Abstract
To estimate the incidence (any mother to child) and rate (from seropositive mother to child) of mother-to-child transmission of Trypanosoma cruzi, a serological census was conducted, targeting pregnant women and infants born to seropositive mothers, in four municipalities of El Salvador. Of 943 pregnant women, 36 (3.8%) were seropositive for T. cruzi. Of 36, 32 proceeded to serological tests of their infants when they became 6–8 months of age. Six infants seropositive at the age of 6–8 months further proceeded to second-stage serological test at the age of 9–16 months. As the result, one infant was congenitally infected. Thus, serological tests at the age of 6–8 months produced five false positives. To ensure earlier effective medication only for true positives, identification of seropositive infants at the age of 9–16 months is crucial. Incidence and rate of mother-to-child transmission were 0.14 (per 100 person-years) and 4.0%, respectively. Estimated number of children infected through mother-to-child transmission in El Salvador (170 per year) was much higher than that of human immunodeficiency virus (HIV; seven per year). It is recommended that serological testing for T. cruzi be integrated into those for HIV and syphilis as part of antenatal care package.
Introduction
Chagas disease remains one of the greatest public health challenges in Latin America. Because of difficulties in identifying cases and treating the disease particularly of adult patients, its prevention and control serve as the key strategies for addressing the Chagas disease.1,2 There are three major routes of transmissions of Trypanosoma cruzi (i.e., vectoral transmission through bug bites, blood-transfusion-based transmission, and mother-to-child transmission). A series of vector control interventions through insecticide spraying and bug-infestation surveillance have been contributing to successful reduction of risks of being exposed to T. cruzi among Latin American populations.3 Systematic blood screening being implemented at local blood banks has been contributing to ensuring safer blood supply, by excluding bloods contaminated with T. cruzi, in Latin America.4,5 The World Health Organization (WHO) encourages Latin American nations to further strengthen and accelerate these interventions in both vector control and blood screening to achieve the interruption of T. cruzi transmissions by 2020.6 However, while recognizing the importance of vector control and blood screening, we must warn ourselves that addressing mother-to-child transmissions has not been adequately committed to but rather left behind, despite an increasing need for early detection of mother-to-child transmissions.7 For instance, in central America, even epidemiologic reality of mother-to-child transmission remains unclear and unknown. Mother-to-child transmission of Chagas disease needs to be equally addressed, to minimize the secondary transmissions of T. cruzi to the next generation, particularly in non-endemic countries where vectoral transmission is nonexistent, in view of globalization of the disease.8–10
Seroprevalence of T. cruzi among pregnant women varies between countries, for example, 0.6% in the United States,11 4.1% in Mexico,12 4.4% in Honduras,13 9.1% in Argentina,14 11.4% in Spain,15 and 42.2% in Bolivia.16 Note that these figures are not necessarily nationally representative seroprevalence among pregnant women, but subnational fragmentary ones measured in the selected communities or provinces of each country. The Pan American Health Organization (PAHO) estimated 200,000 reproductive-aged women are seropositive for T. cruzi and 13,000 children are annually born as the congenitally T. cruzi-positive in Latin America.17 Yet, this statistics does not help estimate the incidence of mother-to-child transmission of T. cruzi (the number of annual cases of seropositive infants per 100 person-years) and mother-to-child transmission rate of T. cruzi (proportion of seropositive infants born to seropositive mothers to total number of infants born to seropositive mothers).
In El Salvador, the number of people infected with T. cruzi is estimated at 232,000, equivalent to 4.0% of its total population. Additional 2.7 million people in the country are at risk of being infected with T. cruzi primarily through vectoral transmission. Of the newborns, 230 were estimated to be congenitally infected with T. cruzi annually through mother-to-child transmission in the country.17 Yet, this estimation is not based on the country-specific incidence and rate of mother-to-child transmission. El Salvador achieved a full coverage of blood screening on T. cruzi at local blood banks by 1996.18 A series of interventions against vectoral transmission (e.g., insecticide spraying, bug-infestation surveillance, housing materials improvement, and health promotion) have been implemented to reduce domestic infestation rate of vector bugs since 2003. As the result, national average domestic infestation rate of vector bugs remarkably reduced from 21.0% at pre-spraying baseline stage to 8.8% at post-spraying surveillance stage during the period from 2003 to 2010.19 Nevertheless, very few have been done to address mother-to-child transmission of T. cruzi in El Salvador.
This study was conducted as a part of the study project that aimed at identifying risk factors for Chagas disease among Salvadorian pregnant women.20 The objectives of this study was to estimate the incidence and rate of mother-to-child transmission of T. cruzi in four municipalities of El Salvador.
Materials and Methods
To estimate the incidence and rate of mother-to-child transmission of T. cruzi in El Salvador, seropositivity tests were conducted, by targeting all the pregnant women and infants born to seropositive pregnant women (i.e., census) in four municipalities of two Chagas disease–endemic provinces.
Study area.
To enable the study to detect infants congenitally infected with T. cruzi, two provinces (Sonsonate and Ahuachapán) in the western region of El Salvador were selected as the study area (Figure 1 ). These two provinces have similar geographic and vegetational characteristics, by mutually bordering in a relatively small territory (20,742 km2) of the country. Both provinces are categorized into Chagas-endemic provinces with high infestation rates of Triatoma dimidiata (the only vector bug species in El Salvador) and high seroprevalence among children under 15 years of age.21,22 Of 16 municipalities in Sonsonate province, three municipalities (Santa Isabel Ishuatán, Armenia, and San Antonio del Monte) were further selected. Of 11 municipalities in Ahuachapán province, one municipality (Guaymango) was further selected. Table 1 shows the rationale for selecting these four municipalities as the study area, along with their geographic and epidemiologic profiles of Chagas disease.
Figure 1.
Four target municipalities in Sonsonate and Ahuachapán provinces, El Salvador.
Table 1.
Selection rationale and characteristics of four target municipalities
| Province/municipality | ||||
|---|---|---|---|---|
| Sonsonate | Ahuachapán | |||
| Santa Isabel Ishuatán | Armenia | San Antonio del Monte | Guaymango | |
| Reasoning for selection | Includes El Mango, a community with the highest seroprevalence of all 29 communities in seven MoH's priority endemic provinces21 | Includes El Provenir, a community with the second highest seroprevalence of all 29 communities in seven MoH's priority endemic provinces21 | The only municipality in Sonsonate province that was selected as a pilot area for the MoH's National Chagas Disease Control Program in 200319 | One maternal death of 17-year-old pregnant woman because of Chagas cardiomyopathy was reported in 2009* |
| Geographic characteristics23 | ||||
| Population | 10,241 | 34,912 | 26,902 | 19,037 |
| Land area (km2) | 95 | 65 | 25 | 60 |
| Altitude (m) | 395 | 588 | 212 | 337 |
| Urban population (%) | 7.9 | 68.7 | 74.3 | 6.0 |
| Recent serological survey among children | ||||
| Number of Trypanosoma cruzi positives | 4 | 3 | 3 | 17 |
| Total number of examinees | 216 | 102 | 399 | 730 |
| Prevalence (%) | 1.9 | 2.9 | 0.8 | 2.3 |
| Target age group (years) | 0–15 | 0–15 | 5–15 | 0–15 |
| Year | 2008 | 2008 | 2009 | 2007 |
MoH = Ministry of Health.
Source: Unidad de Vigilancia de Enfermedades Vectorizadas, Ministerio de Salud de El Salvador (MINSAL).
Study group.
This study targeted all the pregnant women (i.e., census) whose expected dates of delivery were between March 1, 2009 and February 28, 2010 (12 months) in three municipalities of Sonsonate province and between September 1, 2009 and May 31, 2010 (9 months) in one municipality of Ahuachapán. The pregnancy registries at four municipality health centers were referred to and reviewed in order to identify individuals eligible for the study group. The detailed procedure of recruiting the individuals as the study group was adjusted according to locally available resources and possible arrangements in each province as follows.
In three municipalities of Sonsonate province (Santa Isabel Ishuatán, Armenia, and San Antonio del Monte), 1,056 eligible pregnant women were identified in the pregnancy registries. Target women were invited and encouraged to participate in the upcoming blood screening campaign, during health promoters' advanced home visits. The study team made home revisits to those who did not participate in the campaign despite the invitation, to re-encourage them to participate in blood screening during post-campaign antenatal checkup visits. In Guaymango, Ahuachapán province, 146 eligible pregnant women were identified in the pregnancy registry available at Guaymango health center. All the 146 pregnant women were requested to participate in blood screening during their regular antenatal checkup visits to Guaymango health center.
Serological tests in pregnant women.
The methods of serological tests of pregnant women were adjusted between two provinces, according to locally available materials and supplies and possible arrangements. In three municipalities of Sonsonate province, blood samples were collected from target pregnant women on-site at blood screening campaigns during the period from September to November 2009. A few drops of blood were sampled through finger prick and immediately absorbed onto a piece of filter paper. The filter paper pieces were sent to Chagas and Leishmania Unit, the National Laboratory of Doctor Max Bloch in San Salvador, to perform enzyme-linked immunosorbent assay immunoglobulin G (ELISA IgG) by using Chagatest ELISA recombinant v.3.0 (Wiener Lab., Rosario, Argentina). Note that the National Laboratory of Doctor Max Bloch is the top referral laboratory responsible for final confirmation of Chagas disease among likely seropositive blood donors nationwide. Technical quality of works of the laboratory is regularly monitored both internally by conducting a routine self-assessment and externally by requesting a Brazilian institute (Fundação Pró-Sangue, Hemocentro de São Paulo) to annually double-check the results of serological tests.24 For those whose ELISA IgG test results were positive, an additional ELISA IgG test was undertaken by applying Chagatest IHA (Wiener Lab.) to venous blood samples collected during follow-up home visits. We determined that those who showed positive results in both filter paper and venous blood samples were seropositive for T. cruzi, following the National Norm of Prevention and Control of Chagas Disease.25 In Ahuachapán province, blood samples were collected from target pregnant women at Guaymango health center as part of regular antenatal checkup services, during the period from September to November 2009. The rapid test kit Chagas Stat-Pak (Chembio Diagnostic System, Medford, NY) was used for identifying T. cruzi infection among pregnant women by ensuring 15-minute reaction time. When the rapid test result was positive, a venous blood sample was collected by a physician at the health center and sent to the National Laboratory of Doctor Max Bloch for ELISA IgG tests using Chagatest ELISA recombinant v.3.0. Thus, the same antigen was commonly used for ELISA IgG test of blood samples from Sonsonate and Ahuachapán provinces. We determined that those who were positive in both rapid test and ELISA IgG test were seropositive for T. cruzi.
Serological tests in infants.
To accurately identify and determine mother-to-child transmission cases, venous blood samples of infants born to seropositive mothers were collected and tested for T. cruzi infection by applying ELISA IgG tests (Chagatest ELISA recombinant v.3.0), at two different ages. First, when infants born to seropositive mothers became 6–8 months of age, their venous blood samples were examined for T. cruzi infection by ELISA IgG test. Second, when those who were positive in the first ELISA IgG test at 6–8 months of age became 9–16 months of age, follow-up ELISA IgG tests were conducted for their venous blood samples. At each stage of the two-stage serological testing, an additional confirmatory assay was conducted for venous blood sampled during follow-up home visits at the National Laboratory of Doctor Max Bloch. This two-stage serological testing protocol was used to assess the level of possible disappearance of infants' seropositivity between two ages because of loss of maternal antibodies.
Data analysis.
A unique blood sample number was assigned to each blood sample collected for serological tests. The results of serological tests were entered into a dataset and further analyzed, using SPSS for Windows version 22 (IBM/SPSS Inc., Chicago, IL).
Ethical consideration.
The study was designed in accordance with the ethical standards of the Salvadorian Ministry of Health (MoH), and authorized by the Research Committee of the Salvadorian MoH. Because of the sensitive nature of the study, all data were kept confidential. Before conducting blood sampling, the objectives of study were thoroughly explained to target groups using informative leaflet, and a written informed consent was obtained from the pregnant women. When conducting serological tests among infants born to seropositive mothers, an additional informed consent was verbally obtained from their mothers. The infants found infected with T. cruzi were provided with treatment using nifurtimox, in line with the National Norm of Prevention and Control of Chagas Disease.25 The mothers found infected with T. cruzi were referred to the cardiologist for follow-up. Furthermore, the health personnel of the vector control unit in Sonsonate and Ahuachapán provinces visited all houses in which seropositive pregnant women lived, to conduct entomological inspection for triatomine bugs and subsequent insecticide spraying when domestic infestation by Triatoma dimidiata was confirmed, as a part of follow-up.
Results
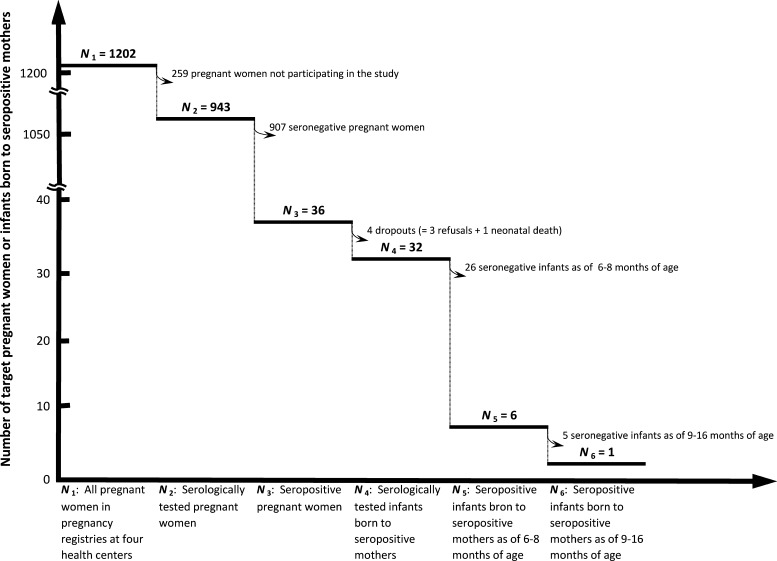
Of 1,202 pregnant women registered at the local health centers in four municipalities (Santa Isabel Ishuatán, Armenia, San Antonio del Monte, and Guymango), 943 (78.5%) participated in the study. Of the 943 participating pregnant women, 36 were identified to be seropositive for T. cruzi (Figure 2 ). Thus, overall seroprevalence of T. cruzi among pregnant women in the four municipalities was 3.8% (36/943). Municipality-specific seroprevalence ranges between 1.3% in Armenia as the lowest and 7.4% in San Antonio del Monte as the highest (Table 2). Of the 36 seropositive pregnant women, 32 proceeded to serological tests of their infants at the age of 6–8 months. Thus, there were four dropouts. One seropositive woman in Santa Isabel Ishuatán and two seropositive women in Guymango refused to proceed to their infants' blood tests for T. cruzi infection at the age of 6–8 months. One infant of a seropositive woman in Guymango could not proceed to her infant's blood test because of neonatal death. Since neither blood test nor autopsy was conducted for the neonate, whether the death was attributed or related to acute Chagas disease is not clear. Of the 32 infants, six infants were identified to be seropositive for T. cruzi at the age of 6–8 months. Then, all the six infants seropositive at 6–8 months of age underwent the second-stage serological test, when becoming 9–16 months of age. It was found that one of six infants from Guymango continued to be seropositive at the second-stage serological test. Therefore, this was the only mother-to-child transmission case identified in this study. This male infant born to a seropositive woman aged 43 years underwent first- and second-stage serological tests at 7 months of age and at 14 months of age, respectively. Though the infant had no clinical symptoms of Chagas disease, nifurtimox was administered to him for 60 days under a physician's supervision at the nearest health center, following the National Norm of Prevention and Control of Chagas Disease.25 As there was no readily available data of his post-medication serological tests at the health center, whether and when he became seronegative were not identified. A staff of vector control unit of Ahuachapán province confirmed that walls and floors of the infant's house were made of blocks and cement and identified no triatomine bug during the post-serological-test home visit. It was found that the house was treated by insecticide spraying after 2003. These serve as the evidence strong enough to determine that the infant had been infected with T. cruzi not through vectoral transmission but through mother-to-child transmission.
Figure 2.
Flow of serological tests among pregnant women and infants born to seropositive mothers.
Table 2.
Results of serological tests among pregnant women and infants born to seropositive mothers
| Province/municipality | Total | ||||
|---|---|---|---|---|---|
| Sonsonate | Ahuachapán | ||||
| Santa Isabel Ishuatán | Armenia | San Antonio del Monte | Guaymango | ||
| Period during which deliveries were expected (months) | 12 | 12 | 12 | 9 | – |
| Pregnant women (N = 943) | |||||
| (+) Trypanosoma cruzi positive | 3 | 5 | 21 | 7 | 36 |
| (−) T. cruzi negative | 132 | 372 | 264 | 139 | 907 |
| Total | 135 | 377 | 285 | 146 | 943 |
| Seroprevalence (%) | 2.2 | 1.3 | 7.4 | 4.8 | 3.8 |
| Infants born to seropositive mothers as of 6–8 months of age (N = 32) | |||||
| (+) T. cruzi positive | 0 | 1 | 4 | 1 | 6 |
| (−) T. cruzi negative | 2 | 4 | 17 | 3 | 26 |
| Total | 2 | 5 | 21 | 4 | 32 |
| Number of dropouts | 1* | 0 | 0 | 3† | 4 |
| Infants born to seropositive mothers as of 9–12 months of age (N = 6) | |||||
| (+) T. cruzi positive | 0 | 0 | 0 | 1 | 1 |
| (−) T. cruzi negative | 0 | 1 | 4 | 0 | 5 |
| Total | 0 | 1 | 4 | 1 | 6 |
| Mother-to-child transmission indicators | |||||
| Incidence of mother-to-child transmission (per 100 person-years)‡ | 0 | 0 | 0 | 0.7 | 0.14 |
| Mother-to-child transmission rate (%)‡ | 0 | 0 | 0 | 25.0 | 4.0 |
In Santa Isabel Ishuatán, one seropositive mother refused to have her infant tested.
In Guaymango, two seropositive mothers refused to have their infants tested and one infant born to a seropositive woman was dead at neonatal stage.
When calculating overall mother-to-child transmission incidence and rate in the four municipalities, the number of pregnant women serologically tested, seropositive pregnant women, and seropositive infants in Guymango were multiplied by 12/9, to standardize the period during which deliveries were expected between the municipalities.
To calculate the incidence and rate of mother-to-child transmission, the period during which pregnant women were expected to deliver must be standardized, since the periods differ between Guymango (9 months) and the other three municipalities (12 months). Thus, the numbers of pregnant women serologically tested, seropositive pregnant women and seropositive infants in Guymango were converted into 12-month basis by multiplying them by 12/9. As a result, overall incidence of mother-to-child transmission in the four municipalities was 0.14 (per 100 person-years). Municipality-specific incidence rate in Guymango, where the only mother-to-child transmission case was identified in this study, was 0.7. Mother-to-child transmission rate in the four municipalities was 4.0%.
Discussion
In this study, one male infant was identified to have been congenitally infected with T. cruzi, as the first mother-to-child transmission case in El Salvador and in central America. Overall seroprevalence among pregnant women in the four municipalities (3.8%) was at the same level as the neighboring countries, that is, 4.1% in Mexico12 and 4.4% in Honduras.13 The seroprevalence among pregnant women (3.8%) was twice as high as seroprevalence among children under 15 years of age (1.9%) in Santa Isabel Ishuatán in 2007–2009 (Table 1) and also national average seroprevalence among blood donors aged 18 years or older (1.7%) in El Salvador in 2011.24 This implies that pregnant women should be the higher priority subgroup in the National Chagas Disease Control Program than other age groups. Note that we cannot determine that pregnant women are exposed to higher risk of being infected with T. cruzi than other age groups, as this study has limitations in assessing whether they had been infected during their pregnancies. One may argue that calculation of overall seroprevalence among pregnant women by combining the results in all the four municipalities could be questionable because of the difference in the initial serological test methods between two provinces. However, ELISA IgG test for blood samples on filter papers (used in Sonsonate province) and Chagas Stat-Pak test (used in Ahuachapán province) are equally sensitive and reliable as the initial test for provisional seropositivity detection. A previous study detected 99.8% of rate of agreement in seropositivity among a total of 5,998 blood samples of Honduran, Salvadorian, and Nicaraguan individuals between Chagas Stat-Pak and Chagatest ELISA.26 Two studies reported that sensitivity and specificity of Chagas Stat-Pak were as high as those of Chagatest ELISA, when testing blood samples in four Latin American countries including Honduras.13,27 Another study in Mexico reported seroprevalence of a group of blood samples were at the same level between Chagas Stat-Pak and Chagatest ELISA,28,29 while low sensitivity of Chagas Stat-Pak was reported when applying to Peruvian populations.29 Therefore, we assumed that comparability of the results of serological tests in our study in El Salvador were ensured between Chagatest ELISA and Chagas Stat-Pak. Moreover, the National Laboratory of Doctor Max Bloch have been skilled in ensuring comparability and reproducibility of the results of serological tests by routinely undertaking ELISA IgG tests of venous blood samples from Sonsonate, Ahuachapán, and other provinces. Thus, the results of serological tests of all the four municipalities of two provinces are comparable enough to be combined into one dataset.
Both the incidence and rate of mother-to-child transmission might have been underestimated for the two reasons. First, of 36 infants born to seropositive mothers, four (11%) who did not proceed to two-stage serological tests could have been infected with T. cruzi. Those mothers are most likely to have decided not to proceed to their infants' blood tests because of psychological dilemma and stress on whether and how they could inform their infants and family members of the possible bad news of T. cruzi infection. Second, all the proportions calculated in this study have consistently no confidence intervals, as the study was designed not as a sampling survey but as a census. Thus, “true” overall incidence could be 0.14 (per 100 person-years) (Table 2) or greater because of potential seropositivity among those infants born to seropositive mothers who did not participate in serological tests. Mother-to-child transmission rate in the four municipalities was 4.0% (Table 2). This rate might have been underestimated similarly for the same reason.
Incidence of mother-to-child transmission in this study (0.14), the first reported incidence from central America, was at similar level to the estimated average incidence of central America (0.123).31 Though this study identified only one seropositive case among infants born to seropositive mothers, the incidence (0.14%) should be reliable as there is no confidence interval because of employment of a census as the survey design. Yet, possibilities of underestimation of the incidence because of four dropout cases of infants born to seropositive mothers (Figure 2) calls for a larger-scale survey to increase precision of the incidence in El Salvador.
Of six infants seropositive as of 6–8 months of age, five (83%) lost the antibodies and only one maintained them as of 9–16 months of age. This implies that seropositivity at the age of 6–8 months does not serve as an adequate scientific justification for determining infection with T. cruzi, but rather indicates maternal antibodies against T. cruzi are likely to disappear by 8 months of age. Our findings on the duration of maternal antibodies against T. cruzi support the previous studies that reported disappearance of maternal antibodies around 8 months of age.32,33 Thus, we propose seropositivity status of infants born to seropositive women should be systematically monitored up to 9–16 months of age to avoid unnecessary treatments of false positives, though earlier medication is generally recommended as treatment of Chagas disease patients.
Mother-to-child transmission rate of T. cruzi identified in this study (4.0%) is lower than Salvadorian national average of mother-to-child transmission rate of human immunodeficiency virus (HIV; 6.9%).34 On the other hand, T. cruzi seroprevalence among pregnant women in this study (3.8%) was 42 times as high as HIV prevalence among pregnant women (0.09%) in the country.33 By assuming our study results are applicable as national average and multiplying the number of pregnant women per year in El Salvador (112,073)35 by these proportions, the number of children infected through mother-to-child transmission per year was estimated and compared between T. cruzi and HIV (Table 3). The estimated total number of children infected with T. cruzi (170) through mother-to-child transmission in El Salvador is much lower than Sosa-Estani's rough estimation (260).13 This indicates the possible overestimation of the number of children infected with T. cruzi by Sosa-Estani and others. Our estimation (170) is 24 times as high as that of children infected with HIV (7) through mother-to-child transmission in the country. Thus, Salvadorian children are exposed to much higher risks of being congenitally infected with T. cruzi than with HIV. Prevention of mother-to-child transmission (PMTCT) of HIV has been globally implemented, as antiretroviral therapy for pregnant and breast-feeding women are effective and efficient. PMTCT of Chagas disease is not feasible, as benznidazole and nifurtimox, typical medications against Chagas disease, should not be applied to pregnant and breast-feeding women because of their severe side effects. However, benznidazole and nifurtimox are very effective in killing T. cruzi during infancy. Therefore, earlier identification of Chagas disease among children born to seropositive mothers serves as one of the key strategies for addressing mother-to-child transmission of T. cruzi.
Table 3.
Comparison of mother-to-child transmission between Trypanosoma cruzi and HIV in El Salvador
| T. cruzi (Chagas disease) | HIV33 | |
|---|---|---|
| Prevalence among pregnant women (%) | 3.8* | 0.09 |
| Mother-to-child transmission rate (%) | 4.0* | 6.9 |
| Estimated number of children infected through mother-to-child transmissions per year* | 170† | 7 |
HIV = human immunodeficiency virus.
The percentage was calculated by using the results of this study.
The number of children infected through mother-to-child transmission per year was estimated by applying the formula: (number of pregnant women per year in El Salvador) × (prevalence among pregnant women) × (mother-to-child transmission rate). As the number of pregnant women per year in El Salvador, 112,073, was used.34
This study has limitations in identifying the general profile or characteristics of those congenitally T. cruzi-seropositive infants, since only one mother-to-child transmission case was detected. There are limitations also in generalizability of the levels of incidence and rate of mother-to-child transmission, as these rates were estimated not by using nationally representative data but the data collected from high-endemic areas of Chagas disease. Although admitting a need for further studies to increase accuracy and precision of these figures, it is recommended the Salvadorian MoH consider taking the necessary steps to formally include systematic blood screening for T. cruzi among pregnant women. Given the fact that most pregnant women seek antenatal care services in El Salvador (e.g., 94% at least once and 78% at least four times),35 the introduction of blood screening for T. cruzi during antennal care visits is logistically efficient and medically effective, by combining blood tests for T. cruzi with those for HIV and syphilis that are already well operationalized. When introduction of systematic blood screening among all pregnant women is not financially feasible, it is recommended that serological tests be selectively conducted targeting Chagas disease high-risk pregnant women (≥ 35 years of age or with anemia) as a part of package of maternal and child health services.20
ACKNOWLEDGMENTS
We express our utmost appreciation to all mothers and children who participated in the study. We gratefully thank the Ministry of Health, El Salvador, particularly all health workers stationed at the health centers of San Isabel Ishuatán, Armenia, San Antonio del Monte, and Guaymango, SIBASI Sonsonate and Ahuachapán, and Western Regional Office of Ministry of Health. A special word of thanks is delivered to Japan International Cooperation Agency (JICA), Esther Elizabeth Hernández, Nahin Isacc Bentitez, Ana Cecilia Muñoz, Koji Oba, and Daniel Ken Inaoka for their tremendous support.
Footnotes
Financial support: This work was supported by the Ministry of Health, El Salvador, and Japan International Cooperation Agency (JICA).
Authors' addresses: Emi Sasagawa and Kiyoshi Kita, Department of Biomedical Chemistry, School of International Health, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan, E-mails: e-sasagawa@umin.ac.jp and kitak@m.u-tokyo.ac.jp. Hirotsugu Aiga, Human Development Department, Japan International Cooperation Agency (JICA), Tokyo, Japan, and Department of Global Health, Milken Institute School of Public Health, The George Washington University, Washington, DC, E-mail: aiga.hirotsugu@jica.go.jp. Edith Yanira Corado Soriano, Sección de Epidemiología, SIBASI Sonsonate, Ministerio de Salud de El Salvador (MINSAL), Sonsonate, El Salvador, E-mail: edith_corado@yahoo.es. Blanca Leticia Cuyuch Marroquín, Programa de Materno Infantil, Dirección Regional de Salud Zona Occidente, Ministerio de Salud de El Salvador (MINSAL), Santa Ana, El Salvador, E-mail: cuyuch@gmail.com. Marta Alicia Hernández Ramírez and Ana Vilma Guevara de Aguilar, Unidad de Vigilancia Laboratorial, Ministerio de Salud de El Salvador (MINSAL), San Salvador, El Salvador, E-mails: martah_ramirez@yahoo.es and anavilmadeaguilar@yahoo.es. José Eduardo Romero Chévez, Unidad de Vigilancia de Enfermedades Vectorizadas, Ministerio de Salud de El Salvador (MINSAL), San Salvador, El Salvador, E-mail: eromerochevez@yahoo.es. Hector Manuel Ramos Hernández, Dirección de Vigilancia Sanitaria, Ministerio de Salud de El Salvador (MINSAL), San Salvador, El Salvador, E-mail: el.cadejo@gmail.com. Rafael Antonio Cedillos, Consejo de Investigaciones Científicas (CIC-UES), Universidad de El Salvador, San Salvador, El Salvador, E-mail: rcedillos@navegante.com.sv. Chizuru Misago, Department of International and Cultural Studies, Tsuda College, Tokyo, Japan, E-mail: cmisago@tsuda.ac.jp.
References
- 1.Dias JCP, Silveira AC, Schofield CJ. The impact of Chagas disease control in Latin America—a review. Mem Inst Oswaldo Cruz. 2002;97:603–612. doi: 10.1590/s0074-02762002000500002. [DOI] [PubMed] [Google Scholar]
- 2.Apt W. Current and developing therapeutic agents in the treatment of Chagas disease. Drug Des Devel Ther. 2010;4:243–253. doi: 10.2147/dddt.s8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dias JCP, Schofield CJ. The evolution of Chagas disease (American trypanosomiasis) control after 90 years since Carlos Chagas' discovery. Mem Inst Oswaldo Cruz. 1999;94((Suppl 1)):103–121. doi: 10.1590/S0074-02761999000700011. [DOI] [PubMed] [Google Scholar]
- 4.Schmunis GA, Zicker F, Pinherio F, Branding-Bennett D. Risk for transfusion-transmitted infectious diseases in Central and South America. Emerg Infect Dis. 1998;4:5–11. doi: 10.3201/eid0401.980102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmunis GA, Rodriguez G, Coenen J, Bellorin EG, Gianella A. Prevention of blood-borne diseases in Bolivia, 1993–2002. Am J Trop Med Hyg. 2008;79:803–808. [PubMed] [Google Scholar]
- 6.WHO . Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases—A Roadmap for Implementation. Geneva, Switzerland: WHO; 2012. [Google Scholar]
- 7.WHO . Chagas Disease: Control and Elimination. Geneva, Switzerland: WHO; 2010. pp. 39–42.http://www.who.int/neglected_diseases/mediacentre/WHA_63.20_Eng.pdf Sixty-third World Health Assembly Resolutions, May 17–21, 2010. Resolution WHA 63.20. Available at. Accessed March 24, 2014. [Google Scholar]
- 8.Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010;115:14–21. doi: 10.1016/j.actatropica.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Yadon ZE, Schmunis GA. Congenital Chagas disease: estimating the potential risk in the United States. Am J Trop Med Hyg. 2009;81:927–933. doi: 10.4269/ajtmh.2009.09-0257. [DOI] [PubMed] [Google Scholar]
- 10.Merino FJ, Martínez-Ruiz R, Olabarrieta I, Merino P, García-Bujalance S, Gastañaga T, Flores-Chavez M, Grupo de Estudio de la Enfermedad de Chagas de la Comunidad de Madrid Control of Chagas disease in pregnant Latin-American women and her children. Rev Esp Quimioter. 2013;26:253–260. [PubMed] [Google Scholar]
- 11.Di Pentima MC, Hwang LY, Skeeter CM, Edwards MS. Prevalence of antibody to Trypanosoma cruzi in pregnant Hispanic women in Houston. Clin Infect Dis. 1999;28:1281–1285. doi: 10.1086/514790. [DOI] [PubMed] [Google Scholar]
- 12.Olivera MA, Guillén OF, Cruz VS, Hernández BN, Pérez GE, Córdova CG, Reyes PA, Monteón VM. Serological and parasitological screening of Trypanosoma cruzi infection in mothers and newborns living in two chagasic areas of Mexico. Arch Med Res. 2006;37:774–777. doi: 10.1016/j.arcmed.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Sosa-Estani S, Gamboa-León MR, Del Cid-Lemus J, Althabe F, Alger J, Almendares O, Cafferata ML, Chippaux JP, Dumonteil E, Gibbons L, Padilla-Raygoza N, Schneider D, Belizán JM, Buekens P, Working Group Use of a rapid test on umbilical cord blood to screen for Trypanosoma cruzi infection in pregnant women in Argentina, Bolivia, Honduras, and Mexico. Am J Trop Med Hyg. 2008;79:755–759. [PubMed] [Google Scholar]
- 14.Sánchez NO, Mora MC, Basombrío MA. High prevalence of congenital Trypanosoma cruzi infection and family clustering in Salta, Argentina. Pediatrics. 2005;115:e668–e672. doi: 10.1542/peds.2004-1732. [DOI] [PubMed] [Google Scholar]
- 15.Barona-Vilar C, Giménez-Martí MJ, Fraile T, González-Steinbauer C, Parada C, Gil-Brusola A, Bravo D, Gómez MD, Navarro D, Perez-Tamarit A, Fernandez-Silveira L, Fullana-Montoro A, Borrás R. Prevalence of Trypanosoma cruzi infection in pregnant Latin American women and congenital transmission rate in a non-endemic area: the experience of the Valencian Health Programme (Spain) Epidemiol Infect. 2012;140:1896–1903. doi: 10.1017/S0950268811002482. [DOI] [PubMed] [Google Scholar]
- 16.Salas NA, Cot M, Schneider D, Mendoza B, Santalla JA, Postigo J, Chippaux JP, Brutus L. Risk factors and consequence of congenital Chagas disease in Yacuiba, south Bolivia. Trop Med Int Health. 2007;12:1498–1505. doi: 10.1111/j.1365-3156.2007.01958.x. [DOI] [PubMed] [Google Scholar]
- 17.PAHO . Estimación cuantitativa de la enfermedad de Chagas en las américas. Montevideo, Uruguay: OPS; 2006. Documento OPS/HDM/CD/425-06. [Google Scholar]
- 18.Schmunis GA, Zicker F, Cruz J, Cuchi P. Safety of blood supply for infectious diseases in Latin American countries, 1994–1997. Am J Trop Med Hyg. 2001;65:924–930. doi: 10.4269/ajtmh.2001.65.924. [DOI] [PubMed] [Google Scholar]
- 19.Ministerio de Salud de El Salvador, Japan International Cooperation Agency . San Salvador, El Salvador: MINSAL/JICA; 2010. Informe de la evaluación final del projecto de control de la enfermedad de Chagas fase 2 en la República de El Salvador [in Spanish] [Google Scholar]
- 20.Sasagawa E, Aiga H, Corado EY, Cuyuch BL, Hernández MA, Guevara AV, Romero JE, Ramos HM, Cedillos RA, Misago C, Kita K. Risk factors for Chagas disease among pregnant women in El Salvador. Trop Med Int Health. 2015;20:268–276. doi: 10.1111/tmi.12440. [DOI] [PubMed] [Google Scholar]
- 21.Aiga H, Sasagawa E, Hashimoto K, Nakamura J, Zúniga C, Romero Chévez JE, Ramos Hernández HM, Nakagawa J, Tabaru Y. Chagas disease: assessing the existence of a threshold for bug infestation rate exist? Am J Trop Med Hyg. 2012;86:972–979. doi: 10.4269/ajtmh.2012.11-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cedillos RA, Francia H, Soundy CJ, Ascencio G, Valcarcel NM. Estudio epidemiológico de la infección por Trypanosoma cruzi en El Salvador, Centro América. Minerva Revista en Línea CIC-UES. 2011;2:35–46. http://www.minerva.cic.ues.edu.sv/makeupwebsitetemplate/articulos/Ciencias%20de%20la%20Salud/T.cruzi.pdf Available at. Accessed March 7, 2014. [Google Scholar]
- 23.Ministerio de Economía de El Salvador . San Salvador, El Salvador: Dirección General de Estadística y Censos, Ministerio de Economía; 2008. VI Censo de Población y V de Vivienda 2007. [Google Scholar]
- 24.Sasagawa E, Aguilar AV, Ramírez MA, Chévez JE, Nakagawa J, Cedillos RA, Misago C, Kita K. Prevalence of Trypanosoma cruzi infection in blood donors in El Salvador from 2001 to 2011. J Infect Dev Ctries. 2014;8:1029–1036. doi: 10.3855/jidc.4035. [DOI] [PubMed] [Google Scholar]
- 25.Ministerio de Salud de El Salvador . Norma Técnica para la Prevención y Control de la Enfermedad de Chagas. 2nd edición. San Salvador, El Salvador: Dirección de Regulación y Legislación en Salud, Unidad de Salud Ambiental, MINSAL; 2011. [Google Scholar]
- 26.Ponce C, Ponce E, Vinelli E, Montoya A, de Aguilar V, Gonzalez A, Zingales B, Rangel-Aldao R, Levin MJ, Esfandiari J, Umezawa ES, Luquetti AO, da Silveira JF. Validation of a rapid and reliable test for diagnosis of Chagas' disease by detection of Trypanosoma cruzi-specific antibodies in blood of donors and patients in Central America. J Clin Microbiol. 2005;43:5065–5068. doi: 10.1128/JCM.43.10.5065-5068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luquetti AO, Ponce C, Ponce E, Esfandiari J, Schijman A, Revollo S, Añez N, Zingales B, Ramgel-Aldao R, Gonzalez A, Levin MJ, Umezawa ES, Franco da Silveira J. Chagas' disease diagnosis: a multicentric evaluation of Chagas Stat-Pak, a rapid immunochromatographic assay with recombinant proteins of Trypanosoma cruzi. Diagn Microbiol Infect Dis. 2003;46:265–271. doi: 10.1016/s0732-8893(03)00051-8. [DOI] [PubMed] [Google Scholar]
- 28.Gamboa-León R, Ramirez-Gonzalez C, Pacheco-Tucuch FS, O'Shea M, Rosecrans K, Pippitt J, Dumonteil E, Buekens P. Seroprevalence of Trypanosoma cruzi among mothers and children in rural Mayan communities and associated reproductive outcomes. Am J Trop Med Hyg. 2014;91:348–353. doi: 10.4269/ajtmh.13-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gamboa-León R, Gonzalez-Ramirez C, Padilla-Raygoza N, Sosa-Estani S, Caamal-Kantun A, Buekens P, Dumonteil E. Do commercial serologic tests for Trypanosoma cruzi infection detect Mexican strains in women and newborns? J Parasitol. 2011;97:338–343. doi: 10.1645/GE-2545.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verani JR, Seitz A, Gilman RH, LaFuente C, Galdos-Cardenas G, Kawai V, de LaFuente E, Ferrufino L, Bowman NM, Pinedo-Cancino V, Levy MZ, Steurer F, Todd CW, Kirchhoff LV, Cabrera L, Verastegui M, Bern C. Geographic variation in the sensitivity of recombinant antigen-based rapid tests for chronic Trypanosoma cruzi infection. Am J Trop Med Hyg. 2009;80:410–415. [PubMed] [Google Scholar]
- 31.Carlier Y, Truyens C. Maternal-fetal transmission of Trypanosoma cruzi. In: Telleria J, Tibayrenc M, editors. American Trypanosomiasis: Chagas Disease, One Hundred Years of Research. Amsterdam, The Netherlands: Elsevier; 2010. pp. 539–581. [Google Scholar]
- 32.Chippaux JP, Clavijo AN, Santalla JA, Postigo JR, Schneider D, Brutus L. Antibody drop in newborns congenitally infected by Trypanosoma cruzi treated with benznidazole. Trop Med Int Health. 2009;15:87–93. doi: 10.1111/j.1365-3156.2009.02431.x. [DOI] [PubMed] [Google Scholar]
- 33.Carlier Y, Torrico F, Sosa-Estani S, Russomando G, Luquetti A, Freilij H, Albajar Vinas P. Congenital Chagas disease: recommendations for diagnosis, treatment and control of newborns, siblings and pregnant women. PLoS Negl Trop Dis. 2011;5:e1250. doi: 10.1371/journal.pntd.0001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.PAHO . Progress Report: Elimination of Mother-to-Child Transmission of HIV and Congenital Syphilis in the Americas, 2012. Washington, DC: PAHO; 2013. [Google Scholar]
- 35.Ministerio de Salud de El Salvador . Informe Nacional sobre el Estado de Situación del VIH en El Salvador en Cumplimiento del Plan Nacional de Nomitoreo y Evaluación Año 2010. San Salvador, El Salvador: Programa Nacional de ITS/VIH/Sida; 2011. [Google Scholar]
- 36.UNICEF . The State of the World's Children: Children with Disabilities. New York, NY: UNICEF; 2013. [Google Scholar]