Abstract
Cases of human visceral leishmaniasis (HVL) were not recorded until recently in the Chaco region of northwestern Argentina. Dogs were surveyed at the sites of infection of two HVL index cases in the Chaco region of Salta province. Canine cases (CanL) were diagnosed by two parasitological methods, two molecular methods targeting mini- and maxicircle DNA, and immunochromatographic dipstick. Among 77 dogs studied, 10 (13%) were found infected with Leishmania spp. In seven dogs and two humans, the infecting species was typed as Leishmania (Leishmania) infantum. The same genotype was detected in the human and two of the CanL. Although several diagnostic methods displayed weak or moderate agreement, the concordance values for serology versus maxicircle PCR were very good (Kappa index = 0.84). Sandflies captured in the area were identified as Lutzomyia migonei and Lu. cortelezzii/Lu. sallesi (cortelezzii complex). The focal appearance of leishmaniasis in dogs and humans in a sylvatic region and its relatively low prevalence of infection suggests that L. (L.) infantum transmission to dogs and humans may, in this region, stem from sylvatic reservoirs.
Introduction
Human visceral leishmaniasis (HVL) is one of the most severe zoonotic infections. The disease is potentially fatal and is prevalent in tropical and subtropical countries in Latin America as well as in the Mediterranean regions of the Old World. It is caused by Leishmania (Leishmania) infantum syn. chagasi and L. (L.) donovani. The parasites are transmitted by phlebotomine sandfly vectors and infect several mammalian species,1,2 of which humans and dogs being the most important from the public health point of view.
This disease has been endemic in northeastern (NE) Brazil for several centuries,3 and the reports of infected dogs and humans have extended to southern Brazil, Paraguay, and, more recently, to NE Argentina.4 Foci of urban transmission have been recorded in the city of Posadas, province of Misiones, Argentina, and occurrence of HVL has extended to the neighboring areas.
The epidemiological picture of leishmaniasis in northwestern (NW) Argentina, where this work was carried out, has been quite different. About 700 km of Chaco forest separate the recognized endemic areas of the NE (humid Chaco) and the NW (dry Chaco). The most frequent clinical form of human leishmaniasis in the latter is tegumentary,5 and the prevailing species of Leishmania isolated from these patients were L. (Viannia) braziliensis and L. (V.) guyanensis.6 No cases of infection by L. (L.) infantum had been reported in dogs of NW Argentina to date. In previous field studies,7,8 we identified a high prevalence (19.4%) of canine leishmaniasis (CanL) caused by L. (V.) braziliensis in dogs sharing the same dwellings with patients suffering from this disease.
Diagnostic methods for HVL and CanL have remarkably improved with the standardization of fine-needle spleen aspirates,9 the use of immunochromatographic dipsticks and cards for DNA storage/shipment, and the development of specific polymerase chain reaction (PCR) techniques.2 These methods have allowed the recognition of particular genotypes within Leishmania spp., information that is useful for understanding transmission cycles.
Several phlebotomine vector species have been identified in NE Argentina, particularly at the sites where HVL and CanL cases were found.4 Lutzomyia longipalpis is the confirmed vector of L. (L.) infantum throughout the Americas.10 Recently, Lu. longipalpis has been found in the city of Tartagal, located in NW Argentina, less than 50 km apart from the sites of infection of the two HVL cases that motivated this work.11
This study addressed the identification of two apparently isolated HVL cases in Salta province, NW Argentina, for which we could obtain data on the probable geographical points where infection occurred. Taking these points as centers of a survey area, we investigated the occurrence of CanL using five different diagnostic methods. The aims of this study were 1) to search for CanL reservoirs and vectors in an area not previously recognized as endemic for VL, 2) to identify the Leishmania spp. causing the canine disease, and 3) to identify the species of phlebotomines captured at the probable sites of infection of humans and dogs.
Materials and Methods
Study area.
Samples were collected within a forest area of ∼5,090 km2, between parallels 22°06′25.15″ S and 22°28′55.31″ S and meridians 63°31′44.94″ W and 62°20′23.27″ W. The entire area was located in the Chaco region of Salta province, which is characterized by uniform, low, dry, xerophytic forest with discrete patches of deforestation for agriculture (dry Chaco).
Study design.
Dogs in close proximity of the sites of infection of two HVL cases were examined and sampled. Only two cases of HVL were observed recently in the study area. One was published12 and the other has so far been reported to the Ministry of Public Health. In this study, the probable sites of infection of these two patients were inferred from detailed interviews. The first patient was a 44-year-old rural worker who reported having been bitten by insects during work at the deforested edge of the jungle. His home was more than 100 km away from the work site, but recruited workers usually sleep in nearby camps during work seasons. The second was a 1-year-old baby that stayed mostly at his home in the forest and, according to his mother's report, had not been at risk of Leishmania infection elsewhere. Both cases were parasitologically diagnosed by observing amastigotes of Leishmania spp. in bone marrow smears. These sites were taken as center points, and a survey of 77 dogs was performed in the surrounding areas (Figure 1). The studied dogs lived from 0 km (same house) up to 29 km from the probable sites of infection of the two human index cases mentioned above. In addition to dogs, 34 apparently healthy humans living in close proximity to the second index case were serologically examined after providing informed consent. They donated a 2-mL blood sample from antebrachial venipuncture.
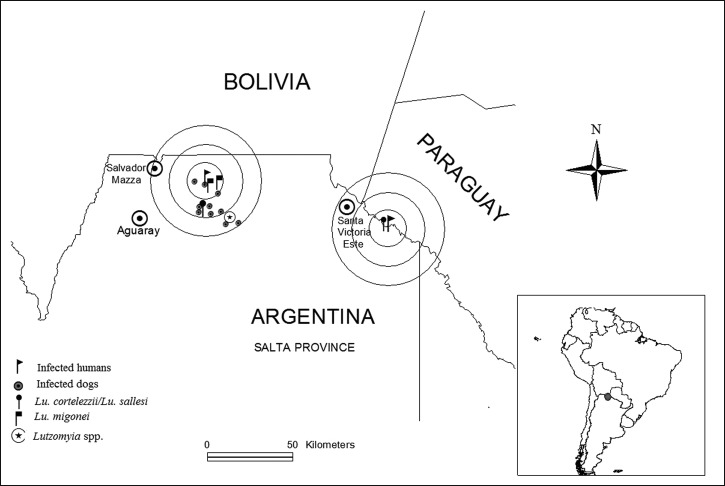
Figure 1.
Schematic map of the sites of infection of the two patients and of detection of vectors and infected dogs. Concentric circles are 10 km apart.
Physical examination and collection of samples.
Procedures recommended in the Declaration of Helsinki and the Guide for the Use and Care of Laboratory Animals13 were followed for physical examination and sampling of dogs. Animals were restrained by the owners and sedated with 0.1 mg/kg acepromazine maleate (Acedán, Holliday-Scott Laboratories, Buenos Aires, Argentina) by intramuscular route. A photograph of the animal was taken and data of the physical examination were recorded. Onychogryposis, eye lesions, lymphadenopathy, weight loss, splenomegaly, alopecia, and other nontraumatic skin lesions were recorded for each dog. Animals were scored as asymptomatic, oligosymptomatic, or polysymptomatic when they displayed none, two or less, or three or more of these signs, respectively. The two human patients were regarded as polysymptomatic.
The splenic puncture was performed as previously described in a study9 by using a 5-mL syringe with 1.5 mL sterile proline balanced salts solution (PBSS). In the field, 0.4 mL of the obtained suspension were inoculated into “Difco” blood agar (DIFCO Argentina, Buenos Aires, Argentina) (USMARU) medium containing 20% defibrinated rabbit blood, and the cultures were transported to the laboratory. About the same amount of the suspension was spread into a circle of FTA™ Classic Cards (Whatman BioScience, Newton Center, MA) following the manufacturer's instructions. The cards were dried and stored at room temperature for the PCR method. Another aliquot of similar volume was added to a 1.5-mL eppendorf tube (Mithril SRL, Buenos Aires, Argentina) containing 1 mL of 96% ethanol for PCR analysis. Finally, a few drops of the spleen aspirates were spread on a slide, air-dried, fixed with methanol for 2 minutes, and transported to the laboratory. Sera from 2 mL peripheral blood were separated by centrifugation at 10,000 rpm for 5 minutes, kept at −20°C and used for the serological reaction as described below.
Parasitological methods.
The spleen aspirate cultures were maintained at 23°C and examined regularly under the microscope for 2 months. The promastigotes isolated from cultures were cryopreserved following the methodology described previously.6 The direct search for Leishmania amastigotes was done on smears of the spleen aspirates14 taken from 76 dogs.
Serological method.
A dipstick, immunochromatographic method (Kalazar Detect, Canine; Inbios International Inc., Seattle, WA) was used throughout, following the manufacturer's instructions. A similar kit, adjusted for humans, was used for 34 human samples.
Amplification of the kinetoplastic minicircle DNA of Leishmania.
Templates were prepared from the spleen aspirate aliquots kept in ethanol, by following a described method.15 PCR amplification of a sequence of the kinetoplastic minicircle DNA conserved among Leishmania spp. were done as described previously.16
Nested PCR and sequencing of the cytochrome b gene of the kinetoplastic maxicircle DNA of Leishmania.
Classic FTA Card filter paper fragments of 2-mm diameter containing spleen aspirate fractions or culture samples were used as a template for a first PCR using the primers for the cytochrome b (cyt b) gene. The nested PCR was performed with the primers for cyt b gene L.cyt-AS (5′-GCGGAGAGRARGAAAAGGC-3′) and L.cyt-AR (5′-CCACTCATAAATATACTATA-3′). One μL from the first PCR product was used as a template for a second PCR using the primers L.cyt-S (5′-GGTGTAGGTTTTAGTYTAGG-3′) and L.cyt-R (5′-CTACAATAAACAAATCATAATATRCAATT-3′). The reaction was concluded with a polymerization step at 72°C for 7 minutes.16 The expected amplicon size was 900 bp. The PCR products were then analyzed on an Applied Biosystems Hitachi 3130 Genetic Analyzer automated sequencer (Applied Biosystems, Foster City, CA).
The procedures described above in the last two sections are referred to as “minicircle PCR” and “maxicircle PCR,” respectively, in this text.
Vector studies.
Phlebotomine sandflies were captured with CDC light minitraps (John W. Hock Co., Gainesville, FL) from 7.00 pm to 8.00 am during two nights, on November 2010 and July 2011. The sampling sites were those where seropositive dogs or HVL index cases were found. Traps were placed at forest edges, peridomicile of human dwellings, surroundings of pig and goat corrals, or water wells. Species were determined by identifying taxonomic markers of the spermatheca and cibarium in females.17
Statistical analysis.
The subjects were first classified into two groups, infected and not infected, following the criteria described in section Diagnostic criteria for CanL and HVL. The cyt b sequences obtained were assembled and edited by Genetyx Mac 11.0.0 (Software Development Co. Ltd., Japan). Data were processed to generate a neighbor-joining tree with 1,000 bootstrap replications using the MLSTest v1.0 software,18 leading to species/genotype identification. Data from field samples were compared with the Argentinean and World Health Organization (WHO) reference strains of the genus Leishmania reported previously.19
Results
Diagnostic criteria for CanL and HVL.
Table 1 displays the number and proportion of dogs positive by each diagnostic test, revealing a higher proportion of positives with molecular and serologic methods than with parasitological (smear and culture) methods.
Table 1.
Numbers and proportions of dogs positive by each diagnostic test
| Assay | Positive | Negative | Positive (%) | Total samples |
|---|---|---|---|---|
| Culture | 3 | 72 | 4.0 | 75 |
| Smear | 2 | 74 | 2.6 | 76 |
| Maxicircle PCR | 7 | 59 | 10.6 | 66 |
| Minicircle PCR | 21 | 52 | 28.8 | 73 |
| rK39 serology | 18 | 59 | 23.4 | 77 |
PCR = polymerase chain reaction.
The criteria for presence or absence of infection by L. (L.) infantum in dogs were based on five laboratory determinations. These were interpreted as follows: a positive result in parasitologic methods (smear or culture), the gold standard, was taken as evidence of infection, even in the absence of other confirmation. Taking into account the low sensitivity of spleen aspirates and culture, a dog was considered as infected only when two or more of the remaining three methods (serology, minicircle PCR, and maxicircle PCR) gave a positive result. The concordance values between serology and parasitological tests were mostly weak or moderate (Kappa index ≤ 0.60), but serology versus maxicircle PCR agreement was very good (Kappa index = 0.84; Table 2).
Table 2.
Concordance values between diagnostic tests
| Kappa index (95% CI) | ||||
|---|---|---|---|---|
| Culture | K39 | Minicircle PCR | Maxicircle PCR | |
| Smear | 0.38 (−0.17, 0.93) | 0.42 (0.02, 0.82) | 0.13 (−0.04, 0.30) | 0.42 (0.02, 0.82) |
| Culture | – | 0.58 (0.21, 0.94) | 0.13 (−0.04, 0.30) | 0.48 (0.05, 0.90) |
| K39 | – | − | 0.24 (0.00, 0.49) | 0.84 (0.62, 1.00) |
| Minicircle PCR | – | − | – | 0.27 (0.04, 0.51) |
CI = confidence interval; PCR = polymerase chain reaction.
Force of concordance: poor < 0.20, weak 0.21–0.40, moderate 0.41–0.60, good 0.61–0.80, very good 0.81–1.00.
Table 3 summarizes the main laboratory and clinical features supporting the diagnosis and parasite identification in two infected patients and 10 infected dogs. Thus, according to the criteria mentioned, the prevalence of CanL in this study reached 13%.
Table 3.
Descriptive listing of the two patients and 10 dogs with leishmaniasis
| Host | Sex | Age (years) | Parasitologic methods | Serology | kPCR | Leishmania species | Cyt b genotype | Symptoms | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Culture | Smear | Mini | Maxi | |||||||
| Human 1 | M | 44 | ND | + | + | + | + | L. (L.) infantum | LiA2 | Poly |
| Human 2 | M | 1 | − | + | + | + | + | L. (L.) infantum | LiA2 | Poly |
| Dog 3 | M | ND | + | − | + | ND | + | L. (L.) infantum | LiA2 | Oligo |
| Dog 8 | F | 6 | + | − | + | + | + | L. (L.) infantum | LiA1 | Asy |
| Dog 15 | M | 4 | − | − | + | + | − | ND | ND | Asy |
| Dog 36 | M | 10 | − | − | + | + | + | L. (L.) infantum | LiA1 | Oligo |
| Dog 37 | F | 6 | − | − | + | + | − | ND | ND | Oligo |
| Dog 40 | M | 7 | − | − | + | + | − | ND | ND | Poly |
| Dog 42 | F | ND | − | + | + | + | + | L. (L.) infantum | LiA1 | Poly |
| Dog 49 | M | 6 | − | − | + | + | + | L. (L.) infantum | LiA1 | Poly |
| Dog 59 | M | 2 | + | + | + | + | + | L. (L.) infantum | LiA2 | Asy |
| Dog 60 | M | 2 | − | − | − | + | + | L. (L.) infantum | LiA1 | Oligo |
Asy = asymptomatic; Cyt b = cytochrome b; F = female; L. (L.) infantum = Leishmania (Leishmania) infantum; M = male; Mini = kinetoplastic minicircle DNA; Maxi = kinetoplastic maxicircle DNA; ND = not determined; Oligo = oligosymptomatic; PCR = polymerase chain reaction; Poly = polysymptomatic; kPCR = kinetoplast PCR.
Relationship between infection and disease.
The association between the presence of infection and the clinical sign score was weak: 70% of the infected dogs were oligo- or polisymptomatic, whereas the corresponding figure for noninfected dogs was 44.8%. The most frequent signs and symptoms were: lymphadenopathy (60%), onychogryphosis (50%), and weight loss (40%).
Geographic distribution of infected humans and dogs.
The probable sites of infection of the two human index cases were both in sylvatic regions. In the area surrounding the first, 10 out of 60 studied dogs were found infected with Leishmania spp. The human dwellings, interspersed in the forest where these infected dogs were detected, were located from 0 up to 29 km from the center point (Figure 1). No infected dogs were found among 17 animals studied in the area surrounding the site of infection of the second human case (the infant), including 14 dogs of the same neighborhood (< 0.2 km from the center point). In addition, 34 human samples from this area were all seronegative.
Typing of Leishmania spp. by nested PCR and sequencing of cytochrome b gene.
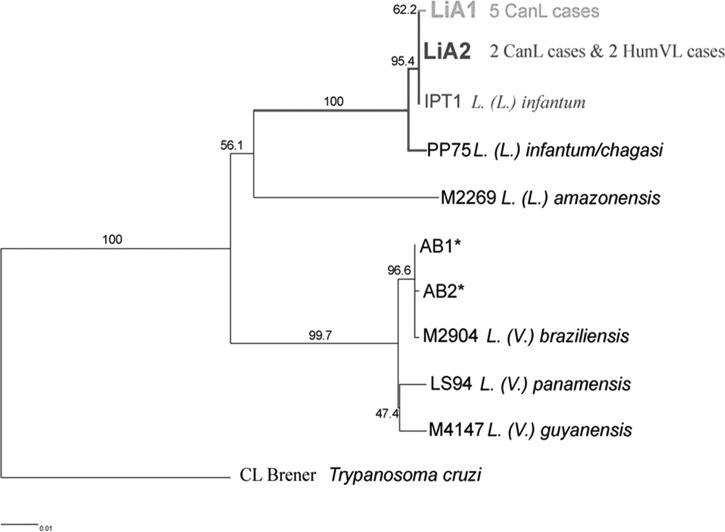
In seven out of the 10 cases diagnosed with CanL, the causal agent was identified by cyt b gene sequencing. The method was performed on templates prepared from seven spleen aspirates and three stocks, spread on FTA cards. Two genotypes were identified, LiA1 in five and LiA2 in two infected cases. They were assigned to L. (L.) infantum, since they showed homology of 99.9% and 100%, respectively, with the WHO reference strain MHOM/TN/80/IPT1. The expected phylogenetic distances between Trypanosoma cruzi and four causal agents of American tegumentary leishmaniasis present in Argentina [L. (L.) amazonensis, L. (V.) braziliensis, L. (V.) guyanensis and L. (V.) panamensis] were verified (Figure 2). The parasites identified from the two HVL cases carried the LiA2 genotype, indistinguishable from the one found in two dogs, suggesting that dogs and humans were involved in the same transmission cycle. In addition, the genotyping performed on the Leishmania stocks and the direct spleen samples obtained from the same infected cases yielded the same results.
Figure 2.
Phylogenetic tree based on Leishmania species typing by Cytochrome b gene sequencing.
Vector studies.
A total of 42 phlebotomines (24 female, 18 male) were captured. They were distributed across all sampling sites. Out of the 24 identified females, 58.3% were classified as Lu. cortelezzii/Lu. sallesi (cortelezzii complex) and 41.7% as Lu. migonei (Figure 1).
Discussion
NW Argentina has been recognized for many years as endemic for tegumentary leishmaniasis caused by L. (V.) braziliensis and L. (V.) guyanensis, but not for HVL.5,19 In this work, field samples for the study of reservoirs and vectors were obtained in the region surrounding the site of two apparently isolated human cases of VL infection in NW Argentina. The area is located far away from previously identified regions of L. (L.) infantum transmission, such as Paraguay and Misiones (Argentina), approximately 700 and 1,000 km away, respectively. Some epidemiologically relevant findings arise from this study: 1) a prevalence of infection of 13% in dogs of the area with Leishmania spp., 2) the identification of L. (L.) infantum, etiologic agent of HVL and CanL, as causative agent in seven infected dogs, and 3) a particular genotype of L. (L.) infantum (LiA2) infecting the two human cases and two of the dogs, suggesting that dogs and humans share the same transmission cycle.
In this study, a seroprevalence of 11.7% for CanL was found by using only the rK39 immunochromatographic dipstick method. By including parasitological and molecular methods, prevalence of CanL increased to 13%. In other studies, the records of sensitivity and specificity of rK39 antigen-based rapid diagnostic tests have been satisfactory, ranging between 65% and 100%. The combined meta-analysis data of sensitivity from several countries around the world was 98.7%.20 The specificity of this method was estimated in 86.6%. The prevalence in this study was also based on reference or direct methods, such as culture, smear, and PCR. The cyt b gene-sequencing method used in this work has been well validated for the identification of Leishmania spp. and genotypes. It was first tested by us with several different reference strains and 17 Argentine Leishmania stocks.19 It was also tested with 30 laboratory strains, mostly of Leishmania21 and further validated in a survey that included 15 clinical specimens and four reference strains of the L. (L.) donovani group, including L. (L.) infantum and L. (L.) chagasi.22
Sequencing of the cyt b gene in Leishmania has allowed the identification of species and also constitutes a valuable tool for tracking transmission cycles, based on isolates from humans, mammalian reservoirs, and insect vectors of a given geographical area. In this work, we found two dogs carrying parasites whose molecular signatures at the cyt b gene had a 100% identity with those of the two human cases that motivated the search, connecting both hosts to the cycle of the LiA2 L. (L.) infantum genotype. According to Chaves and others,23 this observation indicates that humans and dogs have been infected with parasites that stem from the same animal source, but it does not prove by itself that dogs are acting as a reservoir of the parasite in this particular situation. Besides, the low prevalence of CanL in this area is consistent with the view that dogs are not a primary reservoir and other mammals are necessary for maintaining L. (L.) infantum transmission cycle in the region.24
The first human index case led, in the present work, to the discovery of 10 infected dogs, out of 60 studied. These animals were living in human settlements in the jungle and clustered within a 25 km radius from the putative infection site, a point at the edge of the forest where the patient referred to having been bitten by insects during deforestation work. Although his description of the insects was compatible with Lutzomyia (activity in late hours, preferred skin sites of bite), it does not constitute definitive evidence that the patient was attacked by sandflies, but such possibility was supported by our subsequent capture of Lutzomyia specimens at the site. Regarding the second human index case, no other cases of infection were found among 34 humans and 17 dogs living in the area, 14 of which lived in the same house or close surroundings (less than 0.2 km). Our findings would be compatible with a sylvatic mode of transmission of L. (L.) infantum, involving an enzootic cycle where wild mammals would act as primary reservoirs.
The accurate identification of CanL in dogs is hampered by diagnostic difficulties and there is a low agreement of clinical and parasitological data.2 Other Leishmania spp., reacting with generic PCR primers,25 circulate in the area and may infect dogs.7,8 Moreover, in our comparative analysis (Table 1), parasitological methods displayed a very low sensitivity. Thus, diagnostic criteria were developed that took into consideration the fact that highly specific methods are insensitive, and that sensitive molecular methods may bear the risk of false-positive results. The criteria for infection were thus stringent and it cannot be excluded that further studies might confirm as infected some of the dogs bearing less than the complete set of conditions.
In previous vector studies, Lu. longipalpis has been identified as the main vector of L. (L.) infantum in Brazil.10 This species was also reported as the vector of L. (L.) infantum in NE Argentina, particularly in the city of Posadas26 and recently in the city of Tartagal,11 located less than 50 km apart from the probable site of infection of one of our two human index cases. Because our sandfly collection was obtained during the dry season, this study just allowed us to collect and identify a discrete number of sandflies, directly at the sites of probable infection of humans and dogs. In spite of the limited size of samples we did find Lutzomyia specimens, belonged to cortelezzii complex (Lu. cortelezzii/Lu. sallesi) and to Lu. migonei as suspected vectors of L. (L.) infantum in this area. A similar situation was observed in La Banda, Santiago del Estero province, where the suspected vector associated with CanL and HVL cases was Lu. migonei.27 Recently, phlebotomines of cortelezzii complex were reported as vector of L. (V.) braziliensis, but not of L. (L.) infantum in Chaco province of Argentina.28
Current hypotheses on the geographical dissemination of CanL suggest that the spread of this disease is sustained by a domestic transmission cycle, often installed in cities,4,26 where dogs play a major role as reservoirs of L. (L.) infantum.2 Our finding of a 13% prevalence of CanL in a sylvatic region, endemic for cutaneous leishmaniasis7,8 and located far away from the present endemic area of L. (L.) infantum spread,4 is consistent with the view that sylvatic mammals are involved as reservoirs. Domestic dogs would actually play the role of accidental host, similar to that of humans in the cycle of L. (L.) infantum present in the area.
ACKNOWLEDGMENTS
We thank Cecilia Parodi for her help in the collection of specimens. The technical assistance of Federico Ramos and Alejandro Uncos is gratefully acknowledged. Officials, sanitary agents, and van drivers of the Ministry of Public Health provided unrelenting support in fieldwork. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Footnotes
Financial support: This work was supported by grant PICT-2009-0135 of the National Agency for Science and Technology, Argentina, grant no. 2041 of Research Council of the National University of Salta, grant KAKENHI 18-06242 of Japanese Society for the Promotion of Science (JSPS), and grant no. 23256002 of the Ministry of Education, Science, Culture and Sports of Japan.
Authors' addresses: Paola A. Barroso, Jorge D. Marco, María C. Mora, María F. García Bustos, Alejandra B. Barrio, and Miguel A. Basombrío, Instituto de Patología Experimental, Universidad Nacional de Salta/Consejo Nacional de Investigaciones Científicas y Técnicas, Salta, Argentina, E-mails: paobarar@yahoo.com.ar, diegomarcoar@yahoo.com.ar, mariaceliamora@yahoo.com.ar, mariafernandagarcia2008@yahoo.com.ar, aleba05@yahoo.com.ar, and basombri@unsa.edu.ar. Fabricio M. Locatelli and Masataka Korenaga, Kochi University, Nankoku, Japan, E-mails: fm.locatelli@gmail.com and korenaga@kochi-u.ac.jp. Rubén M. Cardozo and Alberto G. Gentile, Dirección de Epidemiología, Ministerio de Salud, Salta, Argentina, E-mails: cardozorm@gmail.com and agentile@salta.gov.ar. Carlos L. Hoyos and Inés López-Quiroga, Instituto de Investigaciones en Enfermedades Tropicales, Sede Regional Orán, Universidad Nacional de Salta, San Ramón de la Nueva Orán, Argentina, E-mails: carloshoyos@gmail.com and ineslq@hotmail.com. Tatsuyuki Mimori, Department of Microbiology, Faculty of Life Sciences, Graduate School of Health Sciences, Kumamoto University, Japan, E-mail: mimori@kumamoto-u.ac.jp. Yoshihisha Hashiguchi, Centro de Biomedicina, Universidad Central de Ecuador, Quito, Ecuador, E-mail: yhashiguchi42@yahoo.co.jp.
References
- 1.Souza TD, Turchetti AP, Fujiwara RT, Paixão TA, Santos RL. Visceral leishmaniasis in zoo and wildlife. Vet Parasitol. 2014;200:233–241. doi: 10.1016/j.vetpar.2013.12.025. [Review] [DOI] [PubMed] [Google Scholar]
- 2.Baneth G, Koutinas AF, Solano-Gallego L, Bourdeau P, Ferrer L. Canine leishmaniosis-new concepts and insights on an expanding zoonosis. Trends Parasitol. 2008;24:324–330. doi: 10.1016/j.pt.2008.04.001. [Review] [DOI] [PubMed] [Google Scholar]
- 3.Quinnel RJ, Courtenay O, Garcez LM, Dye C. Epidemiology of canine leishmaniasis: transmission rates estimated from a cohort study in Amazon Brazil. Parasitology. 1997;115:143–156. doi: 10.1017/s0031182097001200. [DOI] [PubMed] [Google Scholar]
- 4.Salomón OD, Sinagra A, Nevot MC, Barberian G, Paulin P, Estevez JO, Riarte A, Estevez J. First visceral leishmaniasis focus in Argentina. Mem Inst Oswaldo Cruz. 2008;103:109–111. doi: 10.1590/s0074-02762008000100018. [DOI] [PubMed] [Google Scholar]
- 5.Basombrío MA, Taranto NJ. Outbreaks of mucocutaneous leishmaniasis in northern Argentina. Anim Biol. 2000;9:131–133. [Google Scholar]
- 6.Marco JD, Barroso P, Calvopiña M, Kumazawa H, Furuya M, Korenaga M, Cajal SP, Mora MC, Rea MM, Borda CE, Basombrío MA, Taranto NJ, Hashiguchi Y. Species assignation of Leishmania from human and canine American tegumentary leishmaniasis cases by multilocus enzyme electrophoresis in northern Argentina. Am J Trop Med Hyg. 2005;72:606–611. [PubMed] [Google Scholar]
- 7.Marco JD, Padilla AM, Diosque P, Fernandez MM, Malchiodi E, Basombrío MA. Force of infection an evolution of lesions of canine tegumentary leishmaniasis in northwestern Argentina. Mem Inst Oswaldo Cruz. 2001;96:649–652. doi: 10.1590/s0074-02762001000500009. [DOI] [PubMed] [Google Scholar]
- 8.Padilla AM, Marco JD, Diosque P, Segura MA, Mora MC, Fernandez MM, Malchiodi EL, Basombrío MA. Canine infection and the possible role of dogs in the transmission of American tegumentary leishmaniasis in Salta, Argentina. Vet Parasitol. 2002;110:1–10. doi: 10.1016/s0304-4017(02)00330-8. [DOI] [PubMed] [Google Scholar]
- 9.Barrouin-Melo SM, Farias-Laranjeira D, de Andrade-Filho FA, Trigo J, Juliao FS, Franke CR, Palis-Aguiar PH, Conrado-dos-Santos WL, Pontes-de-Carvalho L. Can spleen aspirates be safely used for the parasitological diagnosis of canine visceral leishmaniasis? A study of asymptomatic and polysymptomatic animals. Vet J. 2006;171:331–339. doi: 10.1016/j.tvjl.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Lainson R, Rangel EF. Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil: a review. Mem Inst Oswaldo Cruz. 2005;100:811–827. doi: 10.1590/s0074-02762005000800001. [DOI] [PubMed] [Google Scholar]
- 11.Bravo AG, Quintana MG, Abril M, Salomón OD. The first record of Lutzomyia longipalpis in the Argentine northwest. Mem Inst Oswaldo Cruz. 2013;108:1071–1073. doi: 10.1590/0074-0276130358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrio A, Parodi CM, Locatelli F, Mora MA, Basombrio MA, Korenaga M, Hashiguchi Y, García Bustos MF, Gentile A, Marco JD. Leishmania infantum and visceral leishmaniasis, Argentina. Emerg Infect Dis. 2012;18:354–355. doi: 10.3201/eid1802.110924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. 8th edition. Washington, DC: National Academies Press; 2010. p. 248.http://www.nap.edu/catalog/12910.html Available at. [Google Scholar]
- 14.Marco JD, Barroso PA, Mimori T, Locatelli FM, Tomatani A, Mora MC, Cajal SP, Nasser JR, Parada LA, Taniguchi T, Korenaga M, Basombrío MA, Hashiguchi Y. Polymorphism-specific PCR enhances the diagnostic performance of American tegumentary leishmaniasis and allows the rapid identification of Leishmania species from Argentina. BMC Infect Dis. 2012;12:191–198. doi: 10.1186/1471-2334-12-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higuchi H. Rapid, efficient DNA extraction for PCR from cells or blood. Perkin Elmer Cetus Amplifications. 1990;2:1–3. [Google Scholar]
- 16.Kato H, Uezato H, Katakura K, Calvopiña M, Marco JD, Barroso P, Gomez EA, Mimori T, Korenaga M, Iwata H, Nonaka S, Hashiguchi Y. Detection and identification of Leishmania species within naturally infected sandflies in the Andean areas of Ecuador by polymerase chain reaction. Am J Trop Med Hyg. 2005;72:87–93. [PubMed] [Google Scholar]
- 17.Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae) Mem Am Entomol Inst. 1994;54:1–881. [Google Scholar]
- 18.Tomasini N, Lauthier JJ, Llewellyn MS, Diosque P. MLSTest: novel software for multi-locus sequence data analysis in eukaryotic organisms. Infect Genet Evol. 2013;20:188–196. doi: 10.1016/j.meegid.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 19.Marco JD, Uezato H, Mimori T, Barroso PA, Korenaga M, Nonaka S, Basombrío MA, Taranto NJ, Hashiguchi Y. Are cytochrome B sequencing and polymorphism-specific polymerase-chain reaction as reliable as multilocus enzyme electrophoresis for identifying Leishmania spp. from Argentina? Am J Trop Med Hyg. 2006;75:256–260. [PubMed] [Google Scholar]
- 20.Quinnell RJ, Carson C, Reithinger R, Garcez LM, Courtenay O. Evaluation of rK39 rapid diagnostic tests for canine visceral leishmaniasis: longitudinal study and meta-analysis. PLoS Negl Trop Dis. 2009;7:e1992. doi: 10.1371/journal.pntd.0001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asato Y, Oshiro M, Myint CK, Yamamoto Y, Kato H, Marco JD, Mimori T, Gomez EA, Hashiguchi Y, Uezato H. Phylogenic analysis of the genus Leishmania by cytochrome b gene sequencing. Exp Parasitol. 2009;121:352–361. doi: 10.1016/j.exppara.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Foulet F, Botterel F, Buffet P, Morizot G, Rivollet D, Deniau M, Pratlong F, Costa JM, Bretagne S. Detection and identification of Leishmania species from clinical specimens by using a real-time PCR assay and sequencing of the cytochrome b gene. J Clin Microbiol. 2007;45:2110–2115. doi: 10.1128/JCM.02555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaves LF, Hernandez MJ, Dobson AP, Pascual M. Sources and sinks: revisiting the criteria for identifying reservoirs for American cutaneous leishmaniasis. Trends Parasitol. 2007;23:311–316. doi: 10.1016/j.pt.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Silva ES, Gontijo CM, Melo MN. Contribution of molecular techniques to the epidemiology of neotropical Leishmania species. Trends Parasitol. 2005;21:550–552. doi: 10.1016/j.pt.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Barrio A, Mora MC, Ramos F, Moreno R, Samson R, Basombrío MA. Short report: use of kDNA-based polymerase-chain reaction as sensitive and differentially diagnostic method of American tegumentary leishmaniasis in disease-endemic areas of northern Argentina. Am J Trop Med Hyg. 2007;77:636–639. [PubMed] [Google Scholar]
- 26.Santini MS, Fernandez MS, Perez AA, Sandoval AE, Salomon OD. Lutzomyia longipalpis abundance in the city of Posadas, north-east Argentina: variations at different spatial scales. Mem Inst Oswaldo Cruz. 2012;107:767–771. doi: 10.1590/s0074-02762012000600010. [DOI] [PubMed] [Google Scholar]
- 27.Salomón OD, Quintana MG, Bezzic G, Moranc ML, Bethbederc E, Valdez DV. Short communication: Lutzomyia migonei as putative vector of visceral leishmaniasis in La Banda, Argentina. Acta Trop. 2010;113:84–87. doi: 10.1016/j.actatropica.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 28.Rosa J, Pereira DP, Brazil RP, Filho JD, Salomón O, Szelag E. Natural infection of cortelezzii complex (Diptera: Psychodidae: Phlebotominae) with Leishmania braziliensis in Chaco, Argentina. Acta Trop. 2012;123:128–131. doi: 10.1016/j.actatropica.2012.04.008. [DOI] [PubMed] [Google Scholar]