advises during manuscript preparations. They are grateful to the anonymous reviewers and editors’ helpful suggestions.
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.appliedimmunohist.com.
The authors declare no conflict of interest.
REFERENCES
- 1.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 2.Ali SZ, Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology Definitions, Criteria and Explanatory Notes. 2010New York: Springer. [Google Scholar]
- 3.DeMay RM. The Art & Science of Cytopathology. 2012:2nd edChicago: ASCP Press. [Google Scholar]
- 4.Yang J, Schnadig V, Logrono R, et al. Fine needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer Cytopathol. 2007;111:306–315. [DOI] [PubMed] [Google Scholar]
- 5.Wu HH, Rose C, Elsheikh TM. The Bethesda system for reporting thyroid cytopathology: an experience of 1382 cases in a community practice setting with the implication for risk of neoplasm and risk of malignancy. Diagn Cytopathol. 2012;40:399–403. [DOI] [PubMed] [Google Scholar]
- 6.Jo VY, Stelow EB, Dustin SM, et al. Malignancy risk for fine-needle aspiration of thyroid lesions according to the Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2010;134:450–456. [DOI] [PubMed] [Google Scholar]
- 7.Theoharis CG, Schofield KM, Hammers L, et al. The Bethesda thyroid fine-needle aspiration classification system: year 1 at an academic institution. Thyroid. 2009;19:1215–1223. [DOI] [PubMed] [Google Scholar]
- 8.Yassa L, Cibas ES, Benson CB, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer Cytopathol. 2007;111:508–516. [DOI] [PubMed] [Google Scholar]
- 9.Nayar R, Ivanovic M. The indeterminate thyroid fine needle aspiration: experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer Cytopathol. 2009;3:195–202. [DOI] [PubMed] [Google Scholar]
- 10.Ravetto C, Colombo L, Dottorini ME. Usefulness of fine-needle aspiration in the diagnosis of thyroid carcinoma: a retrospective study in 37,895 patients. Cancer Cytopathol. 2000;90:357–363. [PubMed] [Google Scholar]
- 11.Baloch ZW, Fleisher S, LiVolsi VA, et al. Diagnosis of “follicular neoplasm” a gray zone in thyroid fine-needle aspiration cytology. Diagn Cytopathol. 2002;26:41–44. [DOI] [PubMed] [Google Scholar]
- 12.Jing X, Knoepp SM, Roh MH, et al. Group consensus review minimizes the diagnosis of “follicular lesions of undetermined significance” and improves cytohistologic concordance. Diagn Cytopathol. 2012;40:1037–1042. [DOI] [PubMed] [Google Scholar]
- 13.Chen JC, Pace C, Chen BA, et al. Yield of repeat fine-needle aspiration biopsy and rate of malignancy in patients with atypia or follicular lesion of undetermined significance: the impact of the Bethesda system for reporting thyroid cytopathology. Surgery. 2012;152:1037–1044. [DOI] [PubMed] [Google Scholar]
- 14.Ratour J, Polivka M, Dahan H, et al. Diagnosis of follicular lesions of undetermined significance in fine-needle aspirations of thyroid nodules. J Thyroid Res. 2013;2013:250347.6 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witt RL, Ferris RL, Pribitkin EA, et al. Diagnosis and management of differentiated thyroid cancer using molecular biology. Laryngoscope. 2013;123:1059–1064. [DOI] [PubMed] [Google Scholar]
- 16.Alexander EK, Kennedy GC, Baloch ZW, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367:705–715. [DOI] [PubMed] [Google Scholar]
- 17.Chiu CG, Strugnell SS, Griffith OL, et al. Diagnostic utility of Galectin-3 in thyroid cancer. Am J Pathol. 2010;176:2067–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartolazzi A, Bellotti C, Sciacchitano S. Methodology and technical requirements of the Galectin-3 test for the preoperative characterization of thyroid nodules. Appl Immunohistochem Mol Morphol. 2012;20:2–7. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues HGC, Nogueira de Pontes AA, LFF Adan. Use of molecular markers in samples obtained from preoperative aspiration of thyroid. Endocr J. 2012;59:417–424. [DOI] [PubMed] [Google Scholar]
- 20.Matos LL, Giglio ABD, Matsubayashi CO, et al. Expression of ck-19, galectin-3 and hbme-1 in the differentiation of thyroid lesions: systematic review and diagnostic meta-analysis. Diagn Pathol. 2012;7:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sack MJ, Astengo-Osuna C, Lin BT, et al. HBME-1 immunostaining in thyroid fine-needle aspirations: a useful marker in the diagnosis of carcinoma. Mod Pathol. 1997;10:668–674. [PubMed] [Google Scholar]
- 22.Troncone G, Fulciniti F, Zeppa P, et al. Cyclin-dependent kinase inhibitor p27(Kip1) expression in thyroid cells obtained by fine-needle aspiration biopsy: a preliminary report. Diagn Cytopathol. 2000;23:77–81. [DOI] [PubMed] [Google Scholar]
- 23.Abulkheir IL, Mohammad DB. Value of immunohistochemical expression of p27 and galectin-3 in differentiation between follicular adenoma and follicular carcinoma. Appl Immunohistochem Mol Morphol. 2012;20:131–140. [DOI] [PubMed] [Google Scholar]
- 24.Vitagliano D, Carlomagno F, Motti ML, et al. Regulation of p27Kip1 protein levels contributes to mitogenic effects of the RET/PTC kinase in thyroid carcinoma cells. Cancer Res. 2004;64:3823–3829. [DOI] [PubMed] [Google Scholar]
- 25.Bhatt KV, Spofford LS, Aram G, et al. Adhesion control of cyclin D1 and p27Kip1 levels is deregulated in melanoma cells through BRAF-MEK-ERK signaling. Oncogene. 2005;24:3459–3471. [DOI] [PubMed] [Google Scholar]
- 26.Tallini G, Garcia-Rostan G, Herrero A, et al. Downregulation of p27KIP1 and Ki67/Mib1 labeling index support the classification of thyroid carcinoma into prognostically relevant categories. Am J Surg Pathol. 1999;23:678–685. [DOI] [PubMed] [Google Scholar]
- 27.Rodolico V, Cabibi D, Pizzolanti G, et al. BRAF V600E mutation and p27 kip1 expression in papillary carcinomas of the thyroid < or =1 cm and their paired lymph node metastases. Cancer. 2007;110:1218–1226. [DOI] [PubMed] [Google Scholar]
- 28.Saggiorato E, De Pompa R, Volante M, et al. Characterization of thyroid “follicular neoplasms” in fine-needle aspiration cytological specimens using a panel of immunohistochemical markers: a proposal for clinical application. Endocr Relat Cancer. 2005;12:305–317. [DOI] [PubMed] [Google Scholar]
- 29.Fadda G, Rossi ED, Raffaelli M, et al. Follicular thyroid neoplasms can be classified as low- and high-risk according to HBME-1 and Galectin-3 expression on liquid-based fine-needle cytology. Eur J Endocrinol. 2011;165:447–453. [DOI] [PubMed] [Google Scholar]
- 30.Franco C, Matinez V, Allamand JP, et al. Molecular markers in thyroid fine-needle aspiration biopsy, a prospective study. Appl Immunohistochem Mol Morphol. 2009;17:211–215. [DOI] [PubMed] [Google Scholar]
- 31.Bartolazzi A, Gasbarri A, Papotti M, et al. Application of an immunodiagnostic method for improving preoperative diagnosis of nodular thyroid lesions. Lancet. 2001;357:1644–1650. [DOI] [PubMed] [Google Scholar]
- 32.Maruta J, Hashimoto H, Yamashita H, et al. Immunostaining of Galectin-3 and CD44v6 using fine-needle aspiration for distinguishing follicular carcinoma from adenoma. Diagn Cytopathol. 2004;31:392–396. [DOI] [PubMed] [Google Scholar]
- 33.Rossi ED, Raffaelli M, Minimo C, et al. Immunocytochemical evaluation of thyroid neoplasms on thin-layer smears from fine- needle aspiration biopsies. Cancer Cytopathol. 2005;105:87–95. [DOI] [PubMed] [Google Scholar]
- 34.Collet JF, Hurbain I, Prengel C, et al. Galectin-3 immunodetection in follicular thyroid neoplasms: a prospective study on fine-needle aspiration samples. Br J Cancer. 2005;93:1175–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cochand-Priollet B, Dahan H, Laloi-Michelin M, et al. Immunocytochemistry with cytokeratin 19 and anti-human mesothelial cell antibody (HBME1) increases the diagnostic accuracy of thyroid fine needle aspirations: preliminary report of 150 liquid-based fine-needle aspirations with histological control. Thyroid. 2011;21:1067–1073. [DOI] [PubMed] [Google Scholar]
- 36.Baloch ZW, Abraham S, Roberts S, et al. Differential expression of cytokeratins in follicular variant of papillary carcinoma: an immunohistochemical study and its diagnostic utility. Hum Pathol. 1999;30:1166–1171. [DOI] [PubMed] [Google Scholar]
- 37.Tepeoglu M, Bilezikci B, Bayraktar SG. A histological assessment of the Bethesda system for reporting thyroid cytopathology (2010) abnormal categories: a series of 219 consecutive cases. Cytopathology. 2014;25:39–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


