Abstract
An increased incidence of monkeypox (MPX) infections in the Democratic Republic of the Congo was noted by the regional surveillance system in October 2013. Little information exists regarding how MPX is introduced into the community and the factors associated with transmission within the household. Sixty-eight wild animals were collected and tested for Orthopoxvirus. Two of three rope squirrels (Funisciurus sp.) were positive for antibodies to Orthopoxviruses; however, no increased risk was associated with the consumption or preparation of rope squirrels. A retrospective cohort investigation and a case–control investigation were performed to identify risk factors affecting the introduction of monkeypox virus (MPXV) into the community and transmission within the home. School-age males were the individuals most frequently identified as the first person infected in the household and were the group most frequently affected overall. Risk factors of acquiring MPXV in a household included sleeping in the same room or bed, or using the same plate or cup as the primary case. There was no significant risk associated with eating or processing of wild animals. Activities associated with an increased risk of MPXV transmission all have potential for virus exposure to the mucosa.
Introduction
Monkeypox virus (MPXV), a member of the Orthopoxvirus genus, is a zoonotic virus endemic to western and central Africa. In humans, infection with MPXV can lead to a smallpox-like illness that is characterized by fever, lymphadenopathy, and rash. Following a febrile prodrome, a centrifugal rash appears. At first, the lesions are macular but eventually develop into papules, vesicles, pustules, and, finally, crusts. Fatal outcomes occur in up to 11% of unvaccinated affected individuals.1 Transmission occurs via respiratory droplets or direct contact with lesion exudate2; however, there is evidence that infection may occur by direct inoculation via bite or scratch.3 There are currently no drugs licensed for the treatment of monkeypox (MPX), and although the smallpox vaccine can provide protection, its use is limited because of safety concerns for a live virus vaccine. Therefore, MPX prevention depends on diminishing human contact with infected wild animals and preventing person-to-person spread of the virus.
Previous studies have documented human-to-human transmission within both families and health facilities4–6; however, little information is available about the specific interactions that lead to the transmission of the virus. If specific behaviors were identified, educational programs could be designed to reduce these behaviors and decrease the rate of MPX infection.
The definitive wild animal reservoir of the virus is still unknown; however, several lines of evidence point to small mammals.7–9 Although multiple animals have been shown to have neutralizing Orthopoxvirus antibodies,9–11 live MPXV has only been isolated from sylvatic animals on two occasions, once from a squirrel (Funisciurus sp.) and once from a sooty mangabey (Cercocebus atys).12,13
A significant increase in the number of suspect human MPX cases was noted in the Bokungu Health Zone within Tshuapa District of the Democratic Republic of the Congo (DRC) during 2013. The cases in 2013 represented an increase of over 600% when compared with previous years. During this outbreak frequent transmission events within households were documented as well as a median within-household attack rate of 50%. The current investigation presents an in depth evaluation of transmission during this outbreak, including the risk factors related to both the initial introduction of MPXV into a community, as well as the factors involved in the transmission of the virus within the household. Small mammals were evaluated for evidence of current and past MPXV infection, providing additional information about the possible zoonotic source of the virus.
Methods
MPX cases were identified by the health zone's public health surveillance team and additional cases were identified during the course of the outbreak investigation. For each household with an MPX case, household demographics were collected by local health care providers and investigators.
Case definitions.
The following case definitions were used during the investigation. The symptoms must have occurred between July 1 and December 8, 2013.
Confirmed case.
A person with a history of high fever and a vesicular–pustular rash and with at least one of the following three characteristics: 1) rash on the palms and soles, 2) lymphadenopathy, and/or 3) fever preceding rash; and diagnostic specimens that yielded a positive result for Orthopoxvirus and/or MPXV DNA signatures by polymerase chain reaction (PCR).
Probable case.
A person with a history of high fever and a vesicular–pustular rash and with at least one of the following three characteristics: 1) rash on the palms and soles, 2) lymphadenopathy, and/or 3) fever preceding rash; and history of contact with a person or animal with confirmed MPX in the 14 days preceding illness onset.
Suspect case.
A person with a history of high fever and a vesicular–pustular rash and with at least one of the following three characteristics: 1) rash on the palms and soles, 2) lymphadenopathy, and/or 3) fever preceding rash.
If a case was seen with active disease, a MPX-specific case report form was completed, and vesicular or ocular swab, and/or crust specimens were collected and shipped to Institut National de Recherche Biomedicale (INRB) in Kinshasa for analysis. An Orthopoxvirus-specific real-time PCR assay was performed for diagnostic confirmation and results were reported back to the local health providers.14 Smallpox vaccine status was based on reported history and the presence of a vaccination scar on left arm.
Household contact investigation.
A household investigation was performed to identify specific activities and behaviors potentially associated with an increased risk of MPXV transmission within the household. All individuals who lived in the household of a MPX case were asked to participate in the investigation and complete a questionnaire. If a person was not available for interview but a spouse or parent was available, the interview was conducted using this person as the informant. The person who first showed symptoms of the disease in the household was defined as the primary case in each household, while people who had symptoms later were defined as additional cases. Questionnaires were designed to obtain information regarding the interactions and types of contact between each MPX case in the household and each family member. Questions were designed to elicit information about interactions such as bathing, sleeping, eating, and washing of clothes.
Community case–control investigation.
A second investigation evaluated the activities that were associated with an increased risk of MPX introduction into the community. This community investigation used a case–control design. A minimum of three community controls were matched to the primary case from each household using the following criteria: 1) Age: for cases < 15 years of age, a match was found that was ± 3 years of age. For cases > 15 years of age, a match was found that was ± 10 years of age. 2) Gender: controls were identified that were the same gender as the cases. 3) Location: controls were identified that lived in households situated ≥ 500 m away from the cases' home. 4) Medical history: Potential controls were considered ineligible if they or someone in their family had suspect MPX illness in the past 1 year. At least three community controls and the primary MPX case from each household were interviewed for the case–control investigation. Questionnaires asked about the person's residence, social activities, and exposures to wild animals.
For both the household and community case–control investigations, interviews were performed by local health care staff and investigators from Kinshasa. All interviews were performed at the location of residence in the local language by staff fully proficient in the language. Each participant provided informed consent; parents provided informed consent for children < 18 years of age. The protocol underwent U.S. Centers for Disease Control and Prevention (CDC) human subjects review and was determined not to be research involving human subjects; as such, Institutional Review Board approval was not required.
Statistical analysis.
Data were analyzed using SAS 9.3 TS statistical software (SAS Institute Inc., Cary, NC). For the household investigation, a χ2 analysis was performed. A 5% significance level was used as a cut-off. For the community case–control investigation, odds ratios were calculated using logistic conditional analysis. t-Tests were performed using a two-tailed model with unequal variance.
Geographic distribution analysis.
The locations of cases were collected with Garmin GPS units (Olathe, KS). Nine affected locations were included for analysis. The locations of all human structures in the town of Bokungu and the surrounding area were digitized with a point using a 0.50-m panchromatic Digital Globe image from April 25, 2014. A total of 1,540 structures were identified; however, ~10% of the area was masked by cloud cover and was not able to be digitized accurately. Circles with a radius of 100 and 500 m were made around each of the 11 case locations and 1,529 of the control locations. A 50-m resolution multispectral Landsat composite image obtained from the Central Africa Regional Program for the Environment (CARPE) was classified into five land cover categories with the image classification toolbox in ArcGIS 10.2. A technician with local knowledge of the landscape classified the area into five categories, with each 50 m2 pixel being assigned a specific value; water (rivers, fish pods), forest (represented by tree canopy), disturbed (areas of cleared forest, agricultural lands), developed (areas of human habitation, roads), and flooded (areas showing seasonal flooding). The proportion of pixels from each land cover type was determined with the zonal statistics plugin for Quantum GIS 1.8 (http://qgis.osgeo.org) for each of the three buffer distances. The tables with appended landscape proportions were exported to EpiInfo7 where the means procedure was used to determine any significant differences between regions surrounding the case homes versus the regions surrounding the other homes in the region.
A Global Moran's I test was used to determine spatial autocorrelation of households between 50 and 500 m. The z scores from each 50-m distance were graphed and the peak values were used for distance thresholds in further analyses. A 100-m2 grid was used to summarize the number of structures in each cell. Cell counts were used to perform Gettis-Ord hotspot analysis for areas of statistically high and low population. All analyses were performed with the spatial statistics toolbox in ArcGIS 10.2 (Redlands, CA).
Small mammal collection.
Live trapping of small mammals was conducted with the use of HB Sherman (Tallahassee, FL), victor snap traps, Tomahawk Live Traps (Tallahassee, FL), and museum special traps. The bait for all traps was a mixture of oats, peanut butter, and meal worms. Traps were set inside of eight households belonging to confirmed or suspect cases. In addition, 153 traps were placed in a forested area surrounding an affected village, 30 were set in nearby grassland, and 30 were placed behind the home of a confirmed case. The GPS location of each trap was recorded and orange flagging was used to facilitate collection and re-baiting. Some animals were purchased from local hunters that were reported to have been caught within 16 km of the village of Bokungu.
Captured animals were anesthetized with isoflurane and blood samples were collected via cardiac puncture prior to euthanasia by exsanguination and cervical dislocation. Standard measurements, weights, sex, age class, and reproductive characteristics were documented and a detailed necropsy was performed on each animal. Collected tissues samples (liver, kidney, spleen, heart, lung, brain, serum as well as oral swabs) were preserved in liquid nitrogen and were shipped back to the CDC Atlanta where they were tested by PCR and enzyme-linked immunosorbent assay (ELISA). Dried blood samples were also collected on Nobuto filter paper strips (Advantec, Dublin, CA). This work was conducted under a CDC IACUC-approved protocol (2344RUBMULXZ).
Animal sample processing.
ELISA was used to detect anti-Orthopoxvirus IgG antibodies in serum samples or from dried blood samples. Crude vaccinia virus diluted in bicarbonate buffer at a concentration of 0.01 μg/well was used for coating half of each microtiter plate, the other half of the plate was coated with an equal concentration of BSC-40 cell lysate. The average of the optical density values from the duplicates of a sample in the viral half of the plate, minus the average of the duplicates from the corresponding sample in the cell lysate half of the plate, plus two standard deviations, was used to generate a cut-off value (COV). An animal was confirmed positive to the presence of anti-Orthopoxvirus antibodies if the sample's value was above the COV in at least two consecutive dilutions (1:100 and 1:200). DNA was isolated from liver samples with the Qiagen M48 robot and the Qiagen MagAttract DNA Mini M48 Kit (Qiagen, Hilden, Germany). The presence of viral DNA was assessed using real-time PCR to detect the E9L gene of Orthopoxviruses as described by Li and others.15
Results
A total of 20 confirmed, 19 probable, and 24 suspect cases within 16 households were identified during the course of the outbreak investigation.
Household contact investigation.
A total of 63 cases and 64 unaffected household contacts from 16 households were identified of which 61 cases (100%) and 53 of unaffected contacts (82.8%) completed the interview. The median age of case participants was 10 years, whereas the median age of unaffected household contacts was 20 years (Table 1). Fifty-seven percent of case participants and 38% of unaffected household contacts were male. Among the cases, 17 people were identified as the primary case in their home; one household identified two primary cases, as they developed symptoms on the same day. The median age of primary cases was 9 years and 64% of were male (Table 1). Cases individuals were significantly more likely to be male (P = 0.042) and be students (P = 0.0032). They were also more likely to be younger (P = 0.036), with 67% of those affected and only 45% of those unaffected being less than 15 years of age (P = 0.018).
Table 1.
Participants in the household and community case–control investigations
| Investigation | Type of participant* | Number | Median age (mean)† | Gender (% male) | Vaccinated (%) |
|---|---|---|---|---|---|
| Household | Household contact | 53 | 20 (23) | 38 | 30 |
| Case | 61 | 10 (15.5) | 57 | 15 | |
| Primary | 17 | 9 (15.6) | 64 | 12 | |
| Additional | 44 | 10.5 (15.4) | 54 | 22 | |
| Community | Primary | 16 | 11 (15.9) | 63 | 12 |
| Community Control | 50 | 10 (14.9) | 64 | 12 |
Household contacts are defined as unaffected individuals who lived in the same household as a case. Primary case is defined as the person in a household who first showed symptoms of the disease, whereas additional cases had the onset of symptoms at a later time.
Age in years.
In all, 16.7% of the cases had evidence of vaccination in contrast to 29.8% of the household contacts. There was not a significant difference in vaccination rates between the two groups (P = 0.097).
Behavioral factors that were found to be associated with a significant increase in the risk of acquiring a MPXV infection were sleeping in the same room (P < 0.001) or bed (P = 0.001), sharing food from the same dish (P = 0.015), and drinking out of the same cup (P = 0.003) as the primary case (Table 2). Activities including kissing, assisting with toileting and hygiene and laundering clothes did not show a significant association with acquiring the infection.
Table 2.
Significant behavioral factors associated with an increased risk of acquiring an MPXV infection within a household
| Behavioral factor | χ2 | P value |
|---|---|---|
| Sleeping in the same room | 17.3058 | < 0.001 |
| Sleeping in the same bed | 12.4944 | < 0.001 |
| Drinking from the same cup | 9.0981 | 0.003 |
| Eating from the same dish | 5.9362 | 0.015 |
MPXV = monkeypox virus.
Community case–control investigation.
The community case–control investigation included 16 primary case patients from 15 households and 50 matched community controls. Three community controls were identified for 14 of the cases and 4 controls were identified for 2 of the cases. The average age of cases was 11 years and 10 years for community controls (Table 1). In all, 63% of the cases and 64% of the and controls were male.
Cases were more likely to sleep on the floor (P = 0.032), whereas controls were more likely to live in a house that had a door (P = 0.012), eat duiker (P = 0.018), and prepare wild animal meat to cook (P = 0.05) (Table 3). No significant risk was found associated with having animals in the house, finding dead animals around the house, coming into contact with animal excrement, being bitten or scratched by an animal, or hunting or cooking wild animals. Structural properties of the house, including roofing, wall and floor material, windows and doors, did not demonstrate a significant associated risk. Five cases and one control reported eating animals that were found dead, whereas over 50% from each group reported eating monkey and rat in general. Anecdotally, two case families related stories about their children capturing and playing with live squirrels in the days immediately preceding the first case of MPX in the household.
Table 3.
Significant factors associated with introduction of MPXV into a household
| Odds ratio estimates | Point estimate | 95% Wald confidence limits | P value | |
|---|---|---|---|---|
| Live in house with a door | 0.067 | 0.008 | 0.557 | 0.012 |
| Prepare wild animal for consumption | 0.291 | 0.085 | 0.995 | 0.049 |
| Eat duiker | 0.148 | 0.03 | 0.718 | 0.018 |
| Sleep on floor | 6.062 | 1.164 | 31.574 | 0.032 |
MPXV = monkeypox virus.
Geographic distribution.
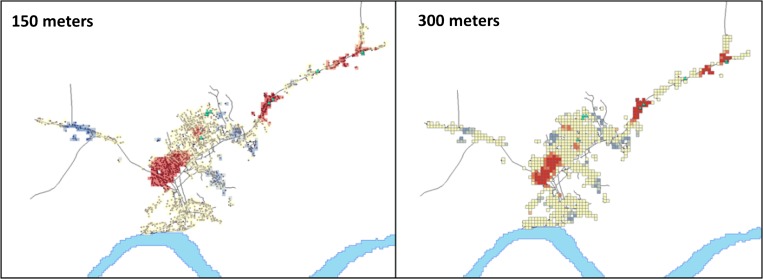
No difference was found in the type of land use in the immediate vicinity around case households and the general community. However, a distance between 0 and 500 m, case households were more likely to live next to disturbed areas and less likely to live next to developed land (Table 4). Tests for global spatial autocorrelation showed that clustering peaked at 150 and 300 m. These distances were used for distance thresholds in further analyses. Five out of nine (55.5%) of MPX households were located in hotspots of high population (Figure 1 ). No case households were located in areas with population density at the lower end of the spectrum (z score less than −1.96).
Table 4.
Geographic regions surrounding case homes vs. general community
| Land use | 100 m | 500 m | ||||
|---|---|---|---|---|---|---|
| Case | Community | P value | Case | Community | P value | |
| Water | 0 | 0.003 | 0.70 | 0 | 0.02 | 0.31 |
| Forest | 0.03 | 0.02 | 0.71 | 0.18 | 0.14 | 0.17 |
| Disturbed | 0.40 | 0.26 | 0.09 | 0.54 | 0.36 | 0.01 |
| Developed | 0.61 | 0.71 | 0.25 | 0.27 | 0.45 | 0.02 |
| Flooded | 0 | 0.01 | 0.48 | 0.01 | 0.03 | 0.21 |
Figure 1.
Density of neighborhoods in Bokungu Health Zone. Cases are represented by light blue triangles. Areas with an z score greater than 1.96 are in red whereas those with an z score less than –1.96 are in blue.
Animal survey.
Sixty-eight animals were collected, representing 3 orders and 12 genera of mammals (Table 5). A total of eight animals were collected from within six households and six animals were collected from behind one of the confirmed case's home. The remaining animals were either captured in the forested area, in the grassland, or were purchased from local hunters. Three rope squirrel specimens were purchased from hunters and were said to be captured within 16 km of the town of Bokungu. Two of the three (66.7%) rope squirrels (Funisciurus sp.) were positive by ELISA for IgG antibodies to Orthopoxviruses. All other samples were negative by ELISA and all samples were negative via real-time PCR specific for Orthopoxviruses.
Table 5.
Small mammals collected in the Bokungu Health Zone during December 2013
| Order | Genus | Number of specimens | Number of animals positive for anti-Orthopoxvirus IgG antibodies (%) |
|---|---|---|---|
| Rodentia | Mastomys | 1 | 0 |
| Rodentia | Lophoromys | 5 | 0 |
| Rodentia | Hybomys | 3 | 0 |
| Rodentia | Mus | 21 | 0 |
| Rodentia | Hylomyscus | 4 | 0 |
| Rodentia | Rattus | 9 | 0 |
| Rodentia | Oenomys | 8 | 0 |
| Rodentia | Funisciurus | 3 | 2 (66.7) |
| Rodentia | Praomys | 8 | 0 |
| Rodentia | Lemniscomys | 3 | 0 |
| Insectivora | Crocidura | 2 | 0 |
| Macroscelidea | Petrodromus | 1 | 0 |
| Total | 68 | 2 (2.9) |
Discussion
Limited information exists regarding transmission of MPXV within households. Each of the households examined here exhibited at least one human-to-human transmission event, with some households having multiple transmission events. Clearly, transmission within a household setting is a major risk factor for MPXV transmission among humans. This emphasizes the importance of identifying factors within the household that affect transmission between family members. A number of activities that require a high level of contact were included in the questionnaire, including sleeping in the same room or bed, sharing dishes or cups, helping to clean the body and clothes, and assisting with toileting. Among these, sleeping in the same room or bed, sharing food on the same dish, and drinking out of the same cup as the primary case were found to be associated with an increased risk of developing MPX. We note that the factors that were associated with an increased risk all had a higher likelihood of virus introduction directly to the oral mucosa when compared with the activities that were not significantly associated with developing MPX. While activities such as washing and helping with toileting involve a great amount of contact with a patient, these activities expose the skin, but not the mucosa, to the virus.
Only a limited number of studies have investigated the initial introduction of MPXV within a community or household. It is generally believed that the initial introduction into a community occurs through the handling and consumption of infected wild animals. Although previous studies have identified the virus in a rope squirrel (Funisciurus sp.), and a sooty mangabey, (Cercocebus sp.),12,13 attempts to definitively determine the reservoir species of MPXV have been persistently unsuccessful.8,9,12,13 Neutralizing Orthopoxvirus antibodies have been found in multiple squirrel species, Gambian rats, elephant shrews, and domestic pigs that have been collected in DRC,9,10,16 as well as a variety of mice, rats, and squirrels (Graphiurus, Xeru, Funisciurus, Heliosciurus, and Cricetomys sp.) collected in Ghana.17 The presence of antibodies indicates that these animals have been exposed to an Orthopoxvirus; however, these results do not confirm that the introduction of MPXV into the human population is through these animals.
Previous reports suggest that the introduction of MPXV into the human population occurs via interactions with infected wildlife, most likely by eating or handling bush meat.9 The present case–control investigation shows that that sleeping on the floor was associated with an increased risk of infection, whereas living in a home with a door, eating duiker, and preparing wild animal meat were identified as protective factors. Multiple different questions were included in the survey that asked about contact with live and dead animals and animal excrement, as well as animal bites and scratches. None of these were shown to significantly change the risk of infection. While it is unclear how the risk factors that were identified are related, we speculate that they may reflect wealth level; with wealthier households more likely to have doors on their houses and sleep off the floor.
In this investigation, data reveal that it was not the hunter or person who prepares the meat that was most likely to introduce the virus into a household, instead it was school-aged males. Our results do not allow us to conclude why this population is the most at risk. It is possible that this is due to an increased susceptibility of this population to infection or it may be due to an activity that puts them at a higher risk of exposure. Such activities could cause a primary introduction, such as interactions with the host animal, or they could result in increased risk of transmission, such as coming in close contact with other young males who are infected. A non-significant correlation between smallpox vaccination status and MPX infection was observed. This association is likely secondarily related to the age of the affected individuals, as smallpox vaccination was stopped ~34 years ago and thus only the older individuals had a positive history of vaccination. Future investigations regarding what activities young males perform that increase their risk of coming into contact with infectious material (either human cases or zoonotic exposures) are needed.
The case–control investigation selected controls to match the cases based on age and gender. If, as postulated above, risk is a related to an individual's age and gender, it is possible that matching on these characteristics masked the activities that put individuals at risk. Future evaluations should consider specifically the activities of young males versus other household members. Nevertheless, knowing that this population is often the group that introduces the infection into the household is important to consider when designing future education and prevention programs.
The placement of a domicile within a community can also affect the type of wild animal species household members may be exposed to. Cases were found to live in areas of higher building density, suggesting that they are more likely to be in high population areas. The land within 500 m of the cases' homes was significantly more likely to be a disturbed habitat and less likely to be developed compared with the general population. Disturbed land is any land that has been cleared of forest, most often for farming. Previous studies suggested that the cleared land creates a habitat preferred by the animals that transmit MPX or, possibly that the disturbed land creates a space where humans are more likely to come in contact with the animals because of either loss of cover or frequency of use.18,19 Observations from this study are consistent with these hypotheses. Further, the animal collection data showed that a variety of rodents were in the areas around affected homes. It will be important to identify animal species that are more frequently encountered around case homes in comparison to control homes.
Mice (Mus sp.) were the animal most frequently caught in the traps around the case houses. Three rope squirrels (Funisciurus sp.) were caught near the village of Bokungu, two of which had previously been exposed to an Orthopoxvirus. Anecdotally, a number of families that participated in the investigations mentioned that their children often catch and play with squirrels. One family related how the first case of MPX in the household occurred 5 days after a captured squirrel died in their home. Although not conclusive, this history suggests a possible source animal for MPXV infection in the family. It is tempting to draw a relationship between these accounts and the many stories of young boys playing with squirrels. Additional research investigating the roles of young boys and small mammals is necessary.
These investigations are limited by our ability to confirm infection and exposure. Although we were able to confirm that the lesions were because of MPXV in 30% of the cases using molecular diagnostics, the remaining cases were identified solely by symptoms. This limitation was because of the fact that most patients were interviewed after their symptoms had resolved. Another limitation is the possibility of immunity within families because of previous exposure to MPXV or another Orthopoxvirus. Older individuals may have been affected to a lesser extent than the children due to preexisting antibodies. In the future we would like to evaluate family members for preexisting antibodies and relate that to exposure and symptoms.
This investigation has added to our basic understanding of MPX and its transmission. Our data have identified a subpopulation that most frequently brings MPX into the household, as well as factors associated with an increased risk of infection within a household and behaviors that increase the probability of transmitting the infection to others. Based on the risk factors identified in this study, communities where MPX occurs should be educated to limit contact with individuals who are sick. This includes having the ill people sleep away from others in the household and designating specific cups and utensils for them to use. Future projects will be designed to further elucidate the risk factors related to MPX infection.
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Authors' addresses: Leisha Diane Nolen, Bacterial Special Pathogens Branch, U.S. Centers for Disease Control and Prevention, Atlanta, GA, E-mail: xdf8@cdc.gov. Lynda Osadebe, Benjamin Monroe, Jeffrey Doty, Joelle Kabamba, Andrea M. McCollum, and Mary G. Reynolds, Poxvirus and Rabies Branch, U.S. Centers for Disease Control and Prevention, Atlanta, GA, E-mails: losadebe@gmail.com, ihd2@cdc.gov, uwb7@cdc.gov, jlz7@cdc.gov, azv4@cdc.gov, and nzr6@cdc.gov. Jacques Katomba and Jacques Likofata, Department of Epidemiology, Ministère de la Sante, Kinshasa, The Democratic Republic of the Congo, E-mails: jxkk2000@gmail.com and jacqueslikofata@gmail.com. Daniel Mukadi, Poxvirus and Rabies Branch, Centers for Disease Control and Prevention, Kinshasa, The Democratic Republic of the Congo, E-mail: drmukadi@gmail.com. Lem's Kalemba and Malekani Jean, Department of Biology, Université de Kinshasa, Kinshasa, The Democratic Republic of the Congo, E-mails: lemskalemba@yahoo.com and elevagefaune@yahoo.fr. Pierre Lokwa Bomponda and Jules Inonga Lokota, Ministère de la Sante, Tshuapa Health District, Tshuapa, The Democratic Republic of the Congo, E-mails: Plbompond@yahoo.fr and JuInLokoto@yahoo.fr. Marcel Pie Balilo and Toutou Likafi, Department of Hemorrhagic Fever and Monkeypox, Ministère de la Sante, Tshuapa Health District, Tshuapa, The Democratic Republic of the Congo, E-mails: marcelbalilopie@yahoo.fr and toutoulikafi@gmail.com. Robert Shongo, Ministry of Health, Surveillance, Kinshasa, The Democratic Republic of the Congo, E-mail: robert_shongo@yahoo.fr. Jean-Jacques Muyembe Tamfum, Institut National de Recherche Biomédicale, Ministry of Health, Kinshasa, The Democratic Republic of the Congo, E-mail: muyembejj@gmail.com. Emile Wemakoy Okitolonda, Center for HIV/AIDS Strategic Information, University of Kinshasa, Kinshasa, The Democratic Republic of the Congo, E-mail: okitow@yahoo.fr.
References
- 1.Jezek Z, Szczeniowski M, Paluku KM, Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987;156:293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 2.Hutson CL, Carroll DS, Gallardo-Romero N, Weiss S, Clemmons C, Hughes CM, Salzer JS, Olson VA, Abel J, Karem KL, Damon IK. Monkeypox disease transmission in an experimental setting: prairie dog animal model. PLoS One. 2011;6:e28295. doi: 10.1371/journal.pone.0028295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds MG, Yorita KL, Kuehnert MJ, Davidson WB, Huhn GD, Holman RC, Damon IK. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773–780. doi: 10.1086/505880. [DOI] [PubMed] [Google Scholar]
- 4.Learned LA, Reynolds MG, Wassa DW, Li Y, Olson VA, Karem K, Stempora LL, Braden ZH, Kline R, Likos A, Libama F, Moudzeo H, Bolanda JD, Tarangonia P, Boumandoki P, Formenty P, Harvey JM, Damon IK. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am J Trop Med Hyg. 2005;73:428–434. [PubMed] [Google Scholar]
- 5.Jezek Z, Arita I, Mutombo M, Dunn C, Nakano JH, Szczeniowski M. Four generations of probable person-to-person transmission of human monkeypox. Am J Epidemiol. 1986;123:1004–1012. doi: 10.1093/oxfordjournals.aje.a114328. [DOI] [PubMed] [Google Scholar]
- 6.Formenty P, Muntasir MO, Damon I, Chowdhary V, Opoka ML, Monimart C, Mutasim EM, Manuguerra JC, Davidson WB, Karem KL, Cabeza J, Wang S, Malik MR, Durand T, Khalid A, Rioton T, Kuong-Ruay A, Babiker AA, Karsani ME, Abdalla MS. Human monkeypox outbreak caused by novel virus belonging to Congo Basin clade, Sudan, 2005. Emerg Infect Dis. 2010;16:1539–1545. doi: 10.3201/eid1610.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds MG, Carroll DS, Karem KL. Factors affecting the likelihood of monkeypox's emergence and spread in the post-smallpox era. Curr Opin Virol. 2012;2:335–343. doi: 10.1016/j.coviro.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arita I, Jezek Z, Khodakevich L, Ruti K. Human monkeypox: a newly emerged Orthopoxvirus zoonosis in the tropical rain forests of Africa. Am J Trop Med Hyg. 1985;34:781–789. doi: 10.4269/ajtmh.1985.34.781. [DOI] [PubMed] [Google Scholar]
- 9.Hutin YJ, Williams RJ, Malfait P, Pebody R, Loparev VN, Ropp SL, Rodriguez M, Knight JC, Tshioko FK, Khan AS, Szczeniowski MV, Esposito JJ. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001;7:434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khodakevich L, Jezek Z, Messinger D. Monkeypox virus: ecology and public health significance. Bull World Health Organ. 1988;66:747–752. [PMC free article] [PubMed] [Google Scholar]
- 11.Sarkar JK, Mitra AC, Mukherjee MK, De SK. Virus excretion in smallpox. 2. Excretion in the throats of household contacts. Bull World Health Organ. 1973;48:523–527. [PMC free article] [PubMed] [Google Scholar]
- 12.Khodakevich L, Jezek Z, Kinzanzka K. Isolation of monkeypox virus from wild squirrel infected in nature. Lancet. 1986;1:98–99. doi: 10.1016/S0140-6736(86)90748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radonic A, Metzger S, Dabrowski PW, Couacy-Hymann E, Schuenadel L, Kurth A, Matz-Rensing K, Boesch C, Leendertz FH, Nitsche A. Fatal monkeypox in wild-living sooty mangabey, Cote d'Ivoire, 2012. Emerg Infect Dis. 2014;20:1009–1011. doi: 10.3201/eid2006.131329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulesh DA, Loveless BM, Norwood D, Garrison J, Whitehouse CA, Hartmann C, Mucker E, Miller D, Wasieloski LP, Jr, Huggins J, Huhn G, Miser LL, Imig C, Martinez M, Larsen T, Rossi CA, Ludwig GV. Monkeypox virus detection in rodents using real-time 3′-minor groove binder TaqMan assays on the Roche LightCycler. Lab Invest. 2004;84:1200–1208. doi: 10.1038/labinvest.3700143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Olson VA, Laue T, Laker MT, Damon IK. Detection of monkeypox virus with real-time PCR assays. J Clin Virol. 2006;36:194–203. doi: 10.1016/j.jcv.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jezek Z, Fenner F. Human monkeypox. Monogr Virol. 1988;17:111–134. [Google Scholar]
- 17.Reynolds MG, Carroll DS, Olson VA, Hughes C, Galley J, Likos A, Montgomery JM, Suu-Ire R, Kwasi MO, Jeffrey Root J, Braden Z, Abel J, Clemmons C, Regnery R, Karem K, Damon IK. A silent enzootic of an orthopoxvirus in Ghana, West Africa: evidence for multi-species involvement in the absence of widespread human disease. Am J Trop Med Hyg. 2010;82:746–754. doi: 10.4269/ajtmh.2010.09-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khodakevich L, Szczeniowski M, Manbu-ma D, Jezek Z, Marennikova S, Nakano J, Messinger D. The role of squirrels in sustaining monkeypox virus transmission. Trop Geogr Med. 1987;39:115–122. [PubMed] [Google Scholar]
- 19.Khodakevich L, Szczeniowski M, Nambu-ma D, Jezek Z, Marennikova S, Nakano J, Meier F. Monkeypox virus in relation to the ecological features surrounding human settlements in Bumba zone, Zaire. Trop Geogr Med. 1987;39:56–63. [PubMed] [Google Scholar]