Abstract
Previous population-based studies have examined treatment impact on Schistosoma-associated urinary tract disease among children, but much less is known about longer-term treatment benefits for affected adult populations in areas where risk of recurrent infection is high. In communities in Msambweni, along the Kenya coast, we identified, using a portable ultrasound, 77 adults (aged 17–85) with moderate-to-severe obstructive uropathy or bladder disease due to Schistosoma haematobium. Treatment response was assessed by repeat ultrasound 1–2 years after praziquantel (PZQ) therapy and compared with interval changes among age- and sex-matched infected/treated control subjects who did not have urinary tract abnormalities at the time of initial examination. Of the 77 affected adults, 62 (81%) had improvement in bladder and/or kidney scores after treatment, 14 (18%) had no change, and one (1.3%) had progression of disease. Of the 77 controls, 75 (97%) remained disease free by ultrasound, while two (3%) had apparent progression with abnormal findings on follow-up examination. We conclude that PZQ therapy for S. haematobium is effective in significantly reducing urinary tract morbidity from urogenital schistosomiasis among adult age groups, and affected adults stand to benefit from inclusion in mass treatment campaigns.
Introduction
Schistosomes infect more than 207 million persons around the world, 85% of them living in Africa.1–3 Schistosoma haematobium, the causative agent of urogenital schistosomiasis, is the most prevalent schistosome species causing human disease.4 Humans infected by contact with cercaria-infested water can harbor Schistosoma adult worms that remain active and discharge eggs for over 25 years.5 The eggs trapped in the tissue continue to trigger inflammatory reaction, and perioval granulomas result in collagen deposition and laminated fibrous tissue replacement of the healthy tissues of the urinary tract.6,7 In the most severe form of S. haematobium–related disease, bladder and ureteral dysfunction leads to ureteral reflux, hydronephrosis, renal impairment, and bladder cancer.8–10 As schistosomiasis control programs based on mass drug treatment have aimed to reduce or prevent morbidity,11 portable ultrasound examination emerged as a useful tool for diagnosing and monitoring schistosome-associated structural urinary tract disease.12–16 Beginning in the 1990s, the World Health Organization (WHO) has developed guidelines to standardize the use of ultrasonography for the assessment of schistosomiasis-related morbidity.17 While earlier studies have reported in some detail on the effects of drug treatment on schistosomiasis-associated urinary tract disease among children,12 relatively little is known about the impact of treatment of affected adults who live in highly endemic areas where the risk of reinfection and recurrent damage is high. Our aim in this was to assess the S. haematobium–associated urinary tract pathology among adults before and after treatment, and to measure the effects of older age and interval reinfection on treatment responses in an adult population living in a high-transmission area.
Materials and Methods
Ethical oversight.
All participants provided individual written informed consent in accord with the guidelines of the Declaration of Helsinki. The research protocol was reviewed and approved by the Ethical Review Board of the Kenya Medical Research Institute (KEMRI/RES/7/3/1) and the Institutional Review Board for Human Investigation of University Hospitals of Cleveland (protocol 03-88-34). All subjects found to be infected with S. haematobium were treated with standard doses of praziquantel (PZQ) (40 mg/kg) immediately after the initial morbidity survey.
Study population.
The study was done as part of a community-based research on S. haematobium transmission in the Msambweni district (now Kwale County) area on the south coast of Kenya. The study was conducted in 2000–2003 in the adjoining villages of Marigiza, Milalani, Mabatani, Nganja, and Vidungeni (aggregate population 4,408). Details of the study environment and its transmission ecology have been published previously.18,19 This area, which is highly endemic for S. haematobium, is 50 km southwest of Mombasa on the Indian Ocean. The area is predominantly rural and the leading occupations are mixed agriculture, cattle herding, and fishing. All available residents > 2 years old, (N = 4,199) from the five S. haematobium endemic villages were surveyed. A complete evaluation was obtained for 3,118/4,199 of these residents or 74% of the targeted age groups.
Inclusion for the current adult morbidity sub-study.
For the present nested case–control analysis, we identified 77 adults aged 17–85 as “cases,” who had moderate-to-severe obstructive uropathy and/or bladder disease due to S. haematobium detected by portable ultrasound. An additional 77 age- and sex-matched Schistosoma-exposed “control” subjects who did not have ultrasound abnormalities were used as a concurrent comparison group. Posttreatment response (in terms of structural morbidity detected by ultrasound) was assessed by repeat ultrasound examination 1–2 years after therapy (PZQ 40 mg/kg given as a single oral dose after the first examination). See Figure 1 for flow chart.
Figure 1.
Flow chart of study enrollment, testing, treatment, and follow-up.
Detection of S. haematobium infection.
Infection status and intensity were determined by duplicate determination of S. haematobium egg counts per 10 mL urine in midday voided urines, using a standard Nuclepore (Nuclepore Corp., Pleasanton, CA) filtration technique.20 Consonant with our previous reports from this highly endemic area, infection intensity was also classified into three groups as light (1–99 eggs/10 mL urine), moderate (100–399 eggs/10 mL urine), or heavy (≥ 400 eggs/10 mL urine).
Ultrasound examination.
Ultrasound examinations are carried out in local village structures under field conditions using a portable ultrasound unit (Sonosite 180, Bothell, WA) run on battery power.16,21 Images were archived either by thermal printing or electronic image capture. Ultrasound examinations are performed and scored by two independent readers of the study team who were unaware of the subject's infection status, using the standardized WHO Niamey protocols for detection of morbidity of the urinary tract caused by S. haematobium.17 In this scoring system, bladder irregularity, thickening, masses/polyps, and shape are individually scored for severity and combined into a urinary bladder intermediate score. Abnormalities of the ureters and renal pelvises are then scored based on standard views and the scores combined for an upper urinary tract intermediate score. A final global morbidity score is then calculated by combining the bladder and upper urinary tract scores. Using this approach, a score of zero means normal or no pathology, while a larger score indicates a greater aggregate level of S. haematobium–associated lesions. As in our previous studies,19,22,23 intra- and inter-observer variation in the study's imaging outcomes was minimized by means of having a highly experienced imaging team adhere closely to the standardized WHO Niamey protocol17 under the supervision of the lead ultrasonographer (Philip Magak).
Statistical analysis.
Subjects were coded according to infection status after therapy, infection intensity before and after therapy, and age group (younger versus older than 25). Ultrasound outcomes were coded according to the subject's bladder scores (irregularity, thickening, deformity, mass, or polyp) as well as their worst kidney score, both before and after therapy; a separate variable encoded the change in these scores between pre- and posttreatment scans. In this qualitative analysis, the subjects who had a reduction in their ultrasound scores between pre- and posttreatment scans were classified as “improved,” while those who progressed to higher individual scores after treatment were classified as “worsened.” The overall scores and the sub-scores for upper and lower urinary tracts were compared for each individual in this fashion. Testing for significant differences in categorical values between study groups (i.e., those affected versus the age-/sex-matched control subject group) and among subgroups was performed by χ2, McNemar, or Mann–Whitney U testing, as appropriate, with the use of SAS statistical software (SAS Institute Inc., Cary, NC). Subjects' ultrasound scores and bladder wall thickness values before and after treatment were compared using Student's paired t test. P values < 0.05 were considered significant.
Results
Subject participation and initial community disease status.
Of all residents surveyed in the target communities (N = 3,118), 1,686 of subjects were adults over the age of 17. Among these, 985 (58%) were women, and 701 (42%) were men. Participation of eligible adult women was 80%, which was significantly higher than that of adult male residents (62%), who were frequently away from the area in pursuit of their employment. Overall, 46% of all community subjects surveyed were found to be egg positive on urine filtration, and so known to be actively infected with S. haematobium. Upon standardized urinary tract sonography17 (performed on all participating study subjects), S. haematobium–related urinary tract abnormalities were present in 17% with bladder inflammation present in 16%, and evidence of hydronephrosis or hydroureter in 2%.23 Overall, 172 adults (10%) had abnormal intake ultrasounds, that is, exams manifesting significant moderate-to-severe pathology detected in bladder, ureters, or kidneys. Of these affected adults, 108 (63%) were male, and the average age was 27 ± 16.5 years. (Full details of the age distributions of pathology are given in our previous publication.19) Among those adults with abnormal ultrasounds, current S. haematobium egg positivity in the urine was 71%, and prevalence of heavy infection (≥ 400 eggs/10 mL urine) was 15%.
Characteristics of participants in paired follow-up surveys.
During the follow-up surveys, 160 of the affected adult group (age range 17–85) were still resident in the study communities, and after consultation with study intake demography and laboratory records, 77 age- and sex-matched case–control pairs were successfully identified, re-consented, and evaluated by repeat parasitological testing and full ultrasound examinations (see Figure 1 for study flow chart). Those adults lost to follow-up and those who were not included in our follow-up surveys were not significantly different from the tested groups in terms of age or sex distribution (Table 1), but they were noted to have had lower prevalence of egg-patent infection and heavy infection than the cases analyzed in this article.
Table 1.
Comparison of Schistosoma haematobium–affected cases and their unaffected age- and sex-matched control group
| Characteristic | Study subject group | ||
|---|---|---|---|
| 77 cases with abnormal intake ultrasound exams | 77 matched controls with normal intake ultrasound exams | 95 with abnormal intake ultrasound exams—not followed | |
| Mean age ± SD (range) | 27.7 ± 16.8 years (17–85) | 27.7 ± 16.7 years (17–85) | 31.2 ± 18.5 years (17–99) |
| Percent male | 65 | 65 | 63 |
| Infection prevalence, pre-Rx | 81% | 59%† | 62%* |
| Infection prevalence, post-Rx | 26% | 19% | − |
| Prevalence of heavy infection, pre-Rx | 22% | 9%* | 8%* |
| Prevalence of heavy infection, post-Rx | 0% | 1% | − |
Rx = praziquantel treatment 40 mg/kg given once; SD = standard deviation.
Significant difference from the cases (ultrasound pathology group included in this report).
P < 0.04.
P < 0.01.
Interval changes in infection status and ultrasound findings after therapy.
During the 1- to 2-year interval after treatment, infection prevalence and prevalence of heavy infection declined, both among cases and controls (Table 1). Whereas cases were more infected and more frequently heavily infected with S. haematobium before treatment than controls, there were no significant differences between the two groups in terms of infection status at the time of follow-up (Table 1). In terms of ultrasound findings, Table 2 shows the mean upper tract, lower tract, and aggregate scores, before and after therapy for both cases and controls. Table 3 shows the qualitative assessment in terms of improved, unchanged, or worsened ultrasound examination outcomes. Of the 77 participating cases, that is, adults who had urinary tract disease detected by pretreatment ultrasound, 62 (81%) had improvement in their bladder and/or kidney scores, whereas 14 (18%) had no change and one (1.3%) had progression of disease. Among the 77 matched controls, who had also received treatment after their initial examinations, 73 (95%) had no change in their baseline examinations, two (3%) had improvement/resolution of mild hydronephrosis, while two (3%) showed new evidence of hydronephrosis at the time of follow-up examination. Seventeen percent (2/12) of subjects with moderate-to-severe upper tract lesions completely resolved their abnormalities, while 93% (65/70) of subjects with bladder abnormalities has resolution of their findings.
Table 2.
Case and control group standardized ultrasound morbidity scores before and after therapy among Msambweni adults aged 17–85 years
| Characteristic | Study subject group | |
|---|---|---|
| 77 cases with abnormal intake ultrasound exams | 77 matched controls with normal intake ultrasound exams | |
| Niamey score* mean ± SD, (range) | Niamey score* mean ± SD, (range) | |
| Pre-Rx upper tract | 1.9 ± 4.9 (0–24) | 0 ± 0 |
| Post-Rx upper tract | 1.0 ± 3.1 (0–16) | 0.08 ± 0.7 (0–6) |
| Pre-Rx lower tract | 2.6 ± 1.4 (0–8) | 0.06 ± 0.4 (0–3) |
| Post-Rx lower tract | 0.17 ± 0.6 (0–4) | 0.03 ± 0.2 (0–2) |
| Pre-Rx global score | 4.5 ± 4.5 (2–24) | 0.06 ± 0.4 (0–3) |
| Post-Rx global score | 1.2 ± 3.2 (0–16) | 0.1 ± 0.7 (0–6) |
Rx = praziquantel treatment 40 mg/kg given once; SD = standard deviation.
Aggregate abnormality score as measured using the Niamey consensus World Health Organization (WHO) ultrasound scoring system.17
Table 3.
Qualitative changes observed in ultrasound scores after praziquantel treatment
| Improved (%) | Unchanged (%) | Worsened (%) | Total* (%) | |
|---|---|---|---|---|
| Cases | ||||
| Combined upper tract score | 9 (12) | 67 (87) | 1 (1) | 77 (100) |
| Combined lower tract score | 62 (81) | 14 (18) | 1 (1) | 77 (100) |
| Global score | 71 (92) | 5 (6) | 1 (1) | 77 (100) |
| Controls | ||||
| Combined upper tract score | 0† (0) | 76 (99) | 1 (1) | 77 (100) |
| Combined lower tract score | 2‡ (3) | 74 (96) | 1 (1) | 77 (100) |
| Global score | 2‡ (3) | 73 (95) | 2 (3) | 77 (100) |
Distribution of posttreatment changes was significantly different between cases and controls.
P < 0.01.
P < 0.001, by Mann–Whitney U test.
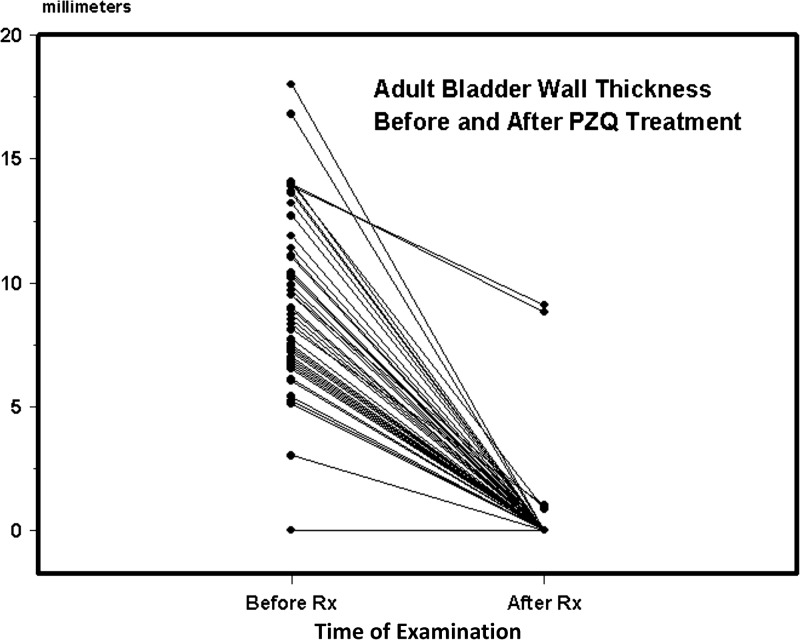
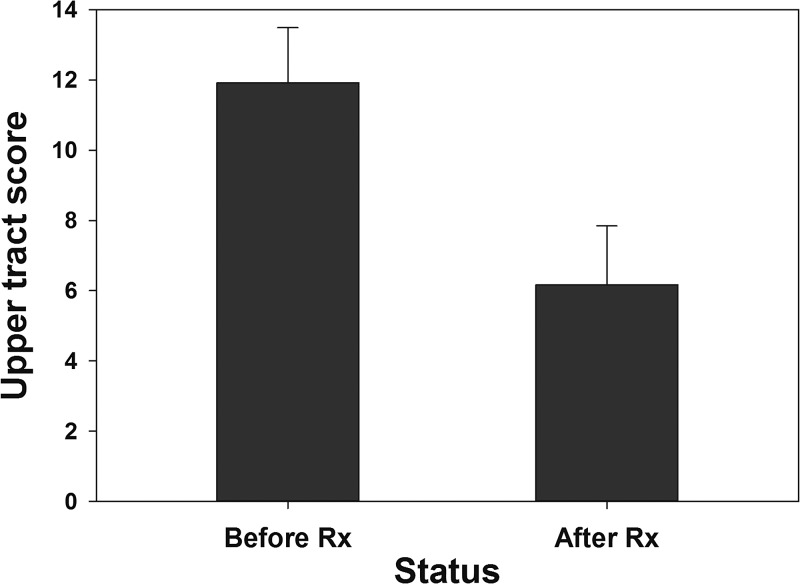
In terms of quantitative outcome assessments, the mean reduction in overall ultrasound score was 3.3 (95% CI = 2.5, 4.0; P < 0.001) for the cases, and −0.04 (95% CI = −0.2, 0.15; nonsignificant [NS]) for the control group. Average (± standard error of the mean) bladder wall thickening among cases declined from 8.2 ± 0.5 to 0.3 ± 0.2 mm (P < 0.001) after therapy (Figure 2 ). Bladder thickening was nil among controls before therapy, and 0.01 ± 0.01 mm after therapy (change NS). Figure 3 indicates the overall improvement in upper tract scores for adults who had significant kidney or ureteral abnormalities before treatment.
Figure 2.
Bladder wall thickening (in millimeters) before and after praziquantel (PZQ) treatment of study cases, the 77 subjects with moderately-to-severely abnormal ultrasound findings detected on intake ultrasound examination at the time of study entry. Lines indicate the change in bladder wall thickening for each subject from before treatment to the posttreatment examination.
Figure 3.
Mean upper urinary tract scores the subset of cases who had significant kidney or ureteral abnormalities on initial examination (N = 12). Bars indicate mean values before and 1–2 years after praziquantel (PZQ) treatment. Error bars indicate standard error of the mean.
Influence of age and recurring infection.
Among the cases, improvement was more likely for those 25 years of age or younger (89% improvement versus 70% for older ages, P < 0.04). In particular, abnormal kidney scores did not improve as often among the older adults (just 40% improvement versus 67% for the younger adults). By contrast, improvement of abnormal bladder scores was equally frequent (> 90%) for both younger and older age groups. Where persisting bladder abnormalities were detected, they were more common among those with persistent infection (or reinfection) at the time of posttreatment evaluation (25% posttreatment abnormalities for those egg positive at the time of follow-up versus zero abnormalities for those without eggs in the urine at the time of follow-up, P < 0.001). Whereas posttreatment infection was more common among younger cases (32%), none of the older cases was egg positive at the time of follow-up, which precluded further analysis of the interaction of age and infection status.
Discussion
The results of this study indicate that when adults living in an S. haematobium–endemic area are treated with standard 40 mg/kg doses of PZQ, those with renal and bladder ultrasound findings typical for schistosomiasis haematobia show regression of these abnormalities over a period of 1–2 years. In contrast, rates of disease progression were quite low both among adults affected by ultrasound abnormalities and matched controls (who were also treated with PZQ). Using WHO guidelines for standardized ultrasound scoring of schistosomiasis-related urinary tract lesions, our study showed that 92% of the affected adult patients with moderate-to-severe obstructive uropathy and/or bladder disease due to S. haematobium improved after treatment.
Early studies using X-ray-contrast excretory pyelography and urography reported very limited regression or even progression of S. haematobium uropathy after treatment.24–28 However, the complexity of X-ray testing meant that study sizes were necessarily limited.25 Also, therapy at the time was with less-effective drugs, tartar emetic, stibocaptate, niridazole, and metrifonate, such that results from those early studies may not reflect the present-day potential for drug treatment benefits at the population level. In that era, Lucas and others24 and Pugh and Gilles28 in Nigeria and MacDonald and others27 in Tanzania found that younger age (< 17 years) was associated with better reduction in the prevalence of urinary tract lesions after antischistosomal treatment in high prevalence areas. By contrast, Davis25 in Nigeria and Farid and others26 in Egypt found minimal net improvement in the prevalence of pathology among S. haematobium–affected adults aged 20–40 years.
In the PZQ era, several large studies have examined the efficacy of drug treatment in improving urinary ultrasound abnormalities due to S. haematobium infection.16,29–38 However, these studies have been primarily focused on responses among children and not adults. Doehring and others documented improvement of bladder lesions associated with schistosomiasis among children within 2 months,29 and found reversibility of urinary tract obstructive findings within 1 year after treatment.30 That study's lesion clearance rates were 93% and 88% for lower and upper urinary tract lesions, respectively. King and others31,39 reported significant reductions in bladder wall lesions among school-age children after 1 year, but no net change in obstructive findings (hydronephrosis/hydroureter), until 2–3 years after the introduction of school-based PZQ or metrifonate treatment in Kenya. Again, younger age was associated with faster clearance of bladder and upper urinary tract lesions. Similar posttreatment improvements in urinary bladder and kidney morbidity scores in children were reported by Medhat and others33 in Egypt and Hatz and others35 in Tanzania. In a study by Delegue and others34 in Senegal, in which 24% of the subjects were over the age of 25, urinary tract lesions were said to regress significantly in the cohort at large 4 months after PZQ therapy, but an age-specific breakdown of outcomes was not given. In a large study in Ghana, Wagatsuma and others36 confirmed that all types of S. haematobium–associated bladder pathology resolve after treatment regardless of patient's age (whether adults or children) and initial intensity of infection. However, many (73%) of their subjects above age 15 who had upper urinary tract pathology did not resolve ureteral and/or kidney abnormalities by 18 months after PZQ therapy. In this study, only 17% of adult subjects completely resolved their upper tract pathology, whereas 93% successfully resolved their bladder pathology.
In aggregate, these studies suggest that current infection prevalence and intensity, as well as age, are important risk factors for urinary tract pathology. In endemic regions, age is a proxy for duration of parasite exposure, and it is believed that S. haematobium–related tissue damage progresses from acute granulomatous injury into a more permanent form of damage caused by cumulative fibrotic changes.7,40 Acute injury is reversible with treatment, and so young children tend to lose their pathological findings quickly unless reinfection occurs.34,35
Our results confirm that bladder lesions regress quickly, even in adults, whereas upper tract lesions tend to regress more slowly, if at all.31,36 Although some of our adult subjects were egg negative, the study was performed in an area where nearly all residents experience significant S. haematobium infection by the age of 15.41 Our subjects' ultrasound findings were typical of schistosomiasis haematobia,17,35 and it is clear that structural urinary tract disease can persist after active infection subsides. Advanced forms of S. haematobium–associated fibrotic injury and tissue calcification tend not to regress, and so adults, who have more of this late form of disease, are believed to be less responsive to treatment. Occlusion of the ureters with hydroureter, hydronephrosis, and renal damage are thought to be the most common cause of S. haematobium–related mortality.9,42 Our data and findings of other studies also confirm that presence of egg-positive infection at the time of follow-up means greater chances of finding persistent/recurrent abnormalities at the time of posttreatment examination.
The study had both strengths and limitations. In focusing on the impact of therapy later in life, the study was designed to include adults only, and with specific interest in those with abnormal ultrasound findings detected in population-based surveys of long-term residents in endemic villages. We used a standardized protocol for assessment, improving comparability to other studies. There was a concurrent comparison group drawn from the same area, and longitudinal evaluation was performed at the individual level for both groups. In terms of limitations, the study's primary focus on affected adults led to a smaller number of included subjects, which limited statistical power and thus precluded analysis of some potential modifying host factors of interest. We did not have interval follow-ups in the period between initial and final examinations, so that some influential intercurrent health events may have been missed. Because of technical and financial limitations, this study did not examine the interval impact of treatment on genital schistosomiasis among men or women, although, given the now well-documented effects of S. haematobium on other pelvic organs and reproductive health, this area is of great research interest.43,44 The study was performed in only one area of Kenya, and regional differences in exposure and parasite strains could lead other studies to have different results in other areas. Finally, this was not a randomized study and there was no untreated control group for comparison; however, we felt that it was inappropriate to delay therapy in diagnosed subjects given current knowledge about the pathophysiology of urogenital schistosomiasis.
We conclude that PZQ therapy for S. haematobium is effective in reducing urinary tract structural morbidity among adult age groups, particularly among younger adults. Given the evidence of slow progression to nonreversible infection-related damage over time, and the association between renal damage and S. haematobium–associated mortality, it seems essential now to also provide basic preventive PZQ treatment to this age group. Furthermore, given evidence of persistent disease-related infection risk among both younger and older adults, they, as well as children, stand to benefit from inclusion in mass treatment campaigns.
ACKNOWLEDGMENTS
We thank the residents of Milalani, Mabatani, Nganja, and Marigiza villages for their gracious participation. We are especially thankful to Anthony Chome and Iddi Masemo as well as Malick Ndzovu, Jackson Muinde, and Joyce Bongo in helping locate subjects for follow-ups. Also, we are thankful for the help from Grace Mathenge in coordinating field operations and data entry.
Footnotes
Financial support: This research was supported by grants from the National Institutes of Health under grants AI-45473 (National Institute of Allergy and Infectious Diseases) and TW/ES01543 (Fogarty International Center).
Authors' addresses: Philip Magak, City X-Ray Services, Nairobi, Kenya, E-mail: magakpw@yahoo.com. Alicia Chang-Cojulun, Unidad De Oncologia Pediatrica (UNOP), Guatemala City, Guatemala, E-mail: aliciachc18@gmail.com. Hilda Kadzo, Kenyatta National Hospital, Nairobi, Kenya, E-mail: hildakadzo@yahoo.com. Edmund Ireri, Kenya Medical Research Institute, Nairobi, Kenya, E-mail: eikareko@hotmail.com. Eric Muchiri, Meru University of Science and Technology, Meru, Kenya, E-mail: ericmmuchiri@gmail.com. Uriel Kitron, Department of Environmental Sciences, Emory University, Atlanta, GA, E-mail: ukitron@emory.edu. Charles H. King, Center for Global Health and Diseases, CWRU School of Medicine, Cleveland, OH, E-mail: chk@cwru.edu.
References
- 1.Engels D, Chitsulo L, Montresor A, Savioli L. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 2002;82:139–146. doi: 10.1016/s0001-706x(02)00045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schur N, Hurlimann E, Garba A, Traore MS, Ndir O, Ratard RC, Tchuem Tchuente LA, Kristensen TK, Utzinger J, Vounatsou P. Geostatistical model-based estimates of schistosomiasis prevalence among individuals aged ≤ 20 years in west Africa. PLoS Negl Trop Dis. 2011;5:e1194. doi: 10.1371/journal.pntd.0001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schur N, Hurlimann E, Stensgaard AS, Chimfwembe K, Mushinge G, Simoonga C, Kabatereine NB, Kristensen TK, Utzinger J, Vounatsou P. Spatially explicit Schistosoma infection risk in eastern Africa using Bayesian geostatistical modelling. Acta Trop. 2013;128:365–377. doi: 10.1016/j.actatropica.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Mutapi F. Improving diagnosis of urogenital schistosome infection. Expert Rev Anti Infect Ther. 2011;9:863–865. doi: 10.1586/eri.11.101. [DOI] [PubMed] [Google Scholar]
- 5.Hueper WC. Occupational and Environmental Cancers of the Urinary System. New Haven, CT: Yale University Press; 1969. [Google Scholar]
- 6.Makar N. Urological Aspects of Bilharziasis in Egypt. Cairo, Egypt: Société Orientale de Publicité; 1955. [Google Scholar]
- 7.Chen MG, Mott KE. Progress in assessment of morbidity due to Schistosoma haematobium infection. Trop Dis Bull. 1989;86:R1–R36. [Google Scholar]
- 8.Sayegh ES, Dimmette RM. The fibrotic contracted urinary bladder associated with schistosomiasis and chronic ulceration: a clinicopathological study including treatment. J Urol. 1956;75:671–679. doi: 10.1016/S0022-5347(17)66862-9. [DOI] [PubMed] [Google Scholar]
- 9.Forsyth DM, Bradley DJ, McMahon J. Death attributed to kidney failure in communities with endemic urinary schistosomiasis. Lancet. 1970;2:472–473. doi: 10.1016/s0140-6736(70)90095-4. [DOI] [PubMed] [Google Scholar]
- 10.WHO . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Schistosomes, Liver Flukes and Helicobacter pylori. Geneva, Switzerland: World Health Organization; 1994. [PMC free article] [PubMed] [Google Scholar]
- 11.WHO . Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 12.Hatz C, Mayombana C, de Savigny D, MacPherson CNL, Koella JC, Degremont A. Ultrasound scanning for detecting morbidity due to Schistosoma haematobium and its resolution following treatment with different doses of praziquantel. Trans R Soc Trop Med Hyg. 1990;84:84–88. doi: 10.1016/0035-9203(90)90392-r. [DOI] [PubMed] [Google Scholar]
- 13.Houston S. Ultrasound: appropriate technology for tropical field work. Trans R Soc Trop Med Hyg. 1991;85:321–323. doi: 10.1016/0035-9203(91)90274-3. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Wahab MF, Ramzy I, Esmat G, el Kafass H, Strickland GT. Ultrasound for detecting Schistosoma haematobium urinary tract complications: comparison with radiographic procedures. J Urol. 1992;148:346–350. doi: 10.1016/s0022-5347(17)36590-4. [DOI] [PubMed] [Google Scholar]
- 15.Doehring-Schwerdtfeger E, Kaiser C, Schlake J, Abdel-Rahim IM, Mohamed-Ali Q, Richter J, Franke D, Kardorff R, Elsheikh M, Ehrich JH. Ultrasound versus clinical examination as indication for Schistosoma mansoni associated morbidity in children. Trop Med Parasitol. 1992;43:245–248. [PubMed] [Google Scholar]
- 16.King CH. Ultrasound monitoring of structural urinary tract disease in S. haematobium infection. Mem Inst Oswaldo Cruz. 2002;97((Suppl 1)):149–152. doi: 10.1590/s0074-02762002000900028. [DOI] [PubMed] [Google Scholar]
- 17.Richter J, Hatz C, Campagne G, Bergquist NR, Jenkins JM. Ultrasound in Schistosomiasis: A Practical Guide to the Standardized Use of Ultrasonography for the Assessment of Schistosomiasis-Related Morbidity. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 18.Clennon JA, King CH, Muchiri EM, Kariuki HC, Ouma JH, Mungai P, Kitron U. Spatial patterns of urinary schistosomiasis infection in a highly endemic area of coastal Kenya. Am J Trop Med Hyg. 2004;70:443–448. [PubMed] [Google Scholar]
- 19.King CH, Blanton RE, Muchiri EM, Ouma JH, Kariuki HC, Mungai P, Magak P, Kadzo H, Ireri E, Koech D. Low heritable component of risk for infection intensity and infection-associated disease in urinary schistosomiasis among Wadigo village populations in Coast Province, Kenya. Am J Trop Med Hyg. 2004;70:57–62. [PubMed] [Google Scholar]
- 20.Peters PAS, Mahmoud AAF, Warren KS, Ouma JH, Siongok TKA. Field studies of a rapid, accurate means of quantifying Schistosoma haematobium eggs in urine samples. Bull World Health Organ. 1976;54:159–162. [PMC free article] [PubMed] [Google Scholar]
- 21.King CH, Lombardi G, Lombardi C, Greenblatt R, Hodder S, Kinyanjui H, Ouma J, Odiambo O, Bryan PJ, Muruka J, Magak P, Weinert D, Mackay W, Ransohoff D, Houser H, Koech D, Siongok TK, Mahmoud AAF. Chemotherapy-based control of schistosomiasis haematobia. I. Metrifonate versus praziquantel in control of intensity and prevalence of infection. Am J Trop Med Hyg. 1988;39:295–305. doi: 10.4269/ajtmh.1988.39.295. [DOI] [PubMed] [Google Scholar]
- 22.King CH, Muchiri EM, Mungai P, Ouma JH, Kadzo H, Magak P, Koech D. Randomized comparison of low-dose versus standard-dose praziquantel therapy in treatment of urinary tract morbidity due to Schistosoma haematobium infection. Am J Trop Med Hyg. 2002;66:725–730. doi: 10.4269/ajtmh.2002.66.725. [DOI] [PubMed] [Google Scholar]
- 23.Magak P, King CH, Ireri E, Kadzo H, Ouma JH, Muchiri EM. High prevalence of ectopic kidney in Coast Province, Kenya. Trop Med Int Health. 2004;9:595–600. doi: 10.1111/j.1365-3156.2004.01228.x. [DOI] [PubMed] [Google Scholar]
- 24.Lucas AO, Adeniyi-Jones CC, Cockshott WP, Gilles HM. Radiological changes after medical treatment of vesical schistosomiasis. Lancet. 1966;1:631–633. doi: 10.1016/s0140-6736(66)90826-9. [DOI] [PubMed] [Google Scholar]
- 25.Davis A. Radiological changes after treatment of vesical schistosomiasis [letter] Lancet. 1966;ii:546. doi: 10.1016/s0140-6736(66)90826-9. [DOI] [PubMed] [Google Scholar]
- 26.Farid Z, Bassily S, McConnell E, Schulert A, Sabour M, Abdel Wahab MF. Symptomatic, radiological, and functional improvement following treatment of urinary schistosomiasis in Egypt. Lancet. 1967;2:1110–1113. doi: 10.1016/s0140-6736(67)90617-4. [DOI] [PubMed] [Google Scholar]
- 27.Macdonald G, Forsyth DM, Rashid C. Urological complications of endemic schistosomiasis in schoolchildren. 4. As modified by treatment. Trans R Soc Trop Med Hyg. 1968;62:775–781. doi: 10.1016/0035-9203(68)90004-7. [DOI] [PubMed] [Google Scholar]
- 28.Pugh RN, Gilles HM. Malumfashi Endemic Disease Research Project, VIII. Follow-up intravenous urograms of boys infected with Schistosoma haematobium from the Malumfashi area. Ann Trop Med Parasitol. 1979;73:191–192. [PubMed] [Google Scholar]
- 29.Doehring E, Reider F, Schmidt-Ehry G, Ehrich JH. Reduction of pathological findings in urine and bladder lesions in infection with Schistosoma haematobium after treatment with praziquantel. J Infect Dis. 1985;152:807–810. doi: 10.1093/infdis/152.4.807. [DOI] [PubMed] [Google Scholar]
- 30.Doehring E, Ehrich JHH, Bremer HJ. Reversibility of urinary tract abnormalities due to Schistosoma haematobium infection. Kidney Int. 1986;30:582–585. doi: 10.1038/ki.1986.224. [DOI] [PubMed] [Google Scholar]
- 31.King CH, Muchiri EM, Ouma JH. Age-targeted chemotherapy for control of urinary schistosomiasis in endemic populations. Mem Inst Oswaldo Cruz. 1992;87:203–210. doi: 10.1590/s0074-02761992000800031. [DOI] [PubMed] [Google Scholar]
- 32.Kardorff R, Traore M, Doehring-Schwerdtfeger E, Vester U, Ehrich JH. Ultrasonography of ureteric abnormalities induced by Schistosoma haematobium infection before and after praziquantel treatment. Br J Urol. 1994;74:703–709. doi: 10.1111/j.1464-410x.1994.tb07110.x. [DOI] [PubMed] [Google Scholar]
- 33.Medhat A, Zarzour A, Nafeh M, Shata T, Sweifie Y, Attia M, Helmy A, Shehata M, Zaki S, Mikhail N, Ibrahim S, King CL, Strickland GT. Evaluation of an ultrasonographic score for urinary bladder morbidity in Schistosoma haematobium infection. Am J Trop Med Hyg. 1997;57:16–19. doi: 10.4269/ajtmh.1997.57.16. [DOI] [PubMed] [Google Scholar]
- 34.Delegue P, Picquet M, Shaw DJ, Vercruysse J, Sambou B, Ly A. Morbidity induced by Schistosoma haematobium infections, as assessed by ultrasound before and after treatment with praziquantel, in a recently expanded focus (Senegal River basin) Ann Trop Med Parasitol. 1998;92:775–783. doi: 10.1080/00034989859014. [DOI] [PubMed] [Google Scholar]
- 35.Hatz CF, Vennervald BJ, Nkulila T, Vounatsou P, Kombe Y, Mayombana C, Mshinda H, Tanner M. Evolution of Schistosoma haematobium-related pathology over 24 months after treatment with praziquantel among school children in southeastern Tanzania. Am J Trop Med Hyg. 1998;59:775–781. doi: 10.4269/ajtmh.1998.59.775. [DOI] [PubMed] [Google Scholar]
- 36.Wagatsuma Y, Aryeetey ME, Sack DA, Morrow RH, Hatz C, Kojima S. Resolution and resurgence of Schistosoma haematobium-induced pathology after community-based chemotherapy in Ghana, as detected by ultrasound. J Infect Dis. 1999;179:1515–1522. doi: 10.1086/314786. [DOI] [PubMed] [Google Scholar]
- 37.Garba A, Campagne G, Tassie JM, Barkire A, Vera C, Sellin B, Chippaux JP. Long-term impact of a mass treatment by praziquantel on morbidity due to Schistosoma haematobium in two hyperendemic villages of Niger. Bull Soc Pathol Exot. 2004;97:7–11. [PubMed] [Google Scholar]
- 38.Ekwunife CA, Okafor FC, Nwaorgu OC. Ultrasonographic screening of urinary schistosomiasis infected patients in Agulu community, Anambra state, southeast Nigeria. Int Arch Med. 2009;2:34. doi: 10.1186/1755-7682-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King CH, Lombardi G, Lombardi C, Greenblatt R, Hodder S, Kinyanjui H, Ouma J, Odiambo O, Bryan PJ, Muruka J, Magak P, Weinert D, Mackay W, Ransohoff D, Houser H, Koech D, Siongok TK, Mahmoud AAF. Chemotherapy-based control of schistosomiasis haematobia. II. Metrifonate vs. praziquantel in control of infection-associated morbidity. Am J Trop Med Hyg. 1990;42:587–595. doi: 10.4269/ajtmh.1990.42.587. [DOI] [PubMed] [Google Scholar]
- 40.Smith JH, Christie JD. The pathobiology of Schistosoma haematobium infection in humans. Hum Pathol. 1986;17:333–345. doi: 10.1016/s0046-8177(86)80456-7. [DOI] [PubMed] [Google Scholar]
- 41.Bustinduy AL, Sutherland LJ, Chang-Cojulun A, Malhotra I, DuVall AS, Fairley JK, Mungai PL, Muchiri EM, Mutuku F, Kitron U, King CH. Age-stratified profiles of serum IL-6, IL-10, and TNF-α cytokines among Kenyan children having Schistosoma haematobium, Plasmodium falciparum, and other chronic parasitic co-infections. Am J Trop Med Hyg. 2015 doi: 10.4269/ajtmh.14-0444. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehman JS, Jr., Farid Z, Bassily S. Mortality in urinary schistosomiasis. Lancet. 1970;2:822–823. doi: 10.1016/s0140-6736(70)91485-6. [DOI] [PubMed] [Google Scholar]
- 43.Kjetland EF, Mduluza T, Ndhlovu PD, Gomo E, Gwanzura L, Midzi N, Mason PR, Friis H, Gundersen SG. Genital schistosomiasis in women: a clinical 12-month in vivo study following treatment with praziquantel. Trans R Soc Trop Med Hyg. 2006;100:740–752. doi: 10.1016/j.trstmh.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 44.Leutscher P, Ramarokoto CE, Reimert C, Feldmeier H, Esterre P, Vennervald BJ. Community-based study of genital schistosomiasis in men from Madagascar [letter] Lancet. 2000;355:117–118. doi: 10.1016/S0140-6736(99)04856-4. [DOI] [PubMed] [Google Scholar]