Abstract
A cross-sectional study was conducted to provide comprehensive data on the patterns and associated risk factors of soil-transmitted helminth (STH) infections among five Orang Asli subgroups in Peninsular Malaysia. The overall prevalence of STH infections was 59.9% (95% confidence interval [CI] = 56.1–63.7%). Trichuris trichiura (54.3%; 95% CI = 50.4–58.2%) was the predominant species followed by Ascaris lumbricoides (26.7%; 95% CI = 23.3–30.1%) and hookworm (9.1%; 95% CI = 6.9–11.3%). This study showed diversity for STH infections by subgroup with poverty and personal sanitary behavior as important risk factors for infection. Risk profile analyses indicating that Orang Kuala subgroup who has a generally well-developed infrastructure and better quality of life had a low rate of infection. There is a need for poverty reduction and promotion of deworming programs along with mass scale campaigns to create awareness about health and hygiene to reduce STH infections.
Introduction
Soil-transmitted helminth (STH) infections caused by Ascaris lumbricoides, Trichuris trichiura, and hookworm (Necator americanus and Ancylostoma duodenale) have extensive socioeconomic and public health impacts. These infections are most significant in the bottom billion of the world's poorest people (i.e., income < US $1.25/day).1 Humans are infected by ingestion of parasite eggs or contact with larvae that thrive in the warm and moist soil. Adult worm of STH can live for years in the human gastrointestinal tract. The STH infections are one of the world's most important causes of physical impairment, intellectual and cognitive retardation especially in children, thwarting educational advancement and hindering economic development.1,2 The greatest morbidity is among children and women of childbearing age.2,3 To date, ∼2 billion people or 24% of the world's population are infected with at least one species of STH.4 It is estimated that worldwide annual death from STH infections varies widely, from 12,000 to as many as 135,000 deaths.2,3
Since gaining her independence almost 57 years ago, Malaysia has witnessed rapid growth in socioeconomic and development infrastructure. Nevertheless, the country is still plagued with many parasitic diseases notably the perpetual STH infections, especially among poor and socioeconomically deprived communities, particularly the Orang Asli communities.5–9 The “Orang Asli” translated as “original people“ or “first people” are the indigenous minority people of Peninsular Malaysia. According to the latest available records, their population is ∼149,723, contributing to 0.7% of the total population in Malaysia.10 Officially, the Orang Asli is classified into three major ethno-linguistic groups, namely the Senoi, Proto-Malays (Aboriginal Malays), and Negritos and further divided into six subgroups, bringing to a total of 18 different subgroups.10 They are not a homogenous group as each group has its own language and custom, most importantly, perceiving themselves as different from their counterparts.11 They live in the closest possible association with nature from the coastal mangrove swamps to the tropical forest valleys and mountains throughout Peninsular Malaysia.
According to the available literature, the earliest study of STH infections among Orang Asli was recorded in 1938.12 Up to 2013, more than 30 studies on STH infections had been conducted among the Orang Asli communities. Lim and others6 reviewed and summarized studies that had been conducted since 1930 and found that the prevalence rate of STH infections remains largely unchanged with alarming high prevalence, especially among children. Several studies had been conducted in these communities between 2010 and 2013 reporting an overall prevalence of more than 50%, ranging from 52.4% to 92.2%.7–9 With the exception of a study carried out by Dunn,13 almost all of these studies failed to take into consideration the differences in the respective Orang Asli subgroups. This is regrettable because Orang Asli is not a homogenous group as they are divided into various subgroups with different socio-demographic background.
As highlighted by Lim and others,6 although implementation of socioeconomic development programs have some positive impacts on STH infections in certain subgroups, these outcomes were not noticeable in many other subgroups as STH infections are still highly prevalent in these communities. Hence, the disease dynamics in each subgroup need to be reevaluated as the findings will provide beneficial information for the formulation of highly effective control strategies and policies relevant to the Orang Asli communities. Within this context, we conducted this community-based study to estimate the current status and distribution patterns of STH infections along with its covariates among Orang Asli subgroups in Peninsular Malaysia.
Methods
Ethical approval.
The study protocol was approved by the National Institutes of Health, Ministry of Health (MoH) Malaysia (Reference no.: NMRR-10-801-6816) and the Ethics Committee of the University Malaya Medical Center (UMMC) Malaysia (Reference no.: 824.11).
Study design and area.
A cross-sectional study was carried out between 2009 and 2011 among Orang Asli subgroups in Peninsular Malaysia. Eight accessible villages representing five subgroups were randomly selected using a convenient sampling method from the available official listings provided by the Department of Orang Asli Development, Ministry of Rural and Regional Development, Malaysia taking into consideration the following criteria 1) accessibility by road, 2) must be willing to participate, and 3) size of the village (each village having > 100 participants). Villages were excluded if one of these selection criteria was not fulfilled. Sample size calculation was calculated taking the estimated prevalence at 50% for gives the maximum sample size, with a 95% level of confidence and 5% bound on the error of estimation. The minimum sample size required was 370 participants.
Consent and structured questionnaire.
The village headman was given advance notice of the survey timing and a small meeting was held in the village the day before sampling. Parents and their children were given an oral briefing by the investigator on the objective and methodology of the study. Those who agreed to participate proceeded to sign the consent sheet (i.e., written form), whereas those who were illiterate, provided a verbal consent followed by thumb prints. For children and very old participants, especially in cases of ill adults, the consent was completed by their parents or relevant adults who are usually head of the family. After they had completed their consent forms, the participants were then asked to answer a structured questionnaire. The questionnaire was administered to get information on their demographic, socioeconomic, behavioral, sanitation infrastructure, living conditions including water supply, latrine system, and domestic animals ownership and contact. The questionnaire was administrated to the head of each household in the presence of all his/her family members.
Fecal sample collection and laboratory analysis.
Following completion of the questionnaire, a dry, clean, and leak proof screw capped pre-labeled fecal containers with names and codes were distributed to the participants. The filled containers with fecal samples were collected the next day. Participants who were not able to provide their fecal samples on the first day were then asked to do so during the second collection. The fecal samples were stored at ambient temperature and transported back to the laboratory on the same day of collection. Upon reaching the laboratory, the fecal samples were preserved in 2.5% potassium dichromate and refrigerated at 4°C for microscopy examination. The fecal samples were processed using a standard wet smear and formalin ethyl acetate concentration technique within 24 hours after collection.14 In brief, 1 to 2 g of the fecal sample was mixed with 7 mL of formalin and 3 mL ethyl acetate, centrifuged, examined with normal saline, and stained in 0.85% iodine. The slides were microscopically examined initially under low power (10×) followed by high power (40×) bright field. Detection of STH species was determined on the basis of morphological characteristics of specific species. The result was considered as positive when at least one STH egg or cyst for other intestinal protozoa was observed in any one of the used techniques.
Statistical analysis.
Completed questionnaire and laboratory data were checked regularly to rectify any discrepancy, logical errors, and missing values. The data entry and analysis was carried out using a Statistical Package for the Social Sciences (SPSS) for Windows version 17 (SPSS Inc., Chicago, IL). Categorical variables such as socio-demographic, environmental, personal sanitary behavior, and infection rate were presented as frequencies and percentages. Continuous variable such as age was expressed as median and ranges. Pearson's χ2 (χ2) test on proportion was used to examine the crude associations between binary and independent variables. The level of statistical significance was set as P ≤ 0.05. Additionally, post hoc statistics comparison was also conducted to show how all of the subgroups are compared and which subgroups are significantly different.
A multiple logistic regression analysis was run to explore the independent association of variables with STH infection. All variables with a significant level of P ≤ 0.05 in the univariate analysis were set for multivariable analysis. To ensure that any potentially important predictors were not excluded and also because of a low number of predictor variables, any variables with the borderline significance level of 0.10–0.25 were also included in the multivariate analysis.15 The subset for the final model was then analyzed with multiple logistic regression analysis to test which factors could be dropped from the model and finally determine the risk factors significantly associated with STH infection. The final model was interpreted by using an adjusted odds ratio (OR) and 95% confidence interval (CI).
Results
General characteristics and population structures.
A total of 634 participants from five Orang Asli subgroups participated in this study. Of the 634 participants, 44.0% was Temuan subgroup, followed by Semelai (17.8%), Jakun (15.6%), Orang Kuala (14.0%), and Mah Meri (8.5%). The overall ages ranged from 1 to 80 years with a median age of 11.0 years. By subgroups, the median ages were 11.0, 10.0, 11.0, 11.0, and 13.0 years for Temuan, Semelai, Jakun, Orang Kuala, and Mah Meri, respectively (χ2 = 45.8, P < 0.001) (Table 1). This included 43.5% males and 56.5% females with proportions of 1.7%, 8.8%, 3.9%, 41.3%, 5.2%, and 39.0% for infants (< 1 year), toddlers (1–4 years), preschool children (5–6 years), school-aged children (7–12 years), teenagers (13–17 years), and adults (≥ 18 years), respectively.
Table 1.
Demographic characteristics distribution by Orang Asli subgroups
| Characteristic | Temuan (N = 279) | Semelai (N = 113) | Jakun (N = 99) | Orang Kuala (N = 89) | Mah Meri (N = 54) | Total (N = 634) | *χ2 | P |
|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | |||
| Gender | ||||||||
| Male | 47.7 | 37.2 | 41.4 | 47.2 | 33.3 | 43.5 | ||
| Female | 52.3 | 62.8 | 58.6 | 52.8 | 66.7 | 56.5 | 6.8 | 0.149 |
| Age (years) | ||||||||
| Range | 1–80 | 8–68 | 7–58 | 7–65 | 1–80 | 1–80 | ||
| Median | 11.0 | 10.0 | 11.0 | 11.0 | 13.0 | 11.0 | 45.8 | < 0.001 |
| Age category (years) | ||||||||
| < 1 (infants) | 2.2 | 0 | 3.0 | 0 | 3.7 | 1.7 | ||
| 1–4 (toddlers) | 16.5 | 0 | 6.1 | 0 | 7.4 | 8.8 | ||
| 5–6 (pre-school children) | 7.2 | 0 | 1.0 | 0 | 7.4 | 3.9 | ||
| 7–12 (school aged children) | 20.8 | 69.9 | 36.4 | 80.9 | 31.5 | 41.3 | ||
| 13–17 (teenagers) | 7.5 | 0 | 11.1 | 0 | 1.9 | 5.2 | ||
| 18 and above (adults) | 45.9 | 30.1 | 42.4 | 19.1 | 48.1 | 39.0 | 186.1 | < 0.001 |
Calculated across all subgroups.
Socioeconomic and demographic characteristics.
Socioeconomic and demographic of the populations surveyed were generally poor and appeared to be similar in all subgroups with the exception of Orang Kuala (Table 2). Briefly, each village has a small population and the number of residents in each village was estimated to be 100 to 150. From our personal observation, most of the houses are traditionally made of bamboo or wood planks for the walls and flooring and raised on stilts with a palm roof. With the exemption of Temuan, other subgroups such as Semelai (72.6%), Jakun (79.8%), Orang Kuala (80.9%), and Mah Meri (66.7%) were significantly more likely to receive formal education at least up to the primary level. Further analysis showed that the majority of the Orang Asli children (76.3%) had received some form of formal education such as primary, secondary, or tertiary level, whereas only 23.7% of them never went to school. There was a high number of adults who did not receive formal education (63.6%) leaving only 36.4% who had educational attainment.
Table 2.
Socioeconomic, environmental, and sanitary behavior characteristics distribution by Orang Asli subgroups
| Characteristic | Temuan (N = 279) | Semelai (N = 113) | Jakun (N = 99) | Orang Kuala (N = 89) | Mah Meri (N = 54) | Total (N = 634) | *χ2 | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | |||
| Occupational status | ||||||||||||||
| Working | 92 | 33.0 | 18 | 15.9 | 9 | 9.1 | 7 | 7.9 | 13 | 24.1 | 139 | 21.9 | ||
| Not working | 187 | 67.0 | 95 | 84.1 | 90 | 90.9 | 82 | 92.1 | 41 | 75.9 | 495 | 78.1 | 42.2 | < 0.001 |
| Occupational category | ||||||||||||||
| Not working (housewife/student) | 185 | 66.3 | 95 | 84.1 | 92 | 92.9 | 82 | 92.1 | 41 | 75.9 | 495 | 78.1 | ||
| Jungle product gatherers | 30 | 10.8 | 7 | 6.2 | 7 | 7.1 | 0 | 0 | 12 | 22.2 | 56 | 8.8 | ||
| Palm oil/rubber plantation | 31 | 11.1 | 7 | 6.2 | 2 | 0.7 | 0 | 0 | 0 | 0 | 40 | 6.3 | ||
| Labor (factory/construction sites) | 28 | 10.0 | 4 | 3.5 | 0 | 0 | 7 | 8.5 | 1 | 1.9 | 40 | 6.3 | ||
| Small business (mini sundry shop) | 2 | 0.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3.2 | ||
| Government employee | 1 | 0.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 | ||
| Others (driver, etc.) | 2 | 0.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3.2 | 140.4 | < 0.001 |
| Household income (RM/month) | ||||||||||||||
| < RM 500 (< US $166.7) | 152 | 56.6 | 100 | 88.5 | 86 | 86.9 | 0 | 0 | 32 | 59.3 | 376 | 59.3 | ||
| > RM 500 (> US $166.7) | 121 | 43.4 | 13 | 11.5 | 13 | 13.1 | 89 | 100 | 22 | 40.7 | 258 | 40.7 | 201.6 | < 0.001 |
| Source of water supply | ||||||||||||||
| Untreated (river, mountain water, etc.) | 191 | 68.5 | 90 | 79.6 | 8 | 8.1 | 0 | 0 | 0 | 0 | 289 | 45.6 | ||
| Treated (government pipe water) | 88 | 31.5 | 23 | 20.4 | 91 | 91.9 | 89 | 100 | 54 | 100 | 345 | 54.4 | 287.6 | < 0.001 |
| Latrine facilities | ||||||||||||||
| No | 175 | 62.7 | 76 | 67.3 | 48 | 48.5 | 0 | 0 | 42 | 77.8 | 341 | 53.8 | ||
| Yes | 104 | 37.3 | 37 | 32.7 | 51 | 51.5 | 89 | 100 | 12 | 22.2 | 293 | 46.2 | 134.4 | < 0.001 |
| Type of latrine | ||||||||||||||
| Pour flush toilet | 94 | 33.7 | 2 | 1.8 | 20 | 20.2 | 89 | 100 | 24 | 44.4 | 229 | 36.1 | ||
| Pit latrine/non-pour flush toilet | 51 | 18.3 | 50 | 44.2 | 48 | 48.5 | 0 | 0 | 1 | 1.9 | 150 | 23.7 | ||
| Bush | 100 | 35.8 | 23 | 20.4 | 25 | 25.3 | 0 | 0 | 28 | 51.9 | 176 | 27.8 | ||
| River | 34 | 12.2 | 38 | 33.8 | 6 | 6.1 | 0 | 0 | 1 | 1.9 | 79 | 12.5 | 332.1 | < 0.001 |
| Defecation sites | ||||||||||||||
| Open/indiscriminate | 201 | 72.0 | 113 | 100 | 76 | 76.8 | 0 | 0 | 17 | 31.5 | 407 | 64.2 | ||
| Latrine | 78 | 28.0 | 0 | 0 | 23 | 23.2 | 89 | 100 | 37 | 68.5 | 227 | 35.8 | 262.0 | < 0.001 |
| Walking bare-footed | ||||||||||||||
| No | 136 | 48.7 | 45 | 39.8 | 38 | 38.4 | 0 | 0 | 26 | 48.1 | 245 | 38.6 | ||
| Yes | 143 | 51.3 | 68 | 60.2 | 61 | 61.6 | 89 | 100 | 28 | 51.9 | 389 | 61.4 | 70.0 | < 0.001 |
| Eat with hand without prior washing | ||||||||||||||
| Yes | 261 | 93.5 | 107 | 94.7 | 99 | 100.0 | 10 | 11.2 | 54 | 100 | 531 | 83.8 | ||
| No | 18 | 6.5 | 6 | 5.3 | 0 | 0 | 79 | 88.8 | 0 | 0 | 103 | 16.2 | 403.2 | < 0.001 |
| Close contact with domestic animals | ||||||||||||||
| Yes | 184 | 65.9 | 113 | 100 | 97 | 98.0 | 80 | 89.9 | 43 | 79.6 | 517 | 81.5 | ||
| No | 95 | 34.1 | 0 | 0 | 2 | 2.0 | 9 | 10.1 | 11 | 20.4 | 117 | 18.5 | 92.7 | < 0.001 |
| Garbage disposal | ||||||||||||||
| Indiscriminate | 145 | 52.0 | 55 | 48.7 | 45 | 45.4 | 57 | 64.0 | 28 | 51.9 | 330 | 52.1 | ||
| Collected | 134 | 48.0 | 58 | 51.3 | 54 | 54.4 | 32 | 36.0 | 26 | 48.1 | 304 | 47.9 | 7.4 | 0.017 |
Calculated across all subgroups.
More than half of the overall population surveyed was unemployed. It was also noted that most of the housewives normally helped their husbands in the oil palm or rubber plantations or go into the jungle to collect jungle products to supplement their family income. As for the used participant, a majority of them were engaged with odd jobs such as jungle product collector (8.8%) who often entered the forest for a varying length of time without permanent income. There was 6.3% who were working as unskilled laborers in an oil palm plantation, factory, and construction site with daily income. Only one (0.2%) was a government employee. Further comparisons of all the subgroups using post hoc statistics showed that among the surveyed working respondents, there were significantly fewer Jakun than would be expected compared with other groups.
Semelai and Jakun subgroups were predominantly poor and 88.5% and 86.9% had a monthly household income < RM 500 respectively followed by the Mah Meri subgroup (59.3%) and Temuan (56.6%). Orang Kuala had a monthly household income of > RM 500. Further post hoc analysis supported that among the surveyed respondents, there was a statistically significant fewer Orang Kuala who earned < RM 500 compared with other groups.
Although more than half of the populations surveyed had a treated water supply (54.4%), it was not fully used. Most of their water supply was terminated as a result of outstanding bills. Rivers located nearby to the village remain the main source of water supply for their domestic needs including drinking, cooking, bathing, and washing. Temuan (68.5%) and Semelai (79.5%) had the least number of treated water supplies at home, whereas all Orang Kuala families had a treated water supply. A single latrine usually serves a few families, but it is not frequently used because of poor maintenance. Seventy-seven point eight percent (77.8%) of the Mah Meri families did not have toilet facilities at home, followed by Semelai (67.3%), Temuan (62.7%), and Jakun (51.5%). Further analysis as assessed using post hoc statistics of all the groups showed that among the respondents surveyed, there was significantly fewer Temuan who were using a treated water supply and did not have toilet facilities at home compared with other groups. Post hoc analysis showed that among the respondents surveyed, there was significantly fewer Semelai who were using a proper latrine as the defecation sites compared with other groups.
Temuan, Semelai, Jakun, and Mah Meri were unlikely to wear shoes or sandals outside the house. Eating with hands without proper hand washing and walking barefoot outside the house was observed as general and routine practices across all subgroups. Post hoc comparison revealed that among respondents surveyed, Orang Kuala had significantly fewer respondents walking barefoot and washing hands properly before eating compared with other groups. Dogs and cats are the most common domestic animals. Others include poultry, monkeys, rabbits, and birds. These animals are usually left to roam freely. Further comparisons of all the subgroups using post hoc statistics showed that among the survey respondents, there was significantly fewer Semelai who had close contact with domestic animals than would be expected compared with other groups.
Prevalence of STH infections.
The overall prevalence of STH infections was 59.9% (95% CI = 56.1–63.7%) with T. trichiura (54.3%; 95% CI = 50.4–58.2%) being the predominant species followed by A. lumbricoides (26.7%; 95% CI = 23.3–30.1%), whereas hookworm (9.1%; 95% CI = 6.9–11.3%) had the lowest infection rate (Table 3). Prevalence of other intestinal parasites was 9.5% for Giardia spp. and 9.1% for Entamoeba spp. (data not shown). The distribution of STH infections was analyzed according to the Orang Asli subgroups. Mah Meri had the highest prevalence of STH infections, whereas Orang Kuala had the lowest prevalence rates (P < 0.05).
Table 3.
Prevalence of soil-transmitted helminth (STH) infections according to population characteristic
| Characteristic | T. trichiura | A. lumbricoides | Hookworm | Any STH | ||||
|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Survey population | 54.3 | 50.3–58.2 | 26.7 | 23.3–30.1 | 9.1 | 7.0–11.7 | 59.9 | 56.0–63.8 |
| Subgroup | ||||||||
| Mah Meri | 66.7 | 52.5–78.9 | 40.7 | 27.6–55.0 | 3.7 | 0.5–12.8 | 75.9 | 64.5–87.3 |
| Temuan | 65.9 | 60.1–71.5 | 28.7 | 23.4–34.4 | 11.8 | 8.3–16.2 | 72.0 | 66.4–77.2 |
| Jakun | 59.6 | 49.3–69.3 | 26.3 | 24.8–47.7 | 8.1 | 3.6–15.3 | 64.6 | 54.4–74.0 |
| Semelai | 54.9 | 45.2–64.3 | 36.3 | 27.5–45.9 | 12.4 | 6.9–19.9 | 61.9 | 52.3–71.0 |
| Orang Kuala | 3.4 | 0.7–9.5 | 0 | 0–4.1 | 1.1 | 0.03–6.1 | 4.5 | 1.2–11.1 |
| χ2 | 112.7 | 43.7 | 12.8 | 140.8 | ||||
| P | < 0.001 | < 0.001 | 0.012 | < 0.001 | ||||
STH = soil-transmitted helminth.
There was no significant difference in the prevalence between used and unemployed persons for all STH species. With the exception of hookworm infection, persons from a low household income family were more likely to be infected with T. trichiura, A. lumbricoides, and any STH infections (P < 0.05). The prevalence of all STH infections (i.e., T. trichiura, A. lumbricoides, hookworm, and any STH species) was higher among those who used an untreated water supply (P < 0.05). The prevalence of any STH species was greater in households with no toilet facilities at home and defecating indiscriminately (P < 0.05). Prevalence of hookworm infection was significantly higher among those who had close contact with animals.
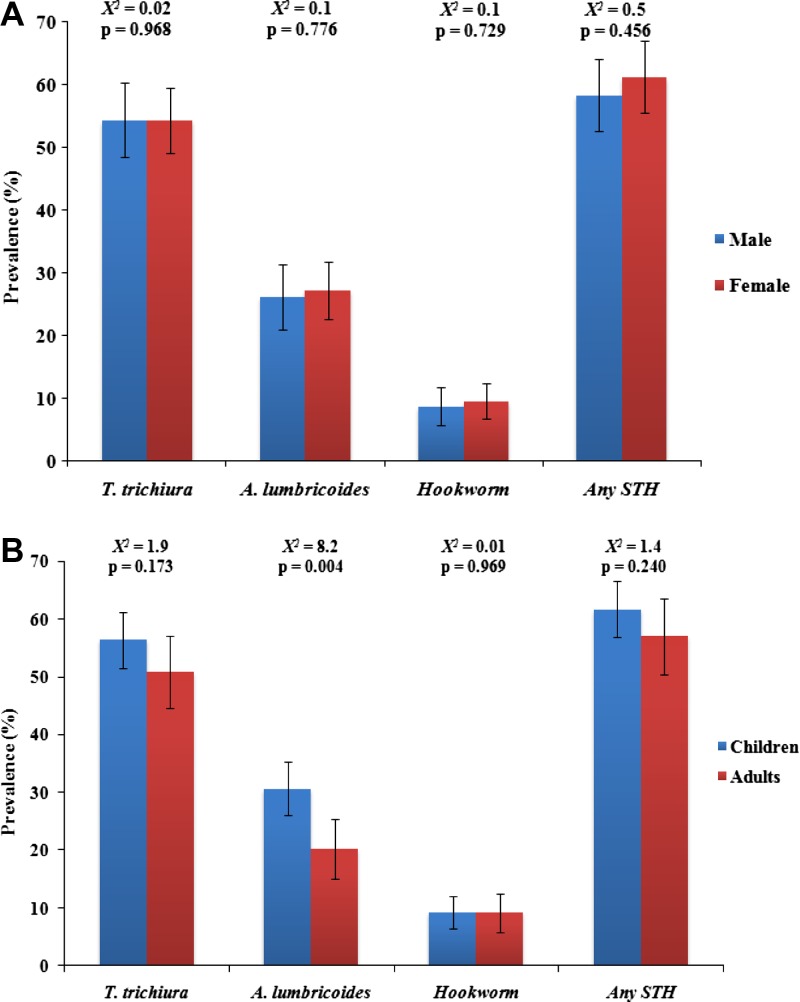
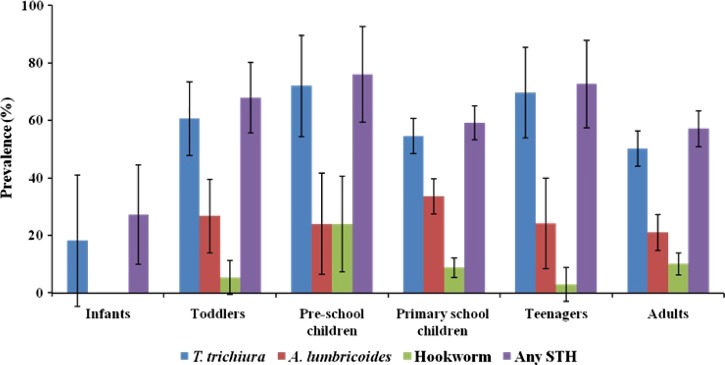
There was no significant difference in the prevalence of all STH species between males and females (Figure 1A ) and between children (≤ 12 years of age) and adults (≥ 13 years of age) with the exception of A. lumbricoides infection (χ2 = 8.2, P = 0.004) (Figure 1B). The prevalence of STH infections was further analyzed according to infant, toddler, preschool children, school-aged children, teenagers, and adults. There was a significant difference between age groups and all types of STH infections (Figure 2 ). With the exception of A. lumbricoides, the prevalence of T. trichiura, hookworm, and any STH species were higher in preschool children (P < 0.05). Although the peak prevalence of A. lumbricoides infection was high among primary school children, it appears to decrease significantly with age.
Figure 1.
Prevalence of soil-transmitted helminth (STH) infections at the community level by (A) gender and (B) age groups. Error bars represent 95% confidence intervals.
Figure 2.
Trend distribution of soil-transmitted helminth (STH) infections at the community level by age category.
Patterns of STH polyparasitism.
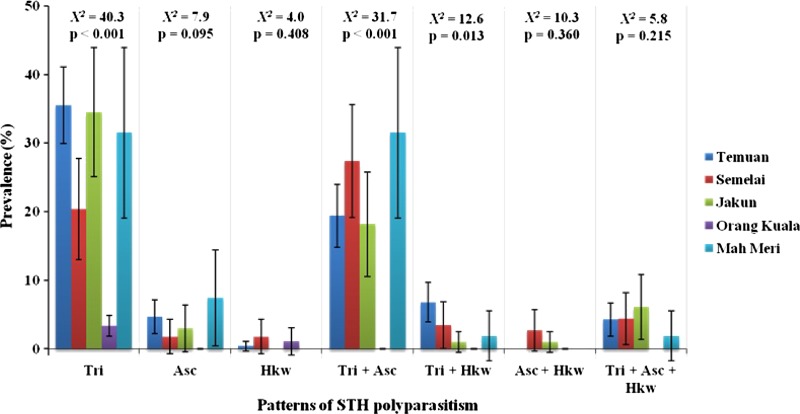
Based on the total sample size of 634 participants, single infection (31.9%; 95% CI = 28.3–35.5%) was the most common followed by double infections (23.5%; 95% CI = 20.2–26.8%) and triple infections (2.8%; 95% CI = 1.5–4.1%) (data not shown). Single infection with T. trichiura (27.8%) was the most predominant followed by A. lumbricoides (3.5%), whereas single infection with hookworm accounted for only 0.6%. Overall prevalence of double infections with T. trichiura and A. lumbricoides (18.9%) was the most common followed by a combination of T. trichiura and hookworm (3.9%) and A. lumbricoides with hookworm (0.6%). The overall prevalence of triple infections with all three STH species was 3.8%. Further analysis based on subgroups showed that the prevalence of single infection with T. trichiura, double infections of T. trichiura with A. lumbricoides and T. trichiura with hookworm were significantly lower in Orang Kuala when compared with other subgroups (Figure 3).
Figure 3.
Prevalence of single, double, and triple infections of soil-transmitted helminth (STH) at the community level by subgroups.
Risk factors of STH infections.
Results of multivariate analyses for the associations of STH infections with socio-demographic, environmental sanitation, and lifestyle characteristics of the population surveyed are shown in Table 4. Low household income family, persons who defecated openly or indiscriminately, walking barefoot outside the house, not washing hands before eating, and close contact with domestic animals were significantly associated with STH infections in Temuan. The final multivariate model confirmed that low family income was at 1.6 higher odds to have STH infections (95% CI = 1.34–2.03; P = 0.050). Households with no toilet facility and persons walking barefoot were also at two times (95% CI = 1.13–3.62; P = 0.018) and 1.8 times (95% CI = 1.01–3.06; P = 0.047) more likely to have STH, respectively. Similarly, close contact with domestic animals was at 1.4 greater odds (95% CI = 1.24–1.74; P = 0.002) to have STH infections.
Table 4.
Risk factors significantly associated with soil-transmitted helminth (STH) infections among the Orang Asli subgroups as determined by multivariate logistic regression model
| Significant risk factors | OR (95% CI) | P |
|---|---|---|
| Temuan subgroup* | ||
| Low household income family | 1.68 (1.32–2.84) | 0.045† |
| No toilet at home | 2.16 (2.03–3.44) | 0.024† |
| Open/indiscriminate defecation | 1.24 (1.02–1.50) | 0.015 |
| Walking barefoot outside the house | 1.37 (1.10–1.87) | 0.032† |
| Not wash hand before eating | 3.10 (1.73–13.19) | 0.010† |
| Close contact with domestic animal | 2.23 (1.30–3.82) | 0.003† |
| Semelai subgroup‡ | ||
| Being a child | 19.91 (7.00–56.66) | < 0.001† |
| No formal education | 2.35 (1.61–3.42) | < 0.001 |
| Non working parents | 1.40 (1.13–1.73) | < 0.001 |
| Low household income family | 4.37 (1.25–15.22) | 0.014 |
| Untreated water supply | 2.17 (1.95–2.45) | 0.018† |
| Not wash hand before eating | 3.07 (1.37–25.41) | 0.020 |
| Jakun subgroup§ | ||
| Being a child | 1.28 (1.07–3.52) | 0.027 |
| Walking barefoot outside the house | 2.05 (1.06–3.98) | 0.019† |
| Close contact with domestic animal | 1.06 (1.01–1.15) | 0.048 |
| Mah Meri subgroup¶ | ||
| Being a child | 4.71 (1.12–19.70) | 0.026 |
| Non working parents | 1.54 (1.19–2.60) | 0.033† |
| Low household income family | 3.09 (1.85–11.21) | 0.030 |
| Defecated openly/indiscriminately | 5.69 (1.49–21.72) | 0.007 |
| Walking barefoot outside the house | 2.43 (1.87–6.80) | 0.038 |
| Close contact with domestic animal | 4.23 (1.57–11.42) | < 0.001† |
Reference groups marked as odds ratio (OR) = 1: high household income (> RM 500 per month); presence of toilet at home; proper latrine; wear shoes/sandal; wash hand properly before eating; absence/no contact with dogs or cats.
Variables were confirmed by multiple logistic regression analysis as significant predictors of STH.
Reference groups marked as OR = 1: adults (≥ 13 years); parent's educational attainment (at least primary school); working parents; high household income (> RM 500 per month); treated/government water supply; wash hands properly before eating.
Reference groups marked as OR = 1: adults (≥ 13 years); wear shoes/sandals; wash hands properly before eating; absence/no contact with dogs or cats.
Reference groups marked as OR = 1: adults (≥ 13 years); working parents; high household income (> RM 500 per month); proper latrine; wear shoes/sandals; wash hands properly before eating; absence/no contact with dogs or cats.
In Semelai, univariate analysis showed that being a child, no formal education, non-working parents, low household income family, and not washing hands before eating were significantly associated with STH infections. Further analysis showed that being a child had 22.4 greater odds (95% CI = 7.56–66.16; P < 0.001) to have STH infections. Likewise, those who used an untreated water supply for their daily chores were three times (95% CI = 1.96–9.41; P < 0.049) more likely to suffer from STH infections as compared with those who used a clean and treated water supply.
In the Jakun subgroup, being a child, walking barefoot, and close contact with domestic animals were significantly associated with the odds of STH infections. The final multivariate model indicated that persons walking barefoot were three times (95% CI = 1.78–7.54; P = 0.021) more likely to have STH infections than those who wore shoes. For Orang Kuala, no risk factors were significantly associated with STH infections. In Mah Meri, six risk factors were significantly associated with STH infections including being a child, non-working parents, low household income family, defecating indiscriminately, walking barefoot, and close contact with domestic animals. Of these six significant risk factors, two were retained in the final multivariate model. The final model showed non-working parents were at 10.4 greater odds (95% CI = 1.89–121.97; P = 0.050) to have STH infections. Similarly, persons who had close contact with domestic animals were also at higher odds to have STH infections (95% CI = 2.14–2.73; P < 0.001).
Discussion
Findings of the current study showed that STH infections are still endemic and continue to be a major public health among the Orang Asli communities in Malaysia with 59.9% of participants infected with at least one STH species. As reviewed by Lim and others,6 the high prevalence of STH infections as reported here was consistent with the previous studies conducted among Orang Asli communities. More than 90% of the studies conducted in the past had reported prevalence rates of more than 50%. Recent studies conducted by Nasr and co-workers.9 among Orang Asli communities reported similar findings with prevalence rates of 78%. Likewise, several studies conducted in recent years reported a high prevalence of STH infections, which ranges from 90% and up to 100% among Orang Asli communities.5–9
Of the three STH species, T. trichiura infection was the most predominant, followed by A. lumbricoides infection and hookworm infection. These findings were consistent with other local studies conducted among the Orang Asli communities where T. trichiura infection had the highest prevalence (range: 26% to 98.2%), followed by A. lumbricoides infection (range: 19% to 67.8%) and hookworm infection (range: 3% to 37%).5–9 Hence, the findings in this study not only show that the prevalence of STH remains high but the distribution pattern of STH also remains unchanged among the Orang Asli communities. However, global data have indicated that A. lumbricoides infection was more prevalent.4,16
A particular observation was the high prevalence of T. trichiura infection either as a single infection or mixed with A. lumbricoides. As both species have a similar mode of transmission, this could be the reason for the high prevalence of these two species. Another possible reason is the low efficacy or choice of anthelminthic drugs against T. trichiura as reported previously.17,18 A combination of drugs with different modes of action as an alternative therapy to improve drug efficacy is highly recommended. Study has shown that a combination of more than one anthelminthic drug is more effective against STH infections than any single drug.18 However, the use of drug combinations as therapeutic agents against STH infections is not practiced in the Orang Asli communities.7 During each visit to the rural clinics in the Orang Asli villages, either albendazole or mebendazole and not both are available at any one time. This means that the Orang Asli is exposed to a single drug treatment of a long duration that could eventually cause ineffective treatment leading to development of drug resistance.19
Age is an important risk factor for STH infections and the preschool and school going children have been reported to be at highest risk of infections.20,21 In our study, significantly high prevalence of T. trichiura and A. lumbricoides were recorded among school children. Our finding was in agreement with the previous epidemiological studies conducted in Malaysia5–9 and abroad.4,22,23 This could be because as the child grows older, the exposure to the sources of infection increases. As for preschool and school-going children, they are more independent, active, and inquisitive and are interested in learning new things in their surroundings. As a result of their young age, many are still not fully aware of personal hygiene and good cleanliness practices or realize the consequence of exposing themselves to pathogenic organisms. The linear association of age within this age group needs further exploration through prospective studies.
Many past epidemiological studies were also focused on heterogeneity in the intensity of STH infections by age.20 Although STH can infect all members of a population, it is clear that there are specific groups who are at greater risk of heavy infections. The distribution of STH infections is strongly age dependent.21 Children 5 to 15 years of age have the most severe infection (i.e., high worm burden and frequency) particularly A. lumbricoides and T. trichiura infections, but declines significantly in adulthood.24 In contrast, hookworm frequently exhibits a steady rise in intensity and frequency of infection with age, peaking in adulthood. It is generally thought that the differences in the levels of hookworm infections are caused by the differences in exposure, as hookworm is generally transmitted in the fields or plantations as opposed to T. trichiura and A. lumbricoides, which are generally transmitted near homes.20
It is evident from this study that the socioeconomic status (SES), environmental and sanitary behavior of the Orang Asli communities was generally poor and appeared to be similar across Temuan, Semelai, Jakun, and Mah Meri subgroups with the exception of Orang Kuala subgroup. Lower SES and poverty were also significant risk factors for STH infections.22,25,26 Non-working parents and family with low household income were taken as a proxy measure of poverty, which was also significantly associated with STH infections. This study reported low household income was a significant predictor for STH infections among Temuan, Semelai and Mah Meri subgroups. Similar findings have also been reported that non-working parents and low household income are significant predictors for acquiring STH infections among Orang Asli communities.5–9
The effect of SES on risk of parasitic diseases in general and STH infections in particular, is complex in nature and could be attributed to several other factors such as lack of access to a clean water supply, poor personal and sanitary hygiene, inadequate basic infrastructure, low level of education attainment caused by financial constraints, overcrowding, and impoverished health services.22,27 In such conditions, STH species are commonly co-endemic.28 As a result, the prevalence and distribution of STH may differ from one region to another and sometimes within the country itself because of the SES and other associated risk factor variations.28 Previous local studies have shown that there was a web of risk factors associating high prevalence and intensity of STH infections with poverty, low level of parental education, and lack of access to treated water among Orang Asli communities,5–9 findings that were consistent with this study. Essentially, improvement in sanitation and provision of a safe water supply are necessary for effective control of STH infections.
Poor environmental sanitation, personal and sanitary behavior including defecation practices, lack of footwear, not washing hands before eating are well documented as significant risk factors for transmission of various infectious diseases including parasitic infections.4,26,29 In this study, Temuan, Jakun, Mah Meri, and Semelai children were noted as defecating indiscriminately close to their houses or within the village confines. Although some parents may advise and discourage their children from defecating in the river, this instruction does not prevent the water from being contaminated with fecal material as children cleanse themselves in the river after defecation. This may indirectly contaminate the water and elevates the risk of contamination. As a majority of the Temuan, Jakun, Mah Meri, and Semelai subgroups still rely on the streams located adjacent to their villages as the major source of water supply, contaminated water could increase the likelihood of transmission of STH infections. Thus, it was not surprising that the prevalence of STH infections was significantly higher among Semelai who still rely on the untreated river water supply for their domestic needs. The finding was consistent with the previous studies conducted among the Orang Asli communities in Malaysia.5–9
The highest rates of indiscriminate defecation were observed among the Temuan and Mah Meri subgroups. It was not surprising that indiscriminate defecation was a significant predictor of infections among the Temuan and Mah Meri subgroups. As a household-based toilet facility was almost nonexistent among these communities, a single pit latrine is usually shared between a few families. It was not frequently used because of poor maintenance (e.g., dirty and foul smelling), which encourages indiscriminate defecation in bushes or river. Those without proper toilet facilities at home were more likely to defecate in the river, pit latrines, and bushes. Pit latrines are usually dug several meters into the ground. These provide direct sources of contamination of the subterraneous water flow that passes through the fecal-infected soil into the river, as shown in several surveys assessing the quality of water from pit latrines in other countries.26,30,31 A recent study to determine the occurrences of Giardia spp. and Cryptosporidium spp. in the river water in Orang Asli communities showed a high concentration of oo (cysts) downstream where most villages are located.32 Thus, further studies must be carried out with regards to the impact of pit latrines on river water quality and confirmation of actual contamination.
Walking barefoot outside the house and presence of free-roaming domestic animals were found to be significantly associated with STH infections, particularly among Temuan, Jakun and Mah Meri subgroups. In this study, many households reported that children often play outside the house barefoot. In many other studies, lack of footwear in association with the presence of free-roaming dogs provide a strong predictor for STH infections, particularly hookworm.33,34 Moreover, more studies are reporting on the zoonotic ancylostomiasis caused by A. ceylanicum in humans in endemic areas in Southeast Asia.23,34–36
A recent genetic study to provide further evidence on zoonotic transmission of A. ceylanicum between humans and animals in Malaysia showed that some of the A. ceylanicum strains from both the human and animal host in the same geographical location are clustered together within the same group.37 This provides evidence that dogs and humans share genetically similar genotypes of A. ceylanicum within the same geographical location. These findings confirmed the previous epidemiological survey indicating that individuals who had close contact with dogs were more likely to be infected with A. ceylanicum.33–35 Although the notion of zoonotic transmission of A. lumbricoides and T. trichiura between humans and dogs is still largely unclear, these animals may act as significant mechanical transmitter of ascariasis in the human population especially in communities where the habit of indiscriminate defecation exist. Molecular-based tool studies have shown that dogs act as disseminators and environmental contaminators of A. lumbricoides in communities where indiscriminate defecation is common.38,39
Findings from this study provide a community-based picture of the current status of STH infections in different Orang Asli subgroups with low SES, poor environmental sanitation, and personal hygiene. There are some other factors such as geographical factors including temperature, vegetation, humidity caused by altitude, population density, and land availability around the village that may result in underestimation of the prevalence and influence of the distribution of the infection.40 In an extensive study carried out by Dunn in 1972 among various Orang Asli living in different forest altitudes showed that groups living at higher and cooler elevations have fewer STH infections probably caused by the lower soil temperature, which may reduce the egg embryonation rate.4,13 Although these variables are not being incorporated into this study, they constitute the aim for future work. More recently, mapping and modeling distribution of STH using geographical information system coupled with remote sensing data in Malaysia reported that temperature and vegetation are significant explanatory variables for A. lumbricoides infection.41
This study has certain limitations that need to be considered while interpreting the results. First, single fecal collection was conducted, which may have underestimated the true prevalence as optimal laboratory diagnosis for STH infections requires multiple fecal examinations of at least three specimens collected over several days.42 Several studies have suggested that examination of three fecal samples resulted in the detection of up to 90% more infections.42,43 Second, only one examination technique method was used, i.e., formalin ethyl acetate concentration technique without egg count. This method alone has poor sensitivity for diagnosing STH infections. It indicates that results obtained are an underestimation of true prevalence, whereby only high and moderate intensity infections are more sensitive for the parasite to be detected.44 Hence, a more accurate estimated prevalence of STH infections would have been obtained if a multiple fecal sample collection and a more sensitive diagnostic protocol such as the Kato-Katz method was used. The most recent study comparing the accuracy of the formalin ether concentration and Kato-Katz techniques showed that Kato-Katz has greater sensitivity in estimating STH infections.45
Third, the inability to control interviewer bias during administration of the questionnaire as more than one interviewer was used in this study. Nevertheless, attempts were made to minimize and control these biases through a series of interview training workshop before the commencement of this study. Additionally, the same interviewers who are familiar with the questionnaires were used throughout this study. In future work, using members of the communities themselves as interviewers may provide unbiased data and allow the participants to be more cooperative and feeling more at ease. Fourth, the risk factor analysis showed a large CI value indicating that the sample size was relatively low. Based on the World Health Organization (WHO) guidelines for evaluating STH at the community level, a total sample size of 200–250 participants would be required to accurately determine the prevalence of STH infections in an ecologically similar environment.23 Nevertheless, several risk factors were able to be detected that were significantly associated with STH infections, although the CI values for the risk analysis were wide. Finally, the inability to draw conclusions on causality of association of different risk factors with STH infections is a common limitation in a cross-sectional study design.
In conclusion, STH infections are still highly prevalent among Orang Asli communities. With the exception of the Orang Kuala subgroup, low SES and poor sanitary behavior are important factors associated with STH infections in Orang Asli communities. Age is also an important predictor for STH infections, particularly among preschool and school-going Orang Asli children. This supports an urgent need to start an integrated and effective control program such as a poverty reduction program, implementing mass scale deworming and health promotions campaign to create awareness about health and hygiene. Findings of this study will assist public health authorities to justify the reassessment of the existing control measures that are relevant to each subgroup. With effective control measures in place, the Orang Asli communities, especially children will have a greater opportunity for a better future in terms of health and educational attainment. These measures will eventually raise their health status and put them on par socially and economically with the general communities in Malaysia.
ACKNOWLEDGMENTS
We express our gratitude to the Ministry of Rural and Regional Development, Malaysia and Head of Orang Asli villages for approving and giving the permission to conduct this study. Special thanks also go to all the villagers who have voluntarily participated in this study and to Wan Hafiz Wan Ismail and Kaharmuzakir Ismail for their assistance during the fieldtrip.
Footnotes
Financial support: This study was supported by the UM/MoHE High Impact Research (H-20001-00-E000061), E-Science (16-02-03-6034), and BKP 007-2014 grant. The funders had no role in study design, data collection, analysis and preparation, or decision to publish the manuscript.
Authors' addresses: Romano Ngui, Roslan Muhammad Aidil, Soo Ching Lee, Tiong Kai Tan, Mahmud Rohela, and Yvonne A. L. Lim, Department of Parasitology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia, E-mails: romano@um.edu.my, aidil_lanang@ymail.com, eustacialee@gmail.com, stanley.monas@gmail.com, arineahmad@um.edu. my.rohela@ummc.edu.my, and limailian@um.edu.my. Shafie Aziz, Department of Geography, Faculty of Arts and Social Sciences, University of Malaya, Kuala Lumpur, Malaysia, E-mail: azizs@um.edu.my. Kek Heng Chua, Department of Biomedical Science, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia, E-mail: khchua@um.edu.my. Mistam Mohd Sani, Department of Orang Asli Development, Ministry of Rural and Regional Development, Wisma Selangor Dredging, Jalan Ampang, Kuala Lumpur, Malaysia, E-mail: romano_sky05@yahoo.com.
References
- 1.Liese B, Rosenberg M, Schratz A. Programmes, partnerships and governance for elimination and control of neglected tropical diseases. Lancet. 2010;375:67–76. doi: 10.1016/S0140-6736(09)61749-9. [DOI] [PubMed] [Google Scholar]
- 2.WHO . Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis. Geneva, Switzerland: World Health Organization; 2002. World Health Organization (WHO) Technical Series Report 912. [PubMed] [Google Scholar]
- 3.WHO . World Health Organization (WHO) Report of the Third Global Meeting of the Partners for Parasite Control. Geneva, Switzerland: World Health Organization; 2005. Deworming for health and development. [Google Scholar]
- 4.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. Soil-transmitted helminthes infections: ascariasis, trichuriasis and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 5.Al-Mekhlafi HM, Atiya AS, Lim YA, Mohammed Mahdy AK, Wan Ariffin WA, Surin J. An unceasing problem: soil-transmitted helminthiases in rural Malaysian communities. Southeast Asian J Trop Med Public Health. 2007;38:998–1008. [PubMed] [Google Scholar]
- 6.Lim YAL, Romano N, Colin N, Chow SC, Smith HV. Intestinal parasitic infections among Orang Asli (indigenous) in Malaysia: has socioeconomic development alleviated the problem? Trop Biomed. 2009;26:110–122. [PubMed] [Google Scholar]
- 7.Ngui R, Saidon I, Chow SK, Rohela M, Lim YAL. Prevalence and risk factors of intestinal parasitism in rural and remote west Malaysia. PLoS Negl Trop Dis. 2011;5:e974. doi: 10.1371/journal.pntd.0000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed A, Al-Mekhlafi HM, Choy SH, Ithoi I, Al-Adhroey AH, Abdulsalam AM, Surin J. The burden of moderate-to-heavy soil-transmitted helminth infections among rural Malaysian aborigines: an urgent need for an integrated control programme. Parasit Vectors. 2011;4:242. doi: 10.1186/1756-3305-4-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasr NA, Al-Mekhlafi HMS, Ahmed A, Roslan MA, Bulgiba A. Towards an effective control programme of soil-transmitted helminth infections among Orang Asli in rural Malaysia. Part 1: prevalence and associated key factors. Parasit Vectors. 2013;6:27. doi: 10.1186/1756-3305-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anon . Data Maklumat Asas Jabatan Hal Ehwal Orang Asli Malaysia. Jabatan Hal Ehwal Orang Asli (JHEOA) Kuala Lumpur, Malaysia: 2006. [Google Scholar]
- 11.Nicholas C. The Orang Asli and the Contest for Resources: Indigenous Politics, Development and Identity in Peninsular Malaysia. Copenhagen, Denmark: IWGIA Publication; 2000. [Google Scholar]
- 12.Nevin HM. Health Survey. Ipoh Branch Laboratory; Malaya States: 1938. pp. 145–148. Rept Inst Med Res Fed. [Google Scholar]
- 13.Dunn FL. Intestinal parasites in Malayan aborigines (Orang Asli) Bull World Health Organ. 1972;46:99–113. [PMC free article] [PubMed] [Google Scholar]
- 14.Cheesbrough M. Parasitological Tests: District Laboratory Practice in Tropical Countries, Part 1. Cambridge, United Kingdom: Cambridge University Press; 1998. [Google Scholar]
- 15.Bendel RB, Afifi AA. Comparison of stopping rules in forward “stepwise” regression. J Am Stat Assoc. 1977;72:46–53. [Google Scholar]
- 16.Yap P, Du ZW, Chen R, Zhang LP, Wu FW, Wang J, Wang XZ, Zhou H, Zhou XN, Utzinger J, Steinmann P. Soil-transmitted helminth infections and physical fitness in school-aged Bulang children in southwest China: results from a cross-sectional survey. Parasit Vectors. 2012;5:50. doi: 10.1186/1756-3305-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett A, Guyatt H. Reducing intestinal nematode infection: efficacy of albendazole and mebendazole. Parasitol Today. 2000;6:71–74. doi: 10.1016/s0169-4758(99)01544-6. [DOI] [PubMed] [Google Scholar]
- 18.Albonico M, Bickle Q, Ramsan M, Montresor A, Savioli L, Taylor M. Efficacy of mebendazole and levamisole alone or in combination against intestinal nematode infections after repeated targeted mebendazole treatment in Zanzibar. Bull World Health Organ. 2003;81:343–352. [PMC free article] [PubMed] [Google Scholar]
- 19.Chan L, Kan SP, Bundy DA. The effect of repeated chemotherapy on the prevalence and intensity infection of Ascaris lumbricoides and Trichuris trichiura. Parasitol. 1992;104:371–377. doi: 10.1017/s0031182000061837. [DOI] [PubMed] [Google Scholar]
- 20.Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford. United Kingdom: Oxford University Press; 1991. [Google Scholar]
- 21.Chan L, Bundy DA, Kan SP. Aggregation and predisposition to Ascaris lumbricoides and Trichuris trichiura at the familial level. Trans R Soc Trop Med Hyg. 1994;88:46–48. doi: 10.1016/0035-9203(94)90492-8. [DOI] [PubMed] [Google Scholar]
- 22.Mehraj V, Hatcher J, Akhtar S, Rafique G, Beg MA. Prevalence and factors associated with intestinal parasitic infection among children in an urban slum of Karachi. PLoS ONE. 2008;3:e3680. doi: 10.1371/journal.pone.0003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conlan JV, Khamlome B, Vongxay K, Elliot A, Pallant L, Sripa B, Blacksell SD, Fenwick A, Thompson RC. Soil-transmitted helminthiasis in Laos: a community-wide cross-sectional study of humans and dogs in a mass drug administration environment. Am J Trop Med Hyg. 2012;86:624–634. doi: 10.4269/ajtmh.2012.11-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilles H. Soil-transmitted helminths (geohelminths) In: Cook G, editor. Tropical Diseases. London, United Kingdom: Manson's. W.B. Saunders: 1996. pp. 1369–1412. [Google Scholar]
- 25.Nematian J, Nematian E, Gholamrezanezhad A, Asgari AA. Prevalence of intestinal parasitic infections and their relation with socio-economic factors and hygienic habits in Tehran primary school students. Acta Trop. 2004;92:179–186. doi: 10.1016/j.actatropica.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Karan A, Chapman GB, Galvani A. The influence of poverty and culture on the transmission of parasitic infections in rural Nicaraguan villages. J Parasitol Res. 2012;2012(478292) doi: 10.1155/2012/478292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harpham T. Measuring child poverty and health: a new international study. J Trop Pediatr. 2002;48:128–131. doi: 10.1093/tropej/48.3.128. [DOI] [PubMed] [Google Scholar]
- 28.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Fung IC, Cairncross S. Ascariasis and hand washing. Trans R Soc Trop Med Hyg. 2009;103:215–222. doi: 10.1016/j.trstmh.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Dzwairoa B, Hokoa Z, Love D, Guzha Assessment of the impacts of pit latrines on groundwater quality in rural areas: a case study from Marondera district, Zimbabwe. Phys Chem Earth Pt A. 2006;31:779–788. [Google Scholar]
- 31.Banks D, Karnachuk OV, Parnachev VP, Holden W, Frengstad B. Groundwater contamination from rural pit latrines: examples from Siberia and Kosova. Water Environ J. 2002;16:147–152. [Google Scholar]
- 32.Lee SC, Ngui R, Tan TK, Roslan MA, Ithoi I, Lim YAL. Aquatic biomonitoring of Giardia cysts and Cryptosporidium oocysts in peninsular Malaysia. Environ Sci Pollut Res Int. 2014;21:445–453. doi: 10.1007/s11356-013-1925-1. [DOI] [PubMed] [Google Scholar]
- 33.Ngui R, Lim YA, Traub R, Mahmud R, Mistam MS. Epidemiological and genetic data supporting the transmission of Ancylostoma ceylanicum among human and domestic animals. PLoS Negl Trop Dis. 2012;6:e1522. doi: 10.1371/journal.pntd.0001522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Traub RJ, Robertson ID, Irwin P, Mencke N, Thompson RC. The role of dogs in transmission of gastrointestinal parasites in a remote tea-growing community in northeastern India. Am J Trop Med Hyg. 2002;67:539–545. doi: 10.4269/ajtmh.2002.67.539. [DOI] [PubMed] [Google Scholar]
- 35.Ngui R, Ching LS, Kai TT, Roslan MA, Lim YA. Molecular identification of human hookworm infections in economically disadvantaged communities in Peninsular Malaysia. Am J Trop Med Hyg. 2012;86:837–842. doi: 10.4269/ajtmh.2012.11-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiraanankul V, Aphijirawat W, Mungthin M, Khositnithikul R, Rangsin R, Traub RJ, Piyaraj P, Naaglor T, Taamari P, Leelayoova S. Incidence and risk factors of hookworm infection in a rural community of central Thailand. Am J Trop Med Hyg. 2011;84:594–598. doi: 10.4269/ajtmh.2011.10-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ngui R, Mahdy MA, Chua KH, Traub R, Lim YAL. Genetic characterization of the partial mitochondrial cytochrome oxidase c subunit I (cox 1) gene of the zoonotic parasitic nematode, Ancylostoma ceylanicum from humans, dogs and cats. Acta Trop. 2013;128:154–157. doi: 10.1016/j.actatropica.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Shalaby HA, Abdel-Shafy S, Derbala AA. The role of dogs in transmission of Ascaris lumbricoides for humans. Parasitol Res. 2010;106:1021–1026. doi: 10.1007/s00436-010-1755-8. [DOI] [PubMed] [Google Scholar]
- 39.Traub RJ, Robertson ID, Irwin PJ, Mencke N, Monis P, Thompson RC. Humans, dogs and parasitic zoonoses—unraveling the relationships in a remote endemic community in Northeast India using molecular tools. Parasitol Res. 2003;90:156–157. doi: 10.1007/s00436-003-0925-3. [DOI] [PubMed] [Google Scholar]
- 40.Brooker S, Michael E. The potential of geographical information systems and remote sensing in the epidemiology and control of human helminth infections. Adv Parasitol. 2000;47:245–288. doi: 10.1016/s0065-308x(00)47011-9. [DOI] [PubMed] [Google Scholar]
- 41.Ngui R, Aziz S, Chua KH, Mistam MS, Al-Mekhlafi HM, Wan Yusoff WS, Rohela M, Lim YA. Mapping and modeling the geographical distribution of soil-transmitted helminthiases in Peninsular Malaysia: implications for control approaches. Geospat Health. 2014;8:365–376. doi: 10.4081/gh.2014.26. [DOI] [PubMed] [Google Scholar]
- 42.Marti HP, Koella JC. Multiple stool examinations for ova and parasites and rate of false-negative results. J Clin Microbiol. 1993;31:3044–3045. doi: 10.1128/jcm.31.11.3044-3045.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinmann P, Zhou XN, Du ZW, Jiang JY, Wang LB, Wang XZ, Li LH, Marti H, Utzinger J. Occurrence of Strongyloides stercoralis in Yunnan Province, China, and comparison of diagnostic methods. PLoS Negl Trop Dis. 2007;1:e75. doi: 10.1371/journal.pntd.0000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glinz D, Silué KD, Knopp S, Lohourignon LK, Yao KP, Steinmann P, Rinaldi L, Cringoli G, N'Goran EK, Utzinger J. Comparing diagnostic accuracy of Kato-Katz, Koga agar plate, ether-concentration, and FLOTAC for Schistosoma mansoni and soil-transmitted helminths. PLoS Negl Trop Dis. 2010;4:e754. doi: 10.1371/journal.pntd.0000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Speich B, Utzinger J, Marti H, Ame SM, Ali SM, Albonico M, Keiser J. Comparison of the Kato-Katz method and ether-concentration technique for the diagnosis of soil-transmitted helminth infections in the framework of a randomized controlled trial. Eur J Clin Microbiol Infect Dis. 2014;33:815–822. doi: 10.1007/s10096-013-2019-1. [DOI] [PubMed] [Google Scholar]