Abstract
Wuchereria bancrofti prevalence and transmission were assessed in six endemic villages in Sikasso, Mali prior to and yearly during mass drug administration (MDA) with albendazole and ivermectin from 2002 to 2007. Microfilaremia was determined by calibrated thick smear of night blood in adult volunteers and circulating filarial antigen was measured using immunochromatographic card test in children < 5 years of age. Mosquitoes were collected by human landing catch from July to December. None of the 686 subjects tested were microfilaremic 12 months after the sixth MDA round. More importantly, circulating antigen was not detected in any of the 120 children tested, as compared with 53% (103/194) before the institution of MDA. The number of infective bites/human/year decreased from 4.8 in 2002 to 0.04 in 2007, and only one mosquito containing a single infective larva was observed 12 months after the final MDA round. Whether this dramatic reduction in transmission will be sustained following cessation of MDA remains to be seen.
Introduction
Lymphatic filariasis (LF) caused by Wuchereria bancrofti is endemic throughout West Africa,1,2 where the predominant vectors are species of the Anopheles gambiae and Anopheles funestus complexes.1 One of the key questions that has emerged as the Global Program for the Elimination of Lymphatic Filariasis (GPELF) has grown globally since 20003–5 is the impact that annual mass drug administration (MDA) of albendazole and ivermectin (Mectizan®) has had on the transmission of the W. bancrofti infection in different epidemiological settings in Africa.
Whereas the long-term impact of combination albendazole/diethylcarbamazine (ALB/DEC) on transmission of LF has been demonstrated in multiple epidemiologic settings,6–8 only a few studies have examined the impact of repeated annual administration of albendazole/ivermectin (ALB/IVER), the regimen used throughout West Africa because of the overlapping geographic distributions of LF and onchocerciasis.9–11 To evaluate the effect of ALB/IVER on W. bancrofti transmission in varied epidemiologic settings in Africa, a multi-country study was initiated in 2001 in Mali and Ghana (West Africa) and Kenya (East Africa). Regular assessments of prevalence in the human population (circulating filarial antigen and microfilarial levels), and in the Anopheles vector population (annual transmission potential [ATP]) were conducted prior to and during six annual MDA using ALB/IVER. We report here the results of the portion of the study conducted in a highly endemic area of Mali.
Methods
Study sites.
The study was undertaken in six villages of the district of Sikasso: Dozanso, Gondaga, Missasso, N'Torla, Niantanso, and Zanadougou. The total population of the study area was 5,120 in 2008, according to demographic information available from the Sikasso Regional Directorate responsible for planning and statistics. The administrative region of Sikasso covers 76,480 km2 in the southern Sudan savannah area. The region has a total population of 2.45 million, the highest population density in the country with 32 inhabitants per km2 in 2008. Prior to this study, there had been no MDA implemented in this area. The study villages have previously been described.12 The mean distance between the villages is approximately 15 km occupied by cotton fields, backwaters, and typical Sudan savanna vegetation. During the study period, the rainfall ranged from 1,000 to 1 500 mm/year with the rainy season extending from July to December.
Study design.
To assess the impact of six consecutive annual MDA rounds on W. bancrofti infection and transmission in these six villages of Sikasso District, a monthly cross-sectional entomological survey was undertaken from July to December each year, as well as a parasitological assessment in July each year just prior to the MDA and the entomological survey, from 2002 to 2008. All six villages received MDA for 6 years. During the seventh year (2008), ALB/IVER was not distributed in two villages with no evidence of ongoing transmission to provide preliminary data in anticipation of stopping MDA in the remaining villages the following year.
Study population.
A complete census, including the name, age, sex, and profession of every inhabitant, was performed in the study villages every year before the parasitological assessment. All subjects ≥ 2 years of age who presented for evaluation were included in the study.
Parasitological and clinical assessment.
Each year, before starting the mosquito collection, a parasitological assessment was performed. Sixty microliters of night blood were obtained by fingerprick from adult volunteers (15 years and above) for preparation of three thick smears. The slides were stained with Giemsa for identification and quantification of W. bancrofti microfilariae. The adult volunteers as well as children ≤ 5 years of age were tested for W. bancrofti circulating antigen using immunochromatographic card test during the first year (at baseline) and after the sixth MDA. The clinical assessment consisted of a brief interview and physical exam focusing on characteristic manifestations of LF, namely lymphedema and hydrocele. Any clinical stage of lymphedema (from reversible pitting edema to elephantiasis) or hydrocele (small, big, unilateral, or bilateral) was considered as a case and recorded without additional information.
Mass drug administration.
ALB/IVER was administered to all eligible subjects (not pregnant or breastfeeding within a week of delivery, taller than 90 cm, and aged 5 years and above) in collaboration with the district and community health care staff using the health workers as drug distributors. MDA coverage rates were calculated based on the number of eligible subjects.
Entomological studies.
Villagers were trained to collect mosquitoes from 6:00 pm to 6:00 am using the human landing catch (HLC) method. A 12-day monthly entomological survey was carried out concomitantly by different teams in each of the six villages to determine village-wide W. bancrofti transmission potential. The parameters assessed included the human biting rate (HBR), infection rate, infectivity rate, ATP, and the entomological inoculation rate (EIR) during the study period. From July to December each year, mosquitoes were collected by two collectors per room in four different rooms in each village at night. The first collection team worked from 6:00 pm to midnight and the second from midnight to 6:00 am in each room. The collector caught the mosquitoes as they tried to land using a mouth aspirator connected to a paper cup as the storage container, as developed by Coluzzi and Petrarca. The mosquitoes that were collected during night were kept in ideal conditions (temperature, relative humidity using wet wipes) and dissected early the following morning.
Laboratory analysis.
Mosquito samples were sorted by species (An. gambiae s.l. and An. funestus) on the basis of morphology.13 Members of the An. gambiae complex were identified in a sample of dissected mosquitoes by polymerase chain reaction (PCR) assays in 2002 (at baseline) as described by Favia and others.14
During the dissection, the head and thorax were dissected separately for each mosquito, and recovered parasite larval stages were categorized into L1, L2, and L3. Parity status was determined by dissecting the ovaries and observing the tracheal coils.15
Multiple entomological parameters were calculated as previously described12,13,16:
-
1)
Infection rate: proportion of mosquitoes found infected after dissection with any W. bancrofti larval stage (L1–L3)
-
2)
Infectivity rate: proportion of mosquitoes found infected with one or more infective larvae (L3)
-
3)
HBR: number of mosquitoes caught during the HLC × 30/(total number of collectors used per collection × number of collection in the month)
-
4)
EIR: HBR × infectivity rate. The results of the monthly HBR (from all night HLC) multiplied by the W. bancrofti infectivity rate for a given species give an estimate of the number of infective bites of W. bancrofti received per human per month.
-
5)
ATP: the sum of the monthly EIR over the year.
Data management and analysis.
All data were recorded on standard data sheets and entered into the computer using SPSS version 12 (Statistical Package for Social Sciences, SPSS Inc., Chicago, IL) and GraphPad Prism version 5. The χ2 test or Fisher's exact test was used as appropriate for the comparison of proportions. The confidence level was set at 95% for all statistical tests.
A collective village-wide oral consent was obtained from the villages' elders as well as a signed individual written consent form from all study participants and/or guardians. Both the Institutional Review Board of the World Health Organization/Tropical Diseases Research and the Ethics committee of the Faculty of Medicine of Bamako in Mali approved the protocol and the consent forms.
Results
Clinical, parasitological, and MDA.
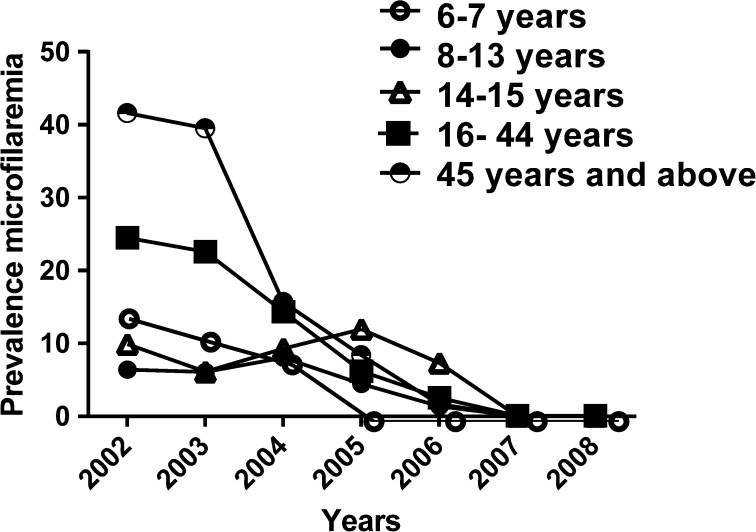
A total of 1,333 subjects from six villages aged 2 years and above have been included in the analysis. Females represented 57.8% of this population. Lymphedema and hydrocele had prevalences of 0.3 and 2.8%, respectively (Table 1). Cross-sectional assessment of the human microfilaremia prevalence rate showed a dramatic decrease in the prevalence of W. bancrofti microfilaremia over the course of the study (P < 10−4) from 21.4% (244/1139) in 2002 (Table 1) before the first MDA to 0.2% (2/856) in 2007 and 0.0% (0/760) in 2008 after the sixth MDA round (data not shown). The geometric mean microfilaria (mf) densities in microfilaria-positive individuals also decreased from 103 mf/mL in 2002 to 63 mf/mL in 2006, 17 mf/mL in 2007 and to 0 mf/mL in 2008 (data not shown). Subjects aged 45 and above had the highest W. bancrofti microfilaremia prevalence (41.6%) and geometric mean microfilaria density (137 mf/mL) (Figure 1). The antigen carriage rate in children also decreased during this period from 53.09% (103/194) in 2002 to 0.0% (0/120) in 2008 (P < 10−4) (Table 2).
Table 1.
Baseline characteristics of the study population
| Total | Male | Female | Lymphedema | Hydrocele | Mf+ | Cag+ | |
|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | ||
| Age group | |||||||
| 2–5 years | 219 | 47.9 | 52.1 | 0 | 0 | ND | 2.2 |
| 6–7 years | 81 | 56.8 | 43.2 | 0 | 0 | 4.5 | 7.1 |
| 8–13 years | 231 | 47.2 | 52.8 | 0 | 0 | 6.1 | 20.3 |
| 14–15 years | 50 | 60 | 40 | 0 | 0 | 2 | 4.4 |
| 16–44 years | 596 | 33.9 | 66.1 | 0.2 | 1.7 | 60.2 | 52.3 |
| 45 years and above | 156 | 53.2 | 46.8 | 1.3 | 14.1 | 26.2 | 13.7 |
| Total | 1,333 | 42.2 | 57.8 | 0.3 | 2.8 | 21.4 | 46.6 |
| Villages* | |||||||
| Dozanso | 120 | 54.2 | 45.8 | 1.7 | 7.7 | 40 | 61.7 |
| Missasso | 207 | 35.3 | 64.7 | 0 | 11 | 20.3 | 36.9 |
| Gondaga | 212 | 45.8 | 54.2 | 0 | 6.2 | 15.1 | 43.4 |
| Niantanso | 202 | 42.1 | 57.9 | 0.5 | 7.1 | 29.7 | 81.8 |
| N'Torla | 196 | 50.5 | 49.5 | 0 | 3 | 13.8 | 40.3 |
| Zanadougou | 202 | 30.7 | 69.3 | 0 | 6.5 | 17.3 | 24.6 |
Cag+ = circulating filarial antigen; Mf+ = microfilaremia, ND = not done.
Village data are restricted to inhabitants > 5 years of age.
Figure 1.
Geometric mean prevalence of Wuchereria bancrofti and microfilaremia prevalence by age over the six consecutive mass drug administration rounds in the study area.
Table 2.
Variation in the Wuchereria bancrofti circulating antigen carriage rate among individuals of 5 years and older in 2002 and 2008
| Localities | Baseline (2002) | Post 6 MDA (2008) | ||||
|---|---|---|---|---|---|---|
| No. tested | Pos | % | No. tested | Pos | % | |
| Dozanso | 120 | 48 | 40 | 86 | 0 | 0 |
| Missasso | 207 | 42 | 20.2 | 138 | 0 | 0 |
| Gondaga | 212 | 32 | 15.1 | 167 | 0 | 0 |
| Niantanso | 202 | 60 | 24.8 | 129 | 0 | 0 |
| N'Torla | 196 | 27 | 13.7 | 141 | 0 | 0 |
| Zanadougou | 202 | 35 | 17.3 | 99 | 0 | 0 |
| Total | 1139 | 244 | 21.4 | 760 | 0 | 0 |
MDA = mass drug administration; No. = number; Pos = positive.
The coverage rate for the eligible population varied from 67% to 78% during the first five MDA. In the four villages treated in 2008, coverage remained high at 89.6% (3201/3574). Mild adverse events were reported by 0.6% (13/2135) of the subjects in 2002 and the frequency decreased over time with only a few cases of mild headache reported in 2008 (data not shown). No severe adverse events were recorded during the study.
Entomology patterns.
An. gambiae complex members represented 90.1% of the vector fauna at baseline. Their mean infectivity rate was 2.3 (372/16,230) whereas An. funestus complex members had an infectivity rate of 2.3% (372/16,230). The annual transmission potential was 76 and 8 infective bites per human per year, respectively, for An. funestus and An. gambiae complexes (Table 3). Among the 15,869 An. gambiae complex members examined by PCR for specific species identification, 99.02% (15,713/15,869) were An. gambiae s.s. and 0.98% (156/15,869) were Anopheles arabiensis. The most common sibling species of An. gambiae s.s. in all localities were the Bamako/Savannah molecular form (S form), which comprised 95.09% (14,942/15,713) of the mosquitoes examined, followed by the Mopti molecular form (M form), which accounted for 3.8% (data not shown).
Table 3.
Baseline vector characteristics in the study area prior to the MDA
| Species | No. of collected (%) | No. of dissected | No. of infected (%) | No. of infective (%) | HBR | EIR | ATP |
|---|---|---|---|---|---|---|---|
| Anopheles gambiae s.l. | 20957 (90.1) | 16230 | 646 (4.0) | 372 (2.3) | 545.8 | 12.6 | 76 |
| Anopheles funestus | 2308 (9.9) | 1471 | 72 (4.9) | 30 (2.0) | 60.1 | 1.2 | 8 |
| Overall | 23265 (100) | 17701 | 718 (4.1) | 402 (2.3) | 605.9 | 13.9 | 84 |
ATP = annual transmission potential; EIR = entomological inoculation rate; HBR = human biting rate; MDA = mass drug administration; No. = number.
The annual vector HBR decreased over time from 605.9 bites per human per year in 2001 (Table 3) to 203.96 bites per human per year in 2007 (Table 4). The vector infection rate (An. gambiae s.l. and An. funestus) also decreased dramatically (by more than 98.11%) from 4.1% (718/17701) in 2001 (Table 3) to 0.04% (2/4680) in 2007, 12 months after the sixth MDA (Table 4). Of the two infected An. gambiae complex mosquitoes, one harboured a single infective L3 larva and the second one, a single non-infective L2 larva. Thus, the mosquito infectivity rate in 2007 was 0.02% (1/4,680) (Table 4). Due to the combination of a decrease in mosquito biting rates and lower numbers of infective mosquitoes, the EIR (number of infective bites per human per year) decreased by 99.7% from 4.8 in 2001 to 0.05 in 2006 and to 0.04 in 2007, 12 months after the sixth MDA (Table 4). A similar effect was noted on the ATP, which decreased by 99.95% (Table 4). For An. funestus complex, the EIR (1.2 infective bites per person per month) and the ATP (seven infective bites per person per year) decreased by 100% by the end of sixth MDA evaluation while for An. gambiae complex, the EIR (12.6 infective bites per person per month) and the ATP (75.3 infective bites per person per year) decreased, respectively, to 0.04 and 0.2 (Supplemental Tables 1 and 2).
Table 4.
Annual variation of the Anopheles gambiae and Anopheles funestus LF transmission level over the six MDA rounds
| Years | Number of mosquito collected | Number of mosquito dissected | HBR | Infection rate (L1/L2 pos) | Infectivity rate (L3 pos) | EIR | ATP |
|---|---|---|---|---|---|---|---|
| % (positive/N) | % (positive/N) | ||||||
| Before (2001) | 23265 | 17701 | 605.9 | 4.1 (718/17701) | 2.3 (402/17701) | 13.9 | 84 |
| MDA 1 (2002) | 12986 | 12986 | 338.2 | 4.6 (597/12986) | 1.4 (181/12986) | 4.8 | 28.1 |
| MDA 2 (2003) | 18394 | 18394 | 479 | 1.2 (222/18394) | 0.2 (44/18394) | 1.1 | 6.9 |
| MDA 3 (2004) | 13021 | 13021 | 339 | 1.1 (143/13021) | 0.1 (16/13021) | 0.4 | 2.5 |
| MDA 4 (2005) | 10622 | 9578 | 276.61 | 0.17 (16/9578) | 0.05 (5/9578) | 0.14 | 0.9 |
| MDA 5 (2006) | 10604 | 10604 | 276.1 | 0.06 (6/10604) | 0.02 (2/10604) | 0.05 | 0.3 |
| MDA 6 (2007) | 7832 | 4680 | 203.96 | 0.04 (2/4680) | 0.02 (1/4680) | 0.04 | 0.3 |
ATP = annual transmission potential; EIR = entomological inoculation rate; HBR = human biting rate; LF = lymphatic filariasis; MDA = mass drug administration; pos = positive.
Of note, the two infected mosquitoes were found in two different villages, Dozanso and Niatanso, resulting in average infection rates of 0.11% (N = 916) and 0.28% (N = 362), respectively. Thus, despite an average EIR of 0.04 infective bites per person during the study period, the EIR in 2007 was 0 in all of the villages except Dozanso, where it was 0.28 (data not shown).
Discussion
Consistent with the data from other studies,9–11 six rounds of MDA with albendazole and ivermectin were extremely effective in reducing the prevalence of W. bancrofti microfilaremia in residents of a highly endemic area of Mali. Although testing for microfilaremia was limited to 53.02% (604/1139) of the total population eligible for MDA in the six villages, it is unlikely that the infection rate in the remaining population was substantially higher than that in the tested subjects. Thus, the observed impact of MDA on W. bancrofti microfilaremia in the present study is compatible with the long-term objective of the GPELF to interrupt transmission using MDA alone.
As previously reported in the baseline study,12 the dominant vector, An. gambiae s.l., continued to account for more than 90% of the mosquito vectors collected in this area over the 7 years of the study, followed by An. funestus (data not shown). The overall trend in any given year was characterized by a high frequency of An. gambiae s.l. early in the rainy season followed by a gradual decrease in An. gambiae s.l. and a gradual increase in the abundance of An. funestus toward the end of the rainy season.12 These changes are related to the climatic conditions over the year and not a result of MDA.13
In addition to seasonal variation, the vector density, and consequently the HBR, showed significant yearly variation from 2001 to 2008. The dramatic decrease in HBR following the first MDA in the study area has previously been reported and was most likely due to increased awareness of the study area population with respect to the role of mosquitoes in disease transmission (resulting in less breeding sites and increased use of insecticide-treated nets) and the effect of ivermectin on mosquito survivorship.12 Long-lasting insecticide-treated nets (LLITNs) were provided for free only to mothers just after delivery at the community health center from 2002 to 2004. Beginning in 2005, LLITN availability in the six villages increased because of the free distribution campaigns for vector control related to malaria prevention. Although yearly variations in HBR are not unusual in Mali and have been observed in the neighboring sites of Pimperena (unpublished data), this would not be expected to have a significant effect on EIR without a concomitant change in the vector infectivity rate.
Despite high levels of transmission prior to the institution of MDA, the vector infection and infectivity rates decreased to a very low, but detectable, level in 2007. Only two captured mosquitoes were infected with W. bancrofti, of which only one was infective, representing a more than 99% reduction in the infectivity rate. No differences were apparent between the two villages that continued to have infected mosquitoes and the four other villages with respect to overall compliance with the program or distance to non-MDA villages.
The mean EIR and ATP were also reduced by more than 99% after six MDA rounds. Although persistence of transmission despite low levels of microfilaremia in the human population has been reported with Culex species that exhibit limitation (decreasing yield of infective larvae per mf as the number of ingested mf increases), An. gambiae and An. funestus complexes demonstrate facilitation (increasing yield of infective larvae per mf as the number of ingested mf increases).17,18 Consequently, the dramatic reduction in transmission intensity (only one infective larvae recovered and an infectivity rate of the anopheles vectors of 0.02% (the cutoff of 0.03% has been proposed) is likely sufficient to interrupt transmission in this rural area of Sikasso district19 as sexual reproduction is required in the human host to produce microfilaria.
Nevertheless, caution should be exercised in stopping MDA as there be might be variation in the efficiency of the different sibling species within the An. gambiae group of mosquitoes; thus continued close surveillance for resurgence of transmission will be essential. In this regard, a staggered approach to stopping, as undertaken in this study, may be most prudent.
In summary, the data to date suggest that six rounds of MDA with albendazole and ivermectin may be sufficient to interrupt transmission in a highly endemic region of Mali where Anopheles is the main vector. Annual evaluation of the human and vector populations for evidence of W. bancrofti infection continues in the study villages following cessation of MDA and will be essential to validate this conclusion. Although the effects of vector control measures, such as impregnated mosquito bednets, were not assessed in this study, such interventions may provide additional benefit, particularly in the maintenance of transmission interruption after MDA is stopped, and should be explored in future studies.
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to the villagers who participated in this study. A special thanks to the Sikasso local health workers, the inhabitants and authorities of Kolokoba rural commune for their collaboration, and Dr Boatin Boakye who visited the study sites and provided with useful advises.
Footnotes
Financial support: This study was funded by the WHO/UNDP/World Bank Special Program for Tropical Diseases grant (ID A00583) to SFK and the Lymphatic Filariasis Support Centre of the Liverpool School of Tropical Medicine, UK through a grant from the UK Department for International Development. This study was funded in part by the Division of Intramural Research, NIAID, NIH.
Authors' addresses: Yaya I. Coulibaly, Benoit Dembele, Abdallah Amadou Diallo, Siaka Konate, Houseini Dolo, Siaka Yamoussa Coulibaly, Salif Seriba Doumbia, Lamine Soumaoro, Michel Emmanuel Coulibaly, and Sekou F. Traore, International Center of Excellence in Research (ICER-Mali), Filariasis Research and Training Unit, Bamako, Bamako, Mali, E-mails: yicoulibaly@icermali.org, dbenedictus@hotmail.com, adaxe@icermali.org, kiforo2002@yahoo.fr, hdolo@icermali.org, yamoussa@icermali.org, Salifdoumbia@icermali.org, soumla@icermali.org, michou@icermali.org, and cheick@icermali.org. Moses J. Bockarie, Liverpool School of Tropical Medicine, Centre for Neglected Tropical Diseases, Liverpool, United Kingdom, E-mail: Moses.Bockarie@lstmed.ac.uk. David Molyneux, Liverpool School of Tropical Medicine, Centre for Neglected Tropical Diseases, Liverpool, United Kingdom, E-mail: David.Molyneux@LSTMed.ac.uk. Thomas B. Nutman, National Institutes of Health, Laboratory of Parasitic Diseases, Helminth Immunology Section, Bethesda, MD, E-mail: tnutman@niaid.nih.gov. Amy D. Klion, National Institutes of Health, Laboratory of Parasitic Diseases, Eosinophil Pathology Section, Bethesda, MD, E-mail: aklion@niaid.nih.gov. Yeya T. Toure, World Health Organization, Vectors, Environment and Society Research, Geneva, Switzerland, E-mail: tourey@who.int.
References
- 1.Brengues J. La Filariose de Brancroft en Afrique de L'Ouest. ORSTOM Paris Memoires ORSTOM No. 79. 1975. p. 293. [Google Scholar]
- 2.Gyapong JO, Kyelem D, Kleinschmidt I, Agbo K, Ahouandogbo F, Gaba J, Owusu-Banahene G, Sanou S, Sodahlon YK, Biswas G, Kale OO, Molyneux DH, Roungou JB, Thomson MC, Remme J. The use of spatial analysis in mapping the distribution of bancroftian filariasis in four West African countries. Ann Trop Med Parasitol. 2002;96:695–705. doi: 10.1179/000349802125001735. [DOI] [PubMed] [Google Scholar]
- 3.Molyneux DH, Zagaria N. Lymphatic filariasis elimination: progress in global programme development. Ann Trop Med Parasitol. 2002;96((Suppl 2)):S15–S40. doi: 10.1179/000349802125002374. [DOI] [PubMed] [Google Scholar]
- 4.Ottesen EA. Lymphatic filariasis: treatment, control and elimination. Adv Parasitol. 2006;61:395–441. doi: 10.1016/S0065-308X(05)61010-X. [DOI] [PubMed] [Google Scholar]
- 5.Ottesen EA, Hooper PJ, Bradley M, Biswas G. The global programme to eliminate lymphatic filariasis: health impact after 8 years. PLoS Negl Trop Dis. 2008;2:e317. doi: 10.1371/journal.pntd.0000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramzy RM, El Setouhy M, Helmy H, Ahmed ES, Abd Elaziz KM, Farid HA, Shannon WD, Weil GJ. Effect of yearly mass drug administration with diethylcarbamazine and albendazole on bancroftian filariasis in Egypt: a comprehensive assessment. Lancet. 2006;367:992–999. doi: 10.1016/S0140-6736(06)68426-2. [DOI] [PubMed] [Google Scholar]
- 7.Supali T, Djuardi Y, Bradley M, Noordin R, Rückert P, Fischer PU. Impact of six rounds of mass drug administration on Brugian filariasis and soil-transmitted helminth infections in eastern Indonesia. PLoS Negl Trop Dis. 2013;7:e2586. doi: 10.1371/journal.pntd.0002586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramaiah KD, Vanamail P, Yuvaraj J, Das PK. Effect of annual mass administration of diethylcarbamazine and albendazole on bancroftian filariasis in five villages in south India. Trans R Soc Trop Med Hyg. 2011;105:431–437. doi: 10.1016/j.trstmh.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Richards FO, Eigege A, Miri ES, Kal A, Umaru J, Pam D, Rakers LJ, Sambo Y, Danboyi J, Ibrahim B, Adelamo SE, Ogah G, Goshit D, Oyenekan OK, Mathieu E, Withers PC, Saka YA, Jiya J, Hopkins DR. Epidemiological and entomological evaluations after six years or more of mass drug administration for lymphatic filariasis elimination in Nigeria. PLoS Negl Trop Dis. 2011;5:e1346. doi: 10.1371/journal.pntd.0001346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Progress toward elimination of lymphatic filariasis–Togo, 2000–2009. MMWR Morb Mortal Wkly Rep. 2011;60:989–991. [PubMed] [Google Scholar]
- 11.Coluzzi M, Petrarca V. Aspirator with paper cup for collecting mosquitoes and other insects. Mosq News. 1973;33:249–250. [Google Scholar]
- 12.Coulibaly YI, Dembele B, Diallo AA, Kristensen S, Konate S, Dolo H, Dicko I, Sangare MB, Keita F, Boatin BA, Traore AK, Nutman TB, Klion AD, Touré YT, Traore SF. Wuchereria bancrofti transmission pattern in southern Mali prior to and following the institution of mass drug administration. Parasit Vectors. 2013;6:247. doi: 10.1186/1756-3305-6-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toure YT, Guimogo D, Petrarca V, Traore SF, Bouare M, Dao A, Carnahan C, Taylor C. Mark Release recapture experiments with Anopheles gambiae s.l. in Banambani village, Mali to determine population size and structure. Med Vet Entomol. 1997;12:74–83. doi: 10.1046/j.1365-2915.1998.00071.x. [DOI] [PubMed] [Google Scholar]
- 14.Favia G, Lanfrancotti A, Spanos L, Sidén-Kiamos I, Louis C. Molecular characterization of ribosomal DNA polymorphisms discriminating among chromosomal forms of Anopheles gambiae s.s. Annu Rev Entomol. 1999;44:131–157. [Google Scholar]
- 15.Detinova TS, Rasnitsyn SP, Markovich NIa, Kupriianova ES, Aksenova AS. Unification of the methods of counting the number of blood-sucking dipteric insects. Med Parazitol (Mosk) 1978;47:84–92. [PubMed] [Google Scholar]
- 16.Appawu MA, Dadzie SK, Baffoe-Wilmot A, Wilson MD. Lymphatic filariasis in Ghana: entomological investigation of transmission dynamics and intensity in communities served by irrigation systems in the Upper East Region of Ghana. Trop Med Int Health. 2001;6:511–516. doi: 10.1046/j.1365-3156.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 17.Southgate BA, Bryan JH. Factors affecting transmission of Wuchereria bancrofti by anopheline mosquitoes. 4. Facilitation, limitation, proportionality and their epidemiological significance. Trans R Soc Trop Med Hyg. 1992;86:523–530. doi: 10.1016/0035-9203(92)90096-u. [DOI] [PubMed] [Google Scholar]
- 18.Pichon G. Limitation and facilitation in the vectors and other aspects of the dynamics of filarial transmission: the need for vector control against Anopheles-transmitted filariasis. Ann Trop Med Parasitol. 2002;96((Suppl 2)):S143–S152. doi: 10.1179/000349802125002509. [DOI] [PubMed] [Google Scholar]
- 19.De Souza DK, Koudou B, Kelly-Hope LA, Wilson MD, Bockarie MJ, Boakye DA. Diversity and transmission competence in lymphatic filariasis vectors in West Africa, and the implications for accelerated elimination of Anopheles-transmitted filariasis. Parasit Vectors. 2012;5:259. doi: 10.1186/1756-3305-5-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.