Abstract
The capacity for self-regulation allows people to control their thoughts, behaviors, emotions, and desires. In spite of this impressive ability, failures of self-regulation are common and contribute to numerous societal problems, from obesity to drug addiction. Such failures frequently occur following exposure to highly tempting cues, during negative moods, or after self-regulatory resources have been depleted. Here we review the available neuroscientific evidence regarding self-regulation and its failures. At its core, self-regulation involves a critical balance between the strength of an impulse and an individual’s ability to inhibit the desired behavior. Although neuroimaging and patient studies provide consistent evidence regarding the reward aspects of impulses and desires, the neural mechanisms that underlie the capacity for control have eluded consensus, with various executive control regions implicated in different studies. We outline the necessary properties for a self-regulation control system and suggest that the use of resting-state functional connectivity analyses may be useful for understanding how people regulate their behavior and why they sometimes fail in their attempts.
Keywords: self-control, frontoparietal network, cingulo-opercular network, reward, resting-state, depletion
Self-regulation allows people to make plans, choose from alternatives, control impulses, inhibit unwanted thoughts, and regulate appetitive behavior (Heatherton 2011). Although humans have a remarkable capacity for self-regulation, at least compared to nonhuman primates, failures are nevertheless common and can arise from a variety of circumstances (Baumeister & Heatherton 1996, Heatherton & Wagner 2011, Wagner & Heatherton 2014). For instance, a recent experience sampling study showed that people failed to resist their temptations on about one in five occasions (Hofmann et al. 2012). Such self-control failures are an important cause of several contemporary societal and health problems---obesity, sexual infidelity, and so on---and are especially important for addiction. Whereas poor self-control puts people at risk for various health and interpersonal problems, those who are best able to self-regulate their behaviors demonstrate better mental health (Duckworth & Seligman 2005, Tangney et al. 2004) and are less at risk for developing addiction problems or engaging in risky sexual behavior (Quinn & Fromme 2010). An understanding of the brain mechanisms underlying self-regulation can provide valuable insights into how people are able to regulate and control their thoughts, behaviors, and emotional states and what happens on those occasions when they fail to do so. Although neuroscience has formed a relatively clear picture of the brain systems that give rise to reward motivation, the field has struggled to coalesce around a unified view of the control mechanisms that support self-regulation.
In healthy adults, self-regulation failures often occur in the presence of a highly desirable reward cue, particularly when the cue follows a precipitating event, such as emotional distress or exhaustion of self-regulatory resources. Successful self-regulation therefore requires a balance between the strength of reward cues and the capacity to keep them in check (Heatherton & Wagner 2011). As such, self-regulation failure can occur in response to an overwhelming impulse or when the capacity to self-regulate is impaired or absent. Three common threats to this balance have been identified: exposure to tempting cues (e.g., food, drugs), emotional and social distress, and depletion of self-regulatory resources.
EXPOSURE TO REWARD CUES
Nonhuman animal studies show that consuming rewards (e.g., foods, drugs) or engaging in rewarding activities (e.g., sex) is associated with activation of the mesolimbic dopamine system (e.g., the ventral tegmental area and nucleus accumbens/ventral striatum) and the orbitofrontal cortex (OFC) (Boileau et al. 2003, Carelli et al. 2000, Damsma et al. 1992, Everitt 1990, Kringelbach 2005, Schilström et al. 1998). In humans, functional neuroimaging research has similarly shown that activity in the ventral striatum and OFC increases during reward consumption (Breiter et al. 1997, Gottfried et al. 2003, Kringelbach et al. 2003)
However, humans are particularly adept at learning to predict reward opportunities. From a very early age, humans are surrounded by potential indulgences, so much so that the dominant form of self-regulation in daily life is likely one of impulse control (Hofmann et al. 2011). In the scientific literature, an impulse is a craving or desire to consume a particular item or engage in a behavior, and the strength of the impulse reflects the likelihood of enactment (Metcalfe & Mischel 1999). The most common way an impulse can arise is when experiencing a reward cue, such as a food advertisement or the sight and smell of a cigarette. Reward cue exposure increases the craving for, and consumption of, the substance (e.g., Carter & Tiffany 1999, Federoff et al. 1997, Harris et al. 2009, Lambert et al. 1991, Sayette et al. 2001). Neuroimaging studies consistently show activation of the ventral striatum and OFC following exposure to reward cues such as food images, drug cues, and attractive faces as well as to secondary reward cues such as money (Cloutier et al. 2008, Due et al. 2002, Garavan et al. 2000, Knutson et al. 2005, Somerville et al. 2011, van der Laan et al. 2011) (Figure 1).
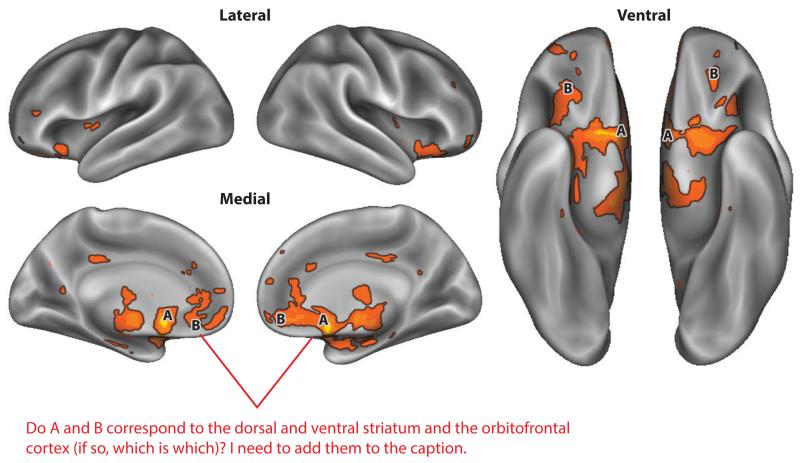
Figure 1.
An automated, large-scale meta-analysis of neuroimaging studies using text mining (Yarkoni et al. 2011) on the term “reward” reveals robust activations in the dorsal and ventral striatum and the orbitofrontal cortex. Activations are displayed on an inflated rendering of the lateral, medial, and ventral cortical surfaces (Van Essen et al. 2012).
The physiological effects of reward cue exposure on arousal and craving can occur even when people are not consciously aware of them. Reward cues can prime people to experience pleasurable thoughts about substance use (Hofmann et al. 2010, Sayette & Hufford 1997), can capture people’s attention when the cues are incidentally presented in the background of films (Lochbuehler et al. 2011), and can even activate motor schemas for enactment [e.g., the mental simulation of holding and smoking a cigarette (Tiffany 1990)]. For example, functional neuroimaging work has shown that when smokers (for whom the manual action of smoking is highly practiced) incidentally view other people smoking, brain regions associated with action observation are engaged (Wagner et al. 2011, Yalachkov et al. 2009).
More recently, the field of neuroscience has made attempts to link reward system cue reactivity to real-world appetitive behaviors, and this work has important implications for individuals who are at risk for obesity or substance abuse problems (Demos et al. 2012, Janes et al. 2010, McClernon et al. 2008, Stice et al. 2010). Demos and colleagues (2012) demonstrated that individual differences in ventral striatum responses to food cues predicted subsequent weight gain in a six-month follow-up, and responses to images of erotic scenes predicted individual differences in sexual interest. These effects were domain specific. Food cue reactivity predicted subsequent weight gain but not sexual interest and vice versa.
Although this study demonstrated the usefulness of reward system cue reactivity in predicting failures to regulate impulses, further work was required to understand the relationship between cue reactivity and the daily battle with desires---the loss of which may lead to enactment. Lopez and colleagues (2014) extended this work using smartphone experience sampling technology and showed that reward activity in response to appetizing food images predicts everyday food desires.
Indeed, measures of reward cue reactivity can reflect a long-lasting sensitivity to rewards, the excess of which can cause a variety of problems for individuals. For instance, early work by Mischel and colleagues (1989) demonstrated that people who had difficulty delaying gratification as children grew up less successful and with more health problems than their peers. Moreover, this inability to delay gratification led to long-term consequences for reward sensitivity. Casey and colleagues (2011) showed that children who had difficulty delaying gratification (e.g., Mischel et al. 1989) exhibited heightened cue reactivity in the ventral striatum when viewing appetitive stimuli over 40 years later.
Behaviorally, we know that momentary lapses of self-control can lead to binge behavior, as is common when dieters or addicts fall off the wagon and consume even more of the forbidden substance following an initial indulgence (Baumeister & Heatherton 1996). In one of the first studies of this form of lapse-activated self-regulation failure, dieters and nondieters were required to consume a high-calorie milkshake under the guise that they were participating in a study on taste perception (Herman & Mack 1975). Whereas nondieters ate less after consuming the milkshake, dieters paradoxically ate more---a finding aptly referred to as the what-the-hell effect (Herman & Polivy 1983). Although this effect has been replicated numerous times in dieters as well as smokers and drug abusers, among whom violations of abstinence are associated with relapse (Marlatt & Gordon 1985), exactly how a small indulgence escalates into binge-like behavior is less clear. Thus far, a single neuroimaging study has examined lapse-activated consumption by forcing dieters to break their diets by consuming a high-calorie milkshake prior to measuring neural food cue reactivity (Demos et al. 2011). In the absence of a dietary violation, dieters demonstrated minimal reward cue reactivity to appetizing food cues in the ventral striatum, suggesting they were successfully self-regulating. Dieters who experienced a momentary diet violation, however, showed robust food cue reactivity. A critical question, however, is whether individual differences in reward cue reactivity reflect differences in the sensitivity of the reward system from one person to the next (i.e., some people have overactive reward systems) or whether individual differences in reward cue reactivity are the consequence of individual differences in self-control.
EMOTIONAL AND SOCIAL DISTRESS
Emotional and social distress can also precipitate self-regulation failure and lead to binge eating or drinking (Haedt-Matt & Keel 2011, Witkiewitz & Villarroel 2009), gambling (Raviv 1993), and even excessive Internet use (LaRose et al. 2003). In behavioral studies, inductions of negative mood or social distress (e.g., social rejection) lead to disinhibited and occasionally even aggressive behavior (e.g., Twenge et al. 2001). For instance, negative mood inductions lead dieters to subsequently overeat (Heatherton et al. 1998, 1991) and smokers to crave cigarettes (Willner & Jones 1996). Negative affect has also been shown to reduce people’s ability to delay gratification, biasing them toward smaller, immediate monetary rewards over larger, delayed payoffs (e.g., Lerner et al. 2013, Mischel et al. 1973, Twenge et al. 2001); has been linked to increased cravings for carbohydrate-rich foods (Christensen & Pettijohn 2001); and, among smokers, increases both the intensity of smoking (McKee et al. 2011) and the amount of pleasure people report from smoking a cigarette (Zinser et al. 1992). Similarly, social rejection has been shown to increase consumption of unhealthy foods, reduce task persistence, and interfere with the ability to sustain attention (Baumeister et al. 2005, Oaten et al. 2008).
Neuroscience studies have shown that emotional and social distress enhance reward cue reactivity (Killgore & Yurgelun-Todd 2006, Wagner et al. 2012). For example, dieters exposed to negative mood inductions show increased food cue reactivity in the OFC (Wagner et al. 2012), a finding that parallels the effects of dietary-restraint violations on food cue reactivity and suggests a potential mechanism for why chronic dieters frequently overeat following negative mood inductions (e.g., Frost et al. 1982; Heatherton et al. 1998, 1991).
One possibility is that emotional and social distress increases the perceived value of reward cues, and as such, they become more difficult to control. Alternatively, emotional and social distress may directly influence self-regulation control mechanisms, the consequence of which would manifest as enhanced sensitivity to rewards.
SELF-REGULATORY DEPLETION
Another common threat to successful self-regulation derives from the long-standing theory that self-regulation is a domain-general resource subject to fatigue (Baumeister & Heatherton 1996). As such, effortful attempts to self-regulate behavior can temporarily deplete the resources needed for additional attempts at self-regulation, thereby leaving individuals vulnerable to temptation. This strength model of self-regulation has received considerable support from numerous behavioral studies (for a meta-analysis, see Hagger et al. 2010) and from real-world settings using experience sampling methods (Hofmann et al. 2011). For example, engaging in a prior, effortful self-control task can subsequently make people more vulnerable to temptations such as food and alcohol (Muraven et al. 2002, Vohs & Heatherton 2000), less able to control their emotions (Schmeichel 2007), and more likely to violate social norms (DeBono et al. 2011, Vohs et al. 2005).
More recently, researchers have suggested that self-regulatory depletion may serve to change people’s motivations such that they shift away from engaging in effortful control and move toward rewarding the self (e.g., Inzlicht et al. 2014). Consistent with this view is the finding that following self-regulatory depletion, people become more sensitive to reward cues (Schmeichel et al. 2010). Research on the neural basis of self-regulatory depletion is limited, with only a handful of studies investigating these effects. However, consistent with behavioral findings that self-regulatory depletion results in enhanced sensitivity to rewards and emotions, neuroimaging studies have demonstrated that self-regulatory depletion can enhance amygdala responsivity to emotional scenes (Wagner & Heatherton 2013) and OFC responsivity to food cues in dieters (Wagner et al. 2013).
COGNITIVE NEUROSCIENCE OF SELF-REGULATION FAILURE: STRIKING A BALANCE
Collectively, studies of self-regulation suggest that a critical balance exists between the strength of an impulse and an individual’s ability to inhibit either the desire itself or enactment upon the desire. One way in which the balance may tip in favor of automatic impulses is via heightened cue reactivity. Following a long period of abstinence, the reward system may respond in an exaggerated manner to an infrequent reward. Such an account could explain the pattern of neural activity in lapse-activated consumption studies (e.g., Demos et al. 2011) and may even offer a neural mechanism for relapse in addiction. Similarly, in the context of emotional or social distress, reward cues may appear more rewarding as evidenced by enhanced activity in these regions (e.g., Wagner et al. 2012). According to this view, self-regulation remains constant while reward value changes as a function of circumstance. An alternative account is that reward is simply reward---that reward-prediction signals in response to learned cues are well-calibrated through repeated experience---and the reason reward cue reactivity changes from one instance to another is because self-regulation processes wax and wane dynamically as a function of circumstance. This latter account is more parsimonious with a strength model of self-regulation, in which heightened reward-system sensitivity from one moment to another reflects the consequence of diminished self-regulation.
In summary, behavioral and neuroscientific investigations of human reward and self-regulation paint a relatively clear picture in terms of how reward cues engage regions of the mesolimbic dopamine system, the OFC, and, in some instances, motor planning regions to motivate real-world behavior. Equally clear are the behavioral and neural consequences of threats to self-regulation---following such threats, reward cue reactivity increases and people give in to temptation. What is noticeably missing, however, is a coherent picture of the source(s) of self-regulatory control, and the primary challenge for neuroscientific research in the years to come will be to better characterize the brain mechanisms that give rise to self-regulation. We now turn to a review of the putative neural correlates of self-regulation.
WHERE IS SELF-REGULATION IN THE BRAIN?
One commonly held view of self-regulation is that humans have evolved specific control systems, particularly within the prefrontal cortex (PFC), that permit superior planning and behavioral flexibility, perhaps owing to the disproportionate amount of cortical expansion in the human PFC (Rilling 2006), increased white-matter PFC connectivity (Schoenemann et al. 2005), or an extended period of prefrontal white-matter development relative to nonhuman primates (Sakai et al. 2011). As human safety and survival has long depended on living in groups, investigators have suggested that humans have a fundamental need to belong (Baumeister & Leary 1995) that motivates them to avoid behaviors that could lead to their expulsion from the group (e.g., theft of common resources), as this would greatly lessen their chances for survival (Goodall 1986, Heatherton 2011).
The PFC supports high-level cognitive abilities necessary for self-regulation, such as working memory, response inhibition, attentional filtering, decision making, and planning (Miller & Cohen 2001, Tranel et al. 1994). Anatomically, the PFC is the portion of the frontal lobe that lies anterior to the primary and secondary motor cortices. Researchers generally agree that the PFC can be divided into several distinct areas, although the precise anatomical boundaries separating subregions of the PFC are debatable. Three PFC subregions have been identified as important for self-regulation: the ventromedial PFC (VMPFC), the lateral PFC (including the dorsal and ventral convexities), and the anterior cingulate cortex (ACC). We discuss each in turn.
Ventromedial Prefrontal Cortex
The exact anatomical boundaries between the VMPFC and OFC are coarsely defined, perhaps owing to the fact that the orbital PFC and VMPFC are cytoarchitectonically similar (Öngür & Price 2000). As a result, these terms are often used interchangeably when referring to regions along the ventral medial wall of the PFC. The superior border of the VMPFC is considered to originate around the genu of the corpus callosum [including the medial aspect of Brodmann areas (BAs) 11, 12, and 25 and the ventral portions of BAs 10 and 32], and the inferior border often overlaps the gyrus rectus and the middle orbital gryus. The VMPFC shares reciprocal connections with subcortical limbic structures such as the amygdala (Amaral & Price 1984) as well as regions typically associated with reward processing, such as the ventral striatum (Haber et al. 1995). Because of its interconnectedness with both the amygdala and ventral striatum, the VMPFC has been implicated in both emotion regulation (for a review, see Quirk & Beer 2006) and self-regulation of social and appetitive behavior (Fehr & Camerer 2007, Hare et al. 2009, Lin et al. 2012).
Perhaps the best evidence of VMPFC involvement in self-regulation comes from case studies of patients with lesions to the VMPFC---the most famous of which is that of Phineas Gauge, a 25-year-old railroad foreman from Lebanon, New Hampshire. In 1848, Gauge suffered extensive damage to his left VMPFC and a portion of his ACC (Harlow 1848) when an explosion drove an iron bar projectile that entered below his cheekbone and exited the top of his skull. Although Gauge survived the accident---indeed, he never lost consciousness throughout his ordeal---his personality and behavior were notably different after his injury. Originally considered to be a dependable, hardworking, honest individual, Gauge was later described to be “gross, profane, coarse, and vulgar to such a degree that [he] was intolerable to decent people” (Anonymous, 1851, p. 89). What emerged from cases like Gauge and others to follow with traumatic brain injuries or lesions involving the VMPFC is that VMPFC damage results in a profound inability to regulate social, emotional, and appetitive behavior. Such patients are often hypersexual and aggressive toward others (Grafman et al. 1996) and may engage in excessive overeating (Erb et al. 1989, Woolley et al. 2007), despite the fact that they are aware of their inappropriate behavior.
Lateral Prefrontal Cortex
The lateral PFC is comprised of both the dorsal and ventral convexities---specifically, BAs 8, 9, and 46 and 44, 45, and 47---and it receives highly processed sensory input from the dorsal and ventral visual streams (i.e., the so-called what and where pathways) (Barbas 1988). It projects to secondary motor regions (Petrides & Pandya 1999), the basal ganglia (Nambu 2008), and the ACC and VMPFC (McDonald et al. 1996). The lateral PFC has been traditionally associated with language functions in the left hemisphere (BAs 44, 45, and 47) and, more generally, with core executive processes such as working memory (Curtis & D’Esposito 2003, Smith & Jonides 1999), response selection (Thompson-Schill et al. 2005), and response inhibition (Garavan et al. 1999). As such, the lateral PFC may play a crucial role in supporting many of the complex cognitive operations needed for successful self-regulation (Cohen & Lieberman 2010). In contrast to the disinhibition of social and appetitive behavior seen following VMPFC damage, patients with lateral PFC lesions struggle to plan, coordinate, and maintain complex goals (Petrides & Milner 1982, Shallice 1982, Shallice & Burgess 1996), fail to adapt to changing goals or circumstances, and have difficulty filtering out goal-irrelevant distractors. Lateral PFC patients show no deficits in understanding appropriate social and emotional behavior (Bar-On et al. 2003), instead performing poorly on neuropsychological tests with switching task demands (Milner 1963) or tests requiring inhibitory control. These patients perseverate on the Wisconsin Card Sorting Test, a task that requires trial-by-error learning of sorting rules that change to a new sorting rule once the current rule is discovered (Milner 1963), and make more errors and take longer on the Stroop task when asked to name the ink color of printed words in trials in which there is a mismatch between the name of the word (e.g., red) and color of the ink (e.g., blue) (Vendrell et al. 1995).
A real-world example of a task that lateral PFC patients fail is Shallice & Burgess’s (1991) multiple errands task, in which patients are given several simple errands requiring them to shop for a list of items, with the additional instructions to spend as little money as possible and to avoid entering a shop unless they plan to purchase an item. The patients were surreptitiously followed by research assistants to assess their performance. Compared to healthy individuals, lateral PFC patients struggled to complete the tasks, visited the same shop multiple times, forgot items on the list, and even failed to pay before leaving shops. Such reports dovetail nicely with the anecdotal report of the famous neurosurgeon Wilder Penfield, who, in the 1930s, operated on his own sister to remove a glioblastoma from her right lateral PFC. Penfield noted that although her personality and ability to express emotion were unchanged postsurgery, she demonstrated marked impairments carrying out what used to be trivial household chores. Of one instance, he wrote, “When the appointed hour arrived she was in the kitchen, the food was all there, one or two things were on the stove, but the salad was not ready, the meat had not been started and she was distressed and confused by her long continued effort alone. It seemed evident that she would never be able to get everything ready at once” (Penfield & Evans 1935, p. 131).
Anterior Cingulate Cortex
The anterior cingulate is the agranular portion of the cingulate, incorporating both the midcingulate and the anterior perigenual cingulate (BAs 24, 25, and 32). Although not strictly a part of the PFC, the ACC is widely associated with cognitive control and conflict monitoring. A functional dissociation along the dorsal and ventral aspects of the ACC has emerged from meta-analyses of neuroimaging studies reporting ACC activity (Bush et al. 2000). Specifically, activity in the dorsal ACC (dACC) often signals the occurrence of cognitive conflicts during a variety of tasks that encourage response competition (for example, the Stroop task), including those that involve the commission of errors (Botvinick et al. 2004, Carter et al. 1998, Kerns et al. 2004). By contrast, activity in the ventral ACC is more typically associated with social and emotional processes (e.g., Somerville et al. 2006, Whalen et al. 1998). This dorsal-cognitive/ventral-affective dissociation within subregions of the ACC is also reflective of their differential pattern of connectivity. For instance, the midcingulate projects to the lateral PFC and motor cortex, whereas the perigenual cingulate projects to the lateral PFC and VMPFC as well as the insula and amygdala (Öngür & Price 2000, Pandya et al. 1981).
Our knowledge of behavioral deficits following ACC damage is somewhat limited. The ACC is less frequently injured during closed-head trauma, so patients with circumscribed lesions to the ACC are rare compared to VMPFC and lateral PFC patients. Some insight into the effects of ACC damage has come from the study of cingulotomy patients undergoing treatment for intractable pain or other psychiatric disorders (Ballantine et al. 1967, Cohen et al. 1999a&b, Corkin 1979, Whitty et al. 1952). Among the more common complaints in patients following cingulotomies are a loss of energy and motivation and blunted emotional affect (Cohen et al. 1999b; Laplane et al. 1981; Wilson & Chang 1974). On neuropsychological tests, patients who underwent cingulotomies demonstrated deficits on the Stroop color-naming task and showed difficulty in spontaneously generating novel responses on a design fluency task (Cohen et al. 1999a).
FUNCTIONAL NEUROIMAGING STUDIES OF SELF-REGULATION
With the possible exception of the ACC, for which limited data exist, studies of patients with PFC lesions coalesce around a fairly unified theme. Whereas the lateral PFC, through its connections with cortical output systems, appears to be critical for enacting the more cognitive aspects of self-regulation, such as goal planning and sequencing, goal maintenance, and attentional filtering of distractors, the VMPFC, with its strong reciprocal connections to subcortical limbic structures, is well positioned to inhibit appetitive or emotional impulses when acting on them would otherwise lead to excessive or socially inappropriate behavior.
Despite the field’s best efforts, the extant neuroimaging literature on self-regulation, including several of our own studies, has struggled to identify consistent activation patterns across studies. For example, some studies provide evidence for a prominent role of the lateral PFC in self-regulation, whereas others implicate the VMPFC or ACC.
Based on our understanding of behavioral impairments following VMPFC damage, and given its interconnectedness to subcortical limbic structures, the VMPFC would appear to be an obvious self-regulation candidate. Indeed, several neuroimaging studies do in fact report heightened VMPFC activity, often in conjunction with heightened lateral PFC activity and in alcoholics (Wrase et al. 2002), drug addicts (Garavan et al. 2000), smokers (David et al. 2005), and obese individuals (Stoeckel et al. 2008). Paradoxically, however, this heightened VMPFC activity is observed in those very individuals who struggle the most to self-regulate appetitive cues, which creates the following conundrum: Both impaired VMPFC functioning (patient studies) and enhanced VMPFC functioning (neuroimaging studies) reflect inadequate self-regulation of appetitive behavior.
Our understanding of the contribution of the VMPFC to self-regulation is further complicated by the region’s seemingly simultaneous role as both a reward evaluator and motivator (Wilson et al. 2004) and an appetitive regulator. Brain imaging studies of reward and self-regulation often obscure the issue to some degree by referring to the region as the OFC in the context of reward and as the VMPFC in the context of self-regulation, but the underlying inconsistency remains. Some researchers have attempted to reconcile the disparate findings by arguing that the VMPFC may function both to inhibit appetitive behaviors when long-term goals or social contexts dictate restraint and to consciously facilitate (i.e., upregulate) and initiate appetitive behaviors when situational factors (e.g., social influences) conspire to promote indulgence. In short, self-regulation and reward motivation need not always compete. The implication here is that alcoholics, smokers, addicts, and obese individuals may alternatively engage self-regulation mechanisms to advance long-term or situational goals to overindulge. To the extent that active goal planning is needed to facilitate indulgence, such an account might also explain the enhanced lateral PFC activity observed in conjunction with VMPFC activity in these studies. College students, for example, may choose to inhibit the temptation to drink and watch movies with friends on a Thursday night before a final exam, but come Friday after the exam, their long-term goal for the evening is to become inebriated.
In line with this possibility, a more recent, alternate hypothesis offers a single-process model of self-regulation whereby self-regulation is the outcome of multiple, subjective value calculations (Inzlicht et al. 2015, Rangel et al. 2008). This hypothesis draws heavily on findings from studies of neuroeconomics and places the VMPFC in the critical position of arbiter as it integrates automatic appetitive and aversive signals alongside other conscious, controlled processes (e.g., situational factors, long-term goals) and then selects the option with the greatest value for enactment (e.g., Hare et al. 2009, Tom et al. 2007). The key distinction between dual-process and single-process models of self-regulation can be appreciated in the college student example described previously. In a dual-process model, the reward motivation signal associated with drinking would increase or decrease depending on whether self-regulation processes were engaged to inhibit (Thursday night) or facilitate (Friday night) the signal. By contrast, the automatic reward motivation signals associated with drinking cues would remain constant in a single-process model. Instead, long-term goals and situational factors would differ from Thursday (e.g., “I need to study to do well in school,” “I shouldn’t drink the night before a final”) to Friday (e.g., “I deserve to celebrate the end of the term”), and these would lead to different subjective value outcomes in the VMPFC.
Although a single-process model of self-regulation is intriguing in that it may offer some potential insight into the distinct roles that the ventral striatum and VMPFC/OFC play in reward motivation and evaluation, other factors make a single-process account of self-regulation unlikely. A key shortcoming of single-process models is that they do not allow for regulation of automatic appetitive and aversive responses in the ventral striatum and amygdala, respectively, yet demonstrations of signal modulations in subcortical limbic structures are numerous (for reviews, see Cohen & Lieberman 2010, Heatherton & Wagner 2011, Ochsner et al. 2012). In addition, lesions to the VMPFC result in disinhibited appetitive behavior. Whereas a dual-process model of self-regulation predicts exactly this outcome, a single-process model would predict that VMPFC damage would result in an inability to calculate the subjective values necessary to drive behavioral outcomes. In essence, VMPFC patients should be paralyzed by indecision, which is a pattern of behavior more characteristic of patients with ACC or lateral PFC lesions.
Neuroimaging studies comparing poor self-regulators (e.g., individuals with eating disorders, smokers, drug-dependent individuals) with controls often report lateral PFC activity associated with self-regulation. Whereas some studies demonstrate enhanced lateral PFC activity associated with impaired self-regulation (David et al. 2005, Garavan et al. 2000, Stoeckel et al. 2008, Wrase et al. 2002), others report the reverse pattern and attribute greater lateral PFC activity to self-regulation success. For example, the lateral PFC is recruited when individuals attempt to inhibit arousal while viewing erotic images, when smokers suppress cravings (Kober et al. 2010), and in dieters in response to food consumption (DelParigi et al. 2007) or when viewing food cues (Demos et al. 2011, Wagner et al. 2013).
Collectively, these studies illustrate one of the many challenges in relating increased or decreased activity in PFC regions to self-regulation success or failure in the absence of independent measures of successful self-regulation, such as reports of craving or desire. On the one hand, enhanced PFC activity during self-regulation could map directly to self-regulation strength (the more active, the better). On the other hand, increased PFC activity during self-regulation could also reflect more effortful but ineffective attempts at self-regulation, such that individuals who struggle to maintain control over automatic appetitive or emotional impulses must engage these systems more robustly to exact the same amount of control as a good self-regulator. In this vein, weak PFC activity during self-regulation may in fact be evidence for efficient, well-practiced self-regulation processes. To the extent that this latter account is true, neuroimaging studies may struggle to use PFC activity as an index of individual differences in self-regulatory ability, as weak PFC activity could be equally indicative of a fluent, efficient self-regulator or an individual who is disinclined to self-regulate at all. As an analogy, a low heart rate is characteristic of both a world-class triathlete on a jog and a couch potato riding an escalator. Indeed, one of the most ubiquitous findings that has emerged from decades of brain imaging research on skill learning and habit formation is the pattern of robust engagement of neural systems when task performance is naive and effortful that, over time, attenuates as a skill or behavior becomes automatic and efficient through practice (Petersen et al. 1998).
To disentangle these competing accounts, researchers have attempted to link changes in PFC activity to changes in reward cue reactivity or real-world self-regulatory behavior. For example, Lopez and colleagues (2014) demonstrated that increased activity in the left inferior frontal gyrus (IFG) during a standard go/no-go self-regulation task predicted successful restraint when people were faced with real-world food temptations. Indeed, individuals with low IFG activity were more than eight times more likely to give in to food temptations. Moreover, the weaker the IFG response, the more the people ate when they succumbed to temptation. The straightforward implication arising from the Lopez et al. (2014) study is that when people are faced with food temptations in their daily lives, the successful ones engage the lateral PFC and inhibit the reward cue reactivity. Unfortunately, such relationships are not readily apparent in neuroimaging studies that pit self-regulation against reward cues. In Lopez et al. (2014), although ventral striatum activity in response to food cues predicted how frequently these individuals gave in to temptation, IFG activity in response to food cues did not similarly predict restraint. Rather, IFG activity during the more deliberate, intentional go/no-go self-regulation task ultimately predicted real-world restraint.
Investigators have observed similar effects in other studies using nearly identical food cue reactivity paradigms. In Demos et al. (2011), dieters who had their diets violated by consuming a high-calorie milkshake demonstrated greater reward cue reactivity in the ventral striatum when viewing food cues. The left IFG was also active in dieters when viewing food cues; however, this activity was observed for all dieters, regardless of whether their diets were violated, and had no influence over reward activity in the ventral striatum. In Wagner et al. (2013), whereas depleted dieters showed greater food cue reactivity in the OFC compared with nondepleted dieters, all dieters showed enhanced IFG activity when viewing food cues.
In each of these studies, incidental viewing of appetitive cues led to heightened reward-related neural responses in the ventral striatum and OFC in the absence of commensurate changes in PFC activity. By contrast, explicit attempts to regulate cravings and desires have been associated with changes in lateral PFC and ACC activity, showing increased activity in response to cigarette paraphernalia when smokers attempt to downregulate responses (Brody et al. 2007, Kober et al. 2010), to food cues among dieters (Siep et al. 2012), to drug cues among substance abusers (Volkow et al. 2010), and to monetary rewards among just about everybody (Delgado et al. 2008). Across all of these studies, engaging in explicit self-regulation resulted in decreased activity in the striatum and OFC.
When considered together, neuroimaging studies show a large degree of variability in the putative neural substrates of self-regulation, and cognitive neuroscience models of self-regulation have drawn upon these findings to champion the role of the VMPFC (Inzlicht Elkins-Brown & Berkman 2015), the lateral PFC (Heatherton & Wagner 2011), the right ventrolateral prefrontal (Cohen & Lieberman 2010) and right inferior frontal cortices (Aron et al. 2014), and the dACC (Botvinick & Braver 2015). Moreover, many of the extant models propose different mechanisms by which self-regulation functions to regulate behavior, with some models suggesting that control regions act to inhibit reward motivation and others suggesting that they act to evaluate motivation signals both for and against behavior, the winner of which results in self-regulation or indulgence.
That said, the struggle to reach consensus on a cognitive neuroscience model of self-regulation should not be considered an indictment of the field of self-regulation research. Indeed, most models are in relative agreement regarding the functional, anatomic correlates of reward processing. Rather, the theoretical differences across models highlight the many challenges inherent to the study of self-regulation. To reconcile theoretical and neuroanatomical differences across theories of self-regulation, the field may need to consider alternatives to single- and dual-process models of self-regulation that wish to ascribe self-regulation failure and success to processing outcomes within a single prefrontal region, as these accounts may not capture all aspects of self-regulation. Instead, what is likely required to advance our understanding of the cognitive neuroscience of self-regulation is a reconsideration of the properties expected from self-regulatory brain systems coupled with a rethinking of the tools used to study self-regulation.
Although traditional functional magnetic resonance imaging (fMRI) is well suited to capture momentary brain activity wedded to the transitory presentation of stimuli such as reward cues, it may struggle to capture more tonic aspects of self-regulation. Put simply, the capacity for self-regulation reflects on-going, well-established efforts to control desires, resist temptations, and achieve personal goals, the temporal dynamics of which are difficult to measure using standard, event-related fMRI paradigms. This may explain, in part, why neural correlates of self-regulation sometimes escape detection during the transient incidental presentations of reward cues yet can manifest in more tonic, deliberative attempts to self-regulate (e.g., Lopez et al. 2014). Event-related imaging studies may capture instances of executive functioning that assist self-regulation, such as detecting conflict and inhibiting a prepotent response, but these transient responses in support of moments of self-regulation should not be mistaken for why some people manage to achieve their goals over time, which involves processes that operate over longer timescales (e.g., chronic monitoring of performance, adjusting ongoing behavior, and planning for the future). In other words, successful self-regulation involves many executive functions, each of which may have a neural signature that differs depending on specific task demands, (e.g., controlling thoughts, reappraising emotion, or inhibiting prepotent responses during a Stroop task), and any one test of executive function may tap only one piece of a larger control system. Thus, a network-level approach may help delineate which systems are important for successful self-regulation.
So what should a self-regulation system look like? A self-regulation system should satisfy the following criteria:
-
■
It must be conscious. Unlike automatic and implicit influences of subcortical reward activity, effective self-regulation relies on conscious, ongoing attempts to regulate behavior (Baumeister & Masicampo 2010, Hofmann et al. 2009, Posner & Rothbart 1998). As a consequence, self-regulation is effortful and slow by comparison. Event-related neuroimaging studies hoping to glimpse momentary instances of self-regulation may fail to do so because a self-regulation system designed in this way would perpetually lose to the faster, automatic subcortical systems. To compensate, a conscious, effortful self-regulation system must be tonically engaged to be ready in advance of incoming reward cues.
-
■
It must understand time. An effective self-regulation system must be capable of understanding what the ventral striatum cannot---that short-term, immediate rewards can have negative long-term consequences. As such, a self-regulation system must be capable of long-term goal planning and goal maintenance to effectively regulate against impulses with long-term negative consequences and to promote behaviors with no short-term immediate reward (e.g., exercising) that are intended to improve long-term well-being (Baumeister & Heatherton 1996).
-
■
It must be configurable. Long-term goals change, and different situations necessitate regulation of different impulses. As cortical real estate is limited, we are not likely to have an independent self-regulation system for each of our vices. An effective self-regulation system must therefore be domain general and capable of swapping and updating goal parameters as situations dictate.
-
■
It must be anatomically positioned to interact with both processing and output systems. If self-regulation systems are domain general and configurable, then they must be interconnected with multiple processing systems to exert control over impulses or motor plans that conflict with long-term goals. In addition, their localization within the cortex should overlap with lesion studies demonstrating the myriad impairments of self-regulation following traumatic brain imaging.
With these key properties in mind, we offer a novel approach predicated on the assumption that event-related fMRI is ill suited to study all aspects of self-regulation. Instead, this approach capitalizes on recently developed applications of network analysis to assess individual differences in resting-state functional connectivity (rs-fc). A key element for us to emphasize is the discovery of functional brain networks---sets of brain regions whose spontaneous activity correlates at rest (i.e., in the absence of explicit task constraints) (Biswal et al. 1995). Reproducibility of functional networks across individuals and laboratories suggests that resting-state connectivity has a common architecture, and yet some identifiable variations in connectivity patterns are likely also related to important individual differences and behavioral outcomes, such as self-regulation (Biswal et al. 2010). In particular, graph theory network analysis of resting-state data is a comprehensive and powerful approach to examining resting-state connectivity that reveals the integrity of brain systems as well as how connectivity between systems functions within wider network contexts (Power et al. 2011). Rs-fcMRI signal correlations are believed to reflect histories of coactivation across brain regions---a pattern of statistical coherence that arises throughout development and provides a measure of the long-term functional relatedness of brain regions (Crossley et al. 2013). Consistent with this notion, rs-fcMRI can successfully predict individual brain maturity across development (Dosenbach et al. 2010). We emphasize here that network-based rs-fcMRI differs from traditional connectivity methods used in brain imaging (e.g., task-based psycho-physiological interactions analyses), which examine momentary rather than tonic connectivity, and that such approaches are complementary.
This network-based approach to understanding brain organization and function (Power et al. 2011) has identified two cortical networks that meet the criteria necessary for a self-regulation system: the frontoparietal network and the cingulo-opercular network (first described by Dosenbach et al. 2006). The frontoparietal network incorporates dorsolateral PFC, posterior parietal, and inferotemporal regions that are commonly activated during cognitive tasks that place demands on goal planning, working memory, and attentional filtering. Dosenbach et al. (2006) defined the cingulo-opercular network as a core system for the implementation of task sets. Activity within three key regions of this system---the left and right anterior insula/frontal operculum and the dorsal anterior cingulate extending into the middle superior frontal cortex---shows a robust, domain-general spike in activity at the beginning of goal-directed task performance that is maintained tonically throughout the task. Activity in these regions further responds transiently in response to task-related feedback. As such, the cingulo-opercular system meets the criteria for a configurable, domain-general self-regulation system that can adapt to changes in long-term goal planning.
Both systems meet the definition of a control system with respect to their interactions with other brain networks. Sensory processing systems demonstrate relatively compartmentalized connectivity patterns within their individual networks (e.g., brain regions within the somatomotor processing network show strong within-network connectedness with other somatomotor system neighbors but weak connectedness with other networks). Control systems, by virtue of their need to flexibly adapt to a diverse set of dynamic task goals, demonstrate the reverse pattern (within-network connectedness is weaker in favor of more robust connectivity patterns to other networks) (Power et al. 2011). The ability to distinguish processing systems from control systems based on compartmentalized, within-network connectedness versus distributed, between-network connectedness may help explain the relatively stable effects observed in studies of reward processing and the more dynamic and variable effects observed in studies of self-regulation.
Importantly, network-based metrics that quantify within-system versus between-system connectivity can also be used to identify network hubs---brain regions that are well positioned to integrate and communicate across multiple systems. Cortical hubs should either demonstrate a high degree of connectivity with multiple brain systems or occur in spatial locations where many subnetworks are represented within a small volume (e.g., areas of overlap between the dorsal attention, visual, frontoparietal control, and default mode systems). Both metrics identify similar cortical hubs in the anterior insula, dorsal medial PFC, medial superior frontal cortex, medial and inferior parietal cortices, and lateral occipitotemporal cortex.
Most of these cortical hubs fall within the frontoparietal and cingulo-opercular systems---networks that meet all of the requirements for a self-regulation system. Moreover, the cortical hubs identified in these control networks overlap with lateral PFC and dACC/medial superior frontal cortex activations reported during self-regulation.
Notably absent as a hub or articulation point of integration, however, is the VMPFC. The VMPFC is functionally connected to the default mode network. Like all regions of this network, the VMPFC demonstrates relatively high within-network connectedness and weak between-network connectedness, a pattern that characterizes this region in the mold of a prototypical processing region, as would be found in the somatomotor or visual processing network (Power et al. 2011). Said differently, the VMPFC behaves more like a bottom-up reward processor than a top-down control hub that functions to integrate processing outcomes across multiple systems (Power et al. 2013).
To the extent that cortical hubs in frontoparietal and cingulo-opercular control systems exert a disproportionate influence over network functioning, lesions to hubs should affect processing across multiple systems and produce profound impairments across multiple domains of cognition. Indeed, recent work by Warren and colleagues (2014) examining cognitive impairments in patients with focal lesions to network hubs provides strong support for this prediction. Damage to network hubs produced widespread cognitive deficits on all tested measures of cognitive and behavioral performance, whereas damage to network regions showing high within-network connectivity and low between-network connectivity, such as the VMPFC, produced more circumscribed cognitive impairment (although these impairments clustered around deficits of executive functioning and personal adjustment or emotional functioning). This domain-general effect could not be accounted for by any factor other than lesion location.
When considered in the context of resting-state networks, the seemingly disparate patterns of activity across self-regulation studies reveal functional organization into three networks: the frontoparietal network, the cingulo-opercular network, and the default mode network (Figure 2). The first two demonstrate network features that are characteristic of control systems, and collectively these two systems are home to most of the brain’s hubs (Power et al. 2013). By contrast, the default network exhibits network properties that are characteristic of processing systems---a finding that may reshape the way we think about the role of the VMPFC in reward and self-regulation. Indeed, a careful consideration of activation patterns from self-regulation studies within the broader context of network systems and their features may challenge existing models of self-regulation and help constrain and improve future theories.
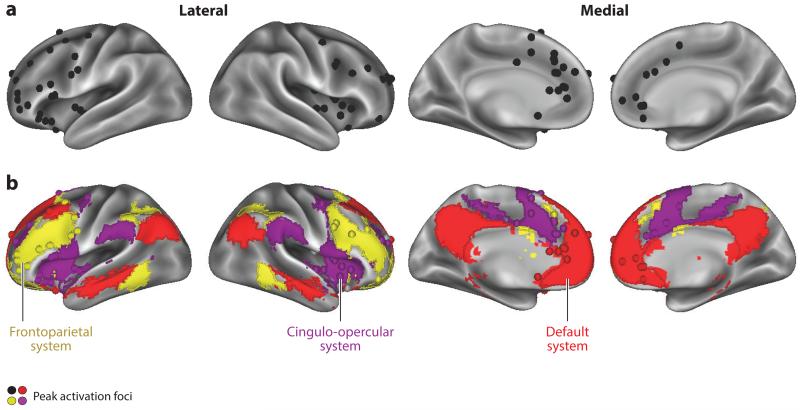
Figure 2.
(a) Peak activation foci reported in prefrontal and anterior cingulate brain regions across neuroimaging studies of reward and self-regulation cited in this review are displayed on an inflated rendering of the medial and lateral cortical surfaces (Van Essen et al. 2012). Activations are broadly distributed throughout the PFC, and these widespread patterns of activation across studies have given rise to differential accounts of a preferential role for either the lateral PFC, VMPFC, or dACC in self-regulation. (b) When considered in the context of brain systems, however, activation foci cluster primarily into three subnetworks (as defined by Power et al. 2011): the frontoparietal system (yellow), the cingulo-opercular system (purple), and the default system (red). Abbreviations: dACC, dorsal anterior cingulate cortex; PFC, prefrontal cortex; VMPFC, ventromedial prefrontal cortex.
FUTURE STUDIES OF SELF-REGULATION
The application of rs-fcMRI to future studies of self-regulation is likely to prove fruitful along several dimensions. Preliminary evidence from seed-based resting-state maps of ventral striatum connectivity suggests the possibility of a human reward subnetwork (Choi et al. 2012, Martino et al. 2008) with strong connectivity to the OFC. To the extent that a functionally connected reward system exists, future studies of self-regulation that capitalize on rs-fcMRI will be well positioned to investigate individual differences in connectivity within and between reward and control networks and their relation to real-world self-regulation outcomes. Researchers have already begun to apply rs-fcMRI as a tool to study individual differences in reward-based memory formation (Hamann et al. 2014), smoking status (Pariyadath et al. 2014), neurofeedback for anxiety (Scheinost et al. 2014), and pain modulation (Kong et al. 2013).
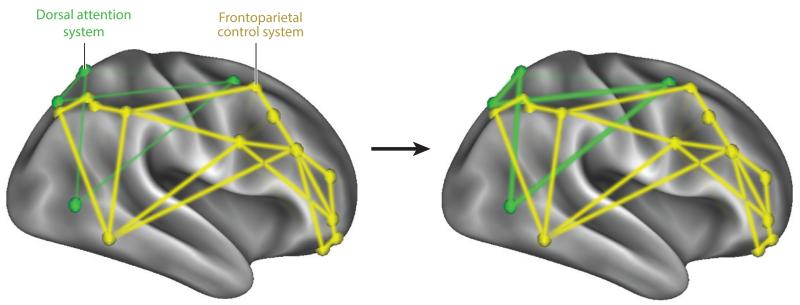
In clinical populations, rs-fcMRI has shown promise as a diagnostic tool in the study of depression in adults (Greicius et al. 2007) and adolescents (Connolly et al. 2013), obesity (Lips et al. 2014), neurodegenerative disorders (Seeley et al. 2009), and schizophrenia (Venkataraman et al. 2012). It may offer novel insights into disruptions of network integrity that give rise to addictive behavior, such as drug use in schizophrenia (Fischer et al. 2014). The ability for rs-fcMRI to predict brain maturation (Dosenbach et al. 2010) is critically important to developmental studies of reward and self-regulation, in that maturation of control systems may lag behind that of the reward system. In particular, individual differences in the network integrity of the frontoparietal control system in children and adolescents may be a key factor in understanding disorders that involve self-regulation failure, such as childhood obesity and attention deficit/hyperactivity disorder (ADHD), both of which have seen a dramatic increase over the past three decades. Interestingly, the recent explosion of media use and multitasking by children during this time has coincided with steep escalations in childhood obesity and ADHD, an observation that suggests a critical linkage between attentional filtering and self-regulation failures in children. Pioneering research by Ophir and colleagues (Ophir, Nass & Wagner, 2009) has documented that frequent media multitaskers struggle to filter irrelevant cues from the environment (Ophir et al. 2009) and show a greater spread of attention to peripheral cues, whether those cues are relevant or not (Cain & Mitroff 2011). A substantial literature now links media multitasking to attentional problems during daily life (Baumgartner et al. 2014, Pea et al. 2012, Ralph et al. 2014, Sanbonmatsu et al. 2013). Researchers speculate that frequent shifting of attention to the periphery may strengthen the dorsal attention network that allocates attention and selectively weaken the frontoparietal control network responsible for filtering extraneous information (Figure 3).
Figure 3.
A heuristic depicting how experience---in this case, increased media multitasking---may strengthen some brain systems [e.g., the dorsal attention system (green)] and weaken others [e.g., the frontoparietal control system (yellow)]. Studies that investigate how experience or training influences dynamic changes at the level of network systems may offer important insights into individual differences in self-regulatory ability that give rise to self-regulation success and failure.
To the extent that practiced behaviors can effect changes in functional connectivity in dynamic control systems, researchers may capitalize on individual differences in resting-state connectivity between so-called good self-regulating brains and bad ones to develop training strategies aimed at improving self-regulation (see also Berkman et al. 2014). In summary, self-regulation is hard---both to achieve and to study. We believe that a systems-level approach to the study of self-regulation afforded by recent advances in brain imaging methods offers the best opportunity to gain new insights into why we fail at self-regulation and what we can do to become better at it.
Acknowledgments
This article was supported by NIH grants DA022582, HL114092, AA021347 and MH59282.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Literature Cited
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J. Comp. Neurol. 1984;230:465–96. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Anonymous Remarkable case of injury. American Phrenological Journal. 1851;13:89. [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends in Cognitive Sciences. 2014;18(4):177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Ballantine HT, Jr., Cassidy WL, Flanagan NB, Marino R., Jr. Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J. Neurosurg. 1967;26:488–95. doi: 10.3171/jns.1967.26.5.0488. [DOI] [PubMed] [Google Scholar]
- Barbas H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J. Comp. Neurol. 1988;276:313–42. doi: 10.1002/cne.902760302. [DOI] [PubMed] [Google Scholar]
- Bar-On R, Tranel D, Denburg NL, Bechara A. Exploring the neurological substrate of emotional and social intelligence. Brain. 2003;126:1790–800. doi: 10.1093/brain/awg177. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, DeWall CN, Ciarocco NJ, Twenge JM. Social exclusion impairs self-regulation. J. Pers. Soc. Psychol. 2005;88:589–604. doi: 10.1037/0022-3514.88.4.589. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Heatherton T. Self-regulation failure: an overview. Psychol. Inq. 1996;7:1–15. [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychol. Bull. 1995;117:497–529. [PubMed] [Google Scholar]
- Baumeister RF, Masicampo EJ. Conscious thought is for facilitating social and cultural interactions: how mental simulations serve the animal-culture interface. Psychol. Rev. 2010;117:945–71. doi: 10.1037/a0019393. [DOI] [PubMed] [Google Scholar]
- Baumgartner SE, Weeda WD, Heijden LL, van der Huizinga M. The relationship between media multitasking and executive function in early adolescents. J. Early Adolesc. 2014;34:1120–44. [Google Scholar]
- Berkman ET, Kahn LE, Merchant JS. Training-induced changes in inhibitory control network activity. J. Neurosci. 2014;34:149–57. doi: 10.1523/JNEUROSCI.3564-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, et al. Toward discovery science of human brain function. PNAS. 2010;107:4734–39. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Boileau I, Assaad J-M, Pihl RO, Benkelfat C, Leyton M, et al. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–31. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Braver T. Motivation and Cognitive Control: From Behavior to Neural Mechanism. Annual Review of Psychology. 2015;66(1):83–113. doi: 10.1146/annurev-psych-010814-015044. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn. Sci. 2004;8:539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, et al. Neural substrates of resisting craving during cigarette cue exposure. Biol. Psychiatry. 2007;62:642–51. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cain MS, Mitroff SR. Distractor filtering in media multitaskers. Perception. 2011;40:1183–92. doi: 10.1068/p7017. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J. Neurosci. 2000;20:4255–66. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–40. [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–49. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Somerville LH, Gotlib IH, Ayduk O, Franklin NT, Askren MK, Shoda Y. Behavioral and neural correlates of delay of gratification 40 years later. Proceedings of the National Academy of Sciences. 2011;108(36):14998–15003. doi: 10.1073/pnas.1108561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EY, Yeo BTT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J. Neurophysiol. 2012;108:2242–63. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen L, Pettijohn L. Mood and carbohydrate cravings. Appetite. 2001;36:137–45. doi: 10.1006/appe.2001.0390. [DOI] [PubMed] [Google Scholar]
- Cloutier J, Heatherton TF, Whalen PJ, Kelley WM. Are attractive people rewarding? Sex differences in the neural substrates of facial attractiveness. J. Cogn. Neurosci. 2008;20:941–51. doi: 10.1162/jocn.2008.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Lieberman MD. The common neural basis of exerting self-control in multiple domains. In: Hassin RR, Ochsner KN, Trope Y, editors. Self Control in Society, Mind, and Brain: Oxford Series in Social Cognition and Social Neuroscience. Oxford Univ. Press; New York: 2010. pp. 141–60. [Google Scholar]
- Cohen RA, Kaplan RF, Moser DJ, Jenkins MA, Wilkinson H. Impairments of attention after cingulotomy. Neurology. 1999a;53:819–24. doi: 10.1212/wnl.53.4.819. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Kaplan RF, Zuffante P, Moser DJ, Jenkins MA, et al. Alteration of intention and self-initiated action associated with bilateral anterior cingulotomy. J. Neuropsychiatry Clin. Neurosci. 1999b;11:444–53. doi: 10.1176/jnp.11.4.444. [DOI] [PubMed] [Google Scholar]
- Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol. Psychiatry. 2013;74:898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkin S. Hidden-figures-test performance: lasting effects of unilateral penetrating head injury and transient effects of bilateral cingulotomy. Neuropsychologia. 1979;17:585–605. doi: 10.1016/0028-3932(79)90034-4. [DOI] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Vértes PE, Winton-Brown TT, Patel AX, et al. Cognitive relevance of the community structure of the human brain functional coactivation network. PNAS. 2013;110:11583–88. doi: 10.1073/pnas.1220826110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn. Sci. 2003;7:415–23. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav. Neurosci. 1992;106:181–91. doi: 10.1037//0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- David SP, Munafò MR, Johansen-Berg H, Smith SM, Rogers RD, et al. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol. Psychiatry. 2005;58:488–94. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBono A, Shmueli D, Muraven M. Rude and inappropriate: the role of self-control in following social norms. Pers. Soc. Psychol. Bull. 2011;37:136–46. doi: 10.1177/0146167210391478. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nat. Neurosci. 2008;11:880–81. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int. J. Obes. 2007;31:440–48. doi: 10.1038/sj.ijo.0803431. [DOI] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J. Neurosci. 2012;32:5549–52. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos KE, Kelley WM, Heatherton TF. Dietary restraint violations influence reward responses in nucleus accumbens and amygdala. J. Cogn. Neurosci. 2011;23:1952–63. doi: 10.1162/jocn.2010.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Power JD, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–61. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth AL, Seligman MEP. Self-discipline outdoes IQ in predicting academic performance of adolescents. Psychol. Sci. 2005;16:939–44. doi: 10.1111/j.1467-9280.2005.01641.x. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am. J. Psychiatry. 2002;159:954–60. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Erb JL, Gwirtsman HE, Fuster JM, Richeimer SH. Bulimia associated with frontal lobe lesions. Int. J. Eat. Disord. 1989;8:117–21. [Google Scholar]
- Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci. Biobehav. Rev. 1990;14:217–32. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- Federoff IDC, Polivy J, Herman CP. The effect of pre-exposure to food cues on the eating behavior of restrained and unrestrained eaters. Appetite. 1997;28:33–47. doi: 10.1006/appe.1996.0057. [DOI] [PubMed] [Google Scholar]
- Fehr E, Camerer CF. Social neuroeconomics: the neural circuitry of social preferences. Trends Cogn. Sci. 2007;11:419–27. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Fischer AS, Whitfield-Gabrieli S, Roth RM, Brunette MF, Green AI. Impaired functional connectivity of brain reward circuitry in patients with schizophrenia and cannabis use disorder: effects of cannabis and THC. Schizophr. Res. 2014;158:176–82. doi: 10.1016/j.schres.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost RO, Goolkasian GA, Ely RJ, Blanchard FA. Depression, restraint and eating behavior. Behav. Res. Ther. 1982;20:113–21. doi: 10.1016/0005-7967(82)90111-5. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho J-K, Sperry L, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am. J. Psychiatry. 2000;157:1789–98. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. PNAS. 1999;96:8301–6. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Belknap Press Harvard Univ. Press; Cambridge, MA: 1986. [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–7. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression: a report of the Vietnam head injury study. Neurology. 1996;46:1231–38. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry. 2007;62:429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J. Neurosci. 1995;15:4851–67. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haedt-Matt AA, Keel PK. Revisiting the affect regulation model of binge eating: a meta-analysis of studies using ecological momentary assessment. Psychol. Bull. 2011;137:660–81. doi: 10.1037/a0023660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagger MS, Wood C, Stiff C, Chatzisarantis NLD. Ego depletion and the strength model of self-control: a meta-analysis. Psychol. Bull. 2010;136:495–525. doi: 10.1037/a0019486. [DOI] [PubMed] [Google Scholar]
- Hamann JM, Dayan E, Hummel FC, Cohen LG. Baseline frontostriatal-limbic connectivity predicts reward-based memory formation. Hum. Brain Mapp. 2014;35:5921–31. doi: 10.1002/hbm.22594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–48. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Harlow JM. Passage of an iron rod through the head. Boston Med. Surg. J. 1848;39:389–93. [PMC free article] [PubMed] [Google Scholar]
- Harlow JM. Recovery from the passage of an iron bar through the head. Publ. Mass. Med. Soc. 1868;2:327–47. [Google Scholar]
- Harris JL, Bargh JA, Brownell KD. Priming effects of television food advertising on eating behavior. Health Psychol. 2009;28:404–13. doi: 10.1037/a0014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF. Neuroscience of self and self-regulation. Annu. Rev. Psychol. 2011;62:363–90. doi: 10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Herman CP, Polivy J. Effects of physical threat and ego threat on eating behavior. J. Pers. Soc. Psychol. 1991;60:138–43. doi: 10.1037//0022-3514.60.1.138. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Striepe M, Wittenberg L. Emotional distress and disinhibited eating: the role of self. Pers. Soc. Psychol. Bull. 1998;24:301–13. [Google Scholar]
- Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends Cogn. Sci. 2011;15:132–39. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman CP, Mack D. Restrained and unrestrained eating. J. Pers. 1975;43:647–60. doi: 10.1111/j.1467-6494.1975.tb00727.x. [DOI] [PubMed] [Google Scholar]
- Herman CP, Polivy J. A boundary model for the regulation of eating. Psychiatr. Ann. 1983;13:918–27. [PubMed] [Google Scholar]
- Hofmann W, Baumeister RF, Förster G, Vohs KD. Everyday temptations: an experience sampling study of desire, conflict, and self-control. J. Pers. Soc. Psychol. 2011;102:1318–35. doi: 10.1037/a0026545. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Friese M, Strack F. Impulse and self-control from a dual-systems perspective. Perspect. Psychol. Sci. 2009;4:162–76. doi: 10.1111/j.1745-6924.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- Hofmann W, van Koningsbruggen GM, Stroebe W, Ramanathan S, Aarts H. As pleasure unfolds: hedonic responses to tempting food. Psychol. Sci. 2010;21:1863–70. doi: 10.1177/0956797610389186. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Vohs KD, Baumeister RF. What people desire, feel conflicted about, and try to resist in everyday life. Psychol. Sci. 2012;23:582–88. doi: 10.1177/0956797612437426. [DOI] [PubMed] [Google Scholar]
- Inzlicht M, Elkins-Brown N, Berkman ET. The neuroscience of “ego depletion” or: How the brain can help us understand why self-control seems limited. In: Harmon-Jones E, Inzlicht M, editors. Social Neuroscience: Biological Approaches to Social Psychology. Psychology; New York: 2015. In press. [Google Scholar]
- Inzlicht M, Schmeichel BJ, Macrae CN. Why self-control seems (but may not be) limited. Trends Cogn. Sci. 2014;18:127–33. doi: 10.1016/j.tics.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, Frederick B deB, Chuzi S, et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol. Psychiatry. 2010;67:722–29. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–26. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. Affect modulates appetite-related brain activity to images of food. Int. J. Eat. Disord. 2006;39:357–63. doi: 10.1002/eat.20240. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J. Neurosci. 2005;25:4806–12. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, et al. Prefrontal--striatal pathway underlies cognitive regulation of craving. PNAS. 2010;107:14811–16. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Jensen K, Loiotile R, Cheetham A, Wey H-Y, et al. Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. PAIN. 2013;154:459–67. doi: 10.1016/j.pain.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat. Rev. Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb. Cortex. 2003;13:1064–71. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Lambert KG, Neal T, Noyes J, Parker C, Worrel P. Food-related stimuli increase desire to eat in hungry and satiated human subjects. Curr. Psychol. 1991;10:297–303. [Google Scholar]
- Laplane D, Degos JD, Baulac M, Gray F. Bilateral infarction of the anterior cingulate gyri and of the fornices: report of a case. J. Neurol. Sci. 1981;51:289–300. doi: 10.1016/0022-510x(81)90107-6. [DOI] [PubMed] [Google Scholar]
- LaRose R, Lin C, Eastin M. Unregulated Internet usage: addiction, habit, or deficient self-regulation? Media Psychol. 2003;5:225–53. [Google Scholar]
- Lerner JS, Li Y, Weber EU. The financial costs of sadness. Psychol. Sci. 2013;24:72–79. doi: 10.1177/0956797612450302. [DOI] [PubMed] [Google Scholar]
- Lin A, Adolphs R, Rangel A. Social and monetary reward learning engage overlapping neural substrates. Soc. Cogn. Affect. Neurosci. 2012;7:274–81. doi: 10.1093/scan/nsr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips MA, Wijngaarden MA, van der Grond J, van Buchem MA, de Groot GH, et al. Resting-state functional connectivity of brain regions involved in cognitive control, motivation, and reward is enhanced in obese females. Am. J. Clin. Nutr. 2014;100:524–31. doi: 10.3945/ajcn.113.080671. [DOI] [PubMed] [Google Scholar]
- Lochbuehler K, Voogd H, Scholte RHJ, Engels RCME. Attentional bias in smokers: exposure to dynamic smoking cues in contemporary movies. J. Psychopharmacol. 2011;25:514–19. doi: 10.1177/0269881110388325. [DOI] [PubMed] [Google Scholar]
- Lopez RB, Hofmann W, Wagner DD, Kelley WM, Heatherton TF. Neural predictors of giving in to temptation in daily life. Psychol. Sci. 2014;25:1337–44. doi: 10.1177/0956797614531492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Gordon J. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. Guilford; New York: 1985. [Google Scholar]
- Martino AD, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, et al. Functional connectivity of human striatum: a resting state fMRI study. Cereb. Cortex. 2008;18:2735–47. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–57. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, et al. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J. Psychopharmacol. 2011;25:490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychol. Rev. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting: the role of the frontal lobes. Arch. Neurol. 1963;9:90–100. [Google Scholar]
- Mischel W, Ebbesen EB, Zeiss AR. Selective attention to the self: situational and dispositional determinants. J. Pers. Soc. Psychol. 1973;27:129–42. [Google Scholar]
- Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244:933–38. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Muraven M, Collins RL, Nienhaus K. Self-control and alcohol restraint: an initial application of the self-control strength model. Psychol. Addict. Behav. 2002;16:113–20. doi: 10.1037//0893-164x.16.2.113. [DOI] [PubMed] [Google Scholar]
- Nambu A. Seven problems on the basal ganglia. Curr. Opin. Neurobiol. 2008;18:595–604. doi: 10.1016/j.conb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Oaten M, Williams KD, Jones A, Zadro L. The effects of ostracism on self-regulation in the socially anxious. J. Soc. Clin. Psychol. 2008;27:471–504. [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N.Y. Acad. Sci. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ophir E, Nass C, Wagner AD. Cognitive control in media multitaskers. PNAS. 2009;106:15583–87. doi: 10.1073/pnas.0903620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya DN, Van Hoesen GW, Mesulam MM. Efferent connections of the cingulate gyrus in the rhesus monkey. Exp. Brain Res. 1981;42:319–30. doi: 10.1007/BF00237497. [DOI] [PubMed] [Google Scholar]
- Pariyadath V, Stein EA, Ross TJ. Machine learning classification of resting state functional connectivity predicts smoking status. Front. Hum. Neurosci. 2014;8:425. doi: 10.3389/fnhum.2014.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pea R, Nass C, Meheula L, Rance M, Kumar A, et al. Media use, face-to-face communication, media multitasking, and social well-being among 8- to 12-year-old girls. Dev. Psychol. 2012;48:327–36. doi: 10.1037/a0027030. [DOI] [PubMed] [Google Scholar]
- Penfield W, Evans J. The frontal lobe in man: a clinical study of maximum removals. Brain. 1935;58:115–33. [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. PNAS. 1998;95:853–60. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20:249–62. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur. J. Neurosci. 1999;11:1011–36. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1998;353:1915–27. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, et al. Functional network organization of the human brain. Neuron. 2011;72:665–78. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Lessov-Schlaggar CN, Petersen SE. Evidence for hubs in human functional brain networks. Neuron. 2013;79:798–813. doi: 10.1016/j.neuron.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Self-regulation as a protective factor against risky drinking and sexual behavior. Psychol. Addict. Behav. 2010;24:376–85. doi: 10.1037/a0018547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr. Opin. Neurobiol. 2006;16:723–27. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Ralph BCW, Thomson DR, Cheyne JA, Smilek D. Media multitasking and failures of attention in everyday life. Psychol. Res. 2014;78:661–69. doi: 10.1007/s00426-013-0523-7. [DOI] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat. Rev. Neurosci. 2008;9:545–56. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviv M. Personality characteristics of sexual addicts and pathological gamblers. J. Gambl. Stud. 1993;9:17–30. [Google Scholar]
- Rilling JK. Human and nonhuman primate brains: Are they allometrically scaled versions of the same design? Evol. Anthropol. Issues News Rev. 2006;15:65–77. [Google Scholar]
- Sakai T, Mikami A, Tomonaga M, Matsui M, Suzuki J, et al. Differential prefrontal white matter development in chimpanzees and humans. Curr. Biol. 2011;21:1397–402. doi: 10.1016/j.cub.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Sanbonmatsu DM, Strayer DL, Medeiros-Ward N, Watson JM. Who multi-tasks and why? Multi-tasking ability, perceived multi-tasking ability, impulsivity, and sensation seeking. PLOS ONE. 2013;8:e54402. doi: 10.1371/journal.pone.0054402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Hufford MR. Effects of smoking urge on generation of smoking-related information. J. Appl. Soc. Psychol. 1997;27:1395–405. [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA. A multi-dimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96:1419–32. doi: 10.1080/09652140120075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Stoica T, Wasylink S, Gruner P, Saksa J, et al. Resting state functional connectivity predicts neurofeedback response. Front. Behav. Neurosci. 2014;8:338. doi: 10.3389/fnbeh.2014.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilström B, Svensson HM, Svensson TH, Nomikos GG. Nicotine and food induced dopamine release in the nucleus accumbens of the rat: putative role of α7 nicotinic receptors in the ventral tegmental area. Neuroscience. 1998;85:1005–9. doi: 10.1016/s0306-4522(98)00114-6. [DOI] [PubMed] [Google Scholar]
- Schmeichel BJ. Attention control, memory updating, and emotion regulation temporarily reduce the capacity for executive control. J. Exp. Psychol. Gen. 2007;136:241–55. doi: 10.1037/0096-3445.136.2.241. [DOI] [PubMed] [Google Scholar]
- Schmeichel BJ, Harmon-Jones C, Harmon-Jones E. Exercising self-control increases approach motivation. J. Pers. Soc. Psychol. 2010;99:162–73. doi: 10.1037/a0019797. [DOI] [PubMed] [Google Scholar]
- Schoenemann PT, Sheehan MJ, Glotzer LD. Prefrontal white matter volume is disproportionately larger in humans than in other primates. Nat. Neurosci. 2005;8:242–52. doi: 10.1038/nn1394. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727–41. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. The domain of supervisory processes and temporal organization of behaviour. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1996;351:1405–12. doi: 10.1098/rstb.1996.0124. [DOI] [PubMed] [Google Scholar]
- Siep N, Roefs A, Roebroeck A, Havermans R, Bonte M, Jansen A. Fighting food temptations: the modulating effects of short-term cognitive reappraisal, suppression and up-regulation on mesocorticolimbic activity related to appetitive motivation. Neuroimage. 2012;60:213–20. doi: 10.1016/j.neuroimage.2011.12.067. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–61. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J. Cogn. Neurosci. 2011;23:2123–34. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat. Neurosci. 2006;9:1007–8. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J. Neurosci. 2010;30:13105–9. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, III, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–47. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Tangney JP, Baumeister RF, Boone AL. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. J. Pers. 2004;72:271–324. doi: 10.1111/j.0022-3506.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Curr. Opin. Neurobiol. 2005;15:219–24. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol. Rev. 1990;97:147–68. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–18. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Tranel D, Anderson SW, Benton A. Development of the concept of “executive function” and its relationship to the frontal lobes. Handb. Neuropsychol. 1994;9:125–48. [Google Scholar]
- Twenge JM, Baumeister RF, Tice DM, Stucke TS. If you can’t join them, beat them: effects of social exclusion on aggressive behavior. J. Pers. Soc. Psychol. 2001;81:1058–69. doi: 10.1037//0022-3514.81.6.1058. [DOI] [PubMed] [Google Scholar]
- Van der Laan LN, de Ridder DTD, Viergever MA, Smeets PAM. The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. Neuroimage. 2011;55:296–303. doi: 10.1016/j.neuroimage.2010.11.055. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Glasser MF, Dierker DL, Harwell J, Coalson T. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb. Cortex. 2012;22:2241–2262. doi: 10.1093/cercor/bhr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrell P, Junqué C, Pujol J, Jurado MA, Molet J, Grafman J. The role of prefrontal regions in the Stroop task. Neuropsychologia. 1995;33:341–52. doi: 10.1016/0028-3932(94)00116-7. [DOI] [PubMed] [Google Scholar]
- Venkataraman A, Whitford TJ, Westin C-F, Golland P, Kubicki M. Whole brain resting state functional connectivity abnormalities in schizophrenia. Schizophr. Res. 2012;139:7–12. doi: 10.1016/j.schres.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohs KD, Baumeister RF, Ciarocco NJ. Self-regulation and self-presentation: regulatory resource depletion impairs impression management and effortful self-presentation depletes regulatory resources. J. Pers. Soc. Psychol. 2005;88:632–57. doi: 10.1037/0022-3514.88.4.632. [DOI] [PubMed] [Google Scholar]
- Vohs KD, Heatherton TF. Self-regulatory failure: a resource-depletion approach. Psychol. Sci. 2000;11:249–54. doi: 10.1111/1467-9280.00250. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Telang F, Logan J, et al. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49:2536–43. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Altman M, Boswell RG, Kelley WM, Heatherton TF. Self-regulatory depletion enhances neural responses to rewards and impairs top-down control. Psychol. Sci. 2013;24:2262–71. doi: 10.1177/0956797613492985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Boswell RG, Kelley WM, Heatherton TF. Inducing negative affect increases the reward value of appetizing foods in dieters. J. Cogn. Neurosci. 2012;24:1625–33. doi: 10.1162/jocn_a_00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Dal Cin S, Sargent JD, Kelley WM, Heatherton TF. Spontaneous action representation in smokers when watching movie characters smoke. J. Neurosci. 2011;31:894–98. doi: 10.1523/JNEUROSCI.5174-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Heatherton TF. Self-regulatory depletion increases emotional reactivity in the amygdala. Soc. Cogn. Affect. Neurosci. 2013;8:410–17. doi: 10.1093/scan/nss082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Heatherton TF. Self-regulation and its failure: the seven deadly threats to self-regulation. In: Mikulincer M, Shaver PR, Borgida E, Bargh JA, editors. APA Handbook of Personality and Social Psychology, Vol. 1: Attitudes and Social Cognition. Am. Psychol. Assoc.; Washington, DC: 2014. pp. 805–42. [Google Scholar]
- Warren DE, Power JD, Bruss J, Denburg NL, Waldron EJ, et al. Network measures predict neuropsychological outcome after brain injury. PNAS. 2014;111:14247–52. doi: 10.1073/pnas.1322173111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, et al. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol. Psychiatry. 1998;44:1219–28. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- Whitty CWM, Duffield JE, Tow PM, Cairns H. Anterior cingulectomy in the treatment of mental disease. Lancet. 1952;259:475–81. doi: 10.1016/s0140-6736(52)90051-2. [DOI] [PubMed] [Google Scholar]
- Willner P, Jones C. Effects of mood manipulation on subjective and behavioural measures of cigarette craving. Behav. Pharmacol. 1996;7:355–63. doi: 10.1097/00008877-199608000-00007. [DOI] [PubMed] [Google Scholar]
- Wilson DH, Chang AE. Bilateral anterior cingulectomy for the relief of intractable pain: report of 23 patients. Confin. Neurol. 1974;36:61–68. doi: 10.1159/000102783. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat. Neurosci. 2004;7:211–14. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Villarroel NA. Dynamic association between negative affect and alcohol lapses following alcohol treatment. J. Consult. Clin. Psychol. 2009;77:633–44. doi: 10.1037/a0015647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Gorno-Tempini M-L, Seeley WW, Rankin K, Lee SS, et al. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology. 2007;69:1424–33. doi: 10.1212/01.wnl.0000277461.06713.23. [DOI] [PubMed] [Google Scholar]
- Wrase J, Grüsser SM, Klein S, Diener C, Hermann D, et al. Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Eur. Psychiatry. 2002;17:287–91. doi: 10.1016/s0924-9338(02)00676-4. [DOI] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Naumer MJ. Brain regions related to tool use and action knowledge reflect nicotine dependence. J. Neurosci. 2009;29:4922–29. doi: 10.1523/JNEUROSCI.4891-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nature Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinser MC, Baker TB, Sherman JE, Cannon DS. Relation between self-reported affect and drug urges and cravings in continuing and withdrawing smokers. J. Abnorm. Psychol. 1992;101:617–29. doi: 10.1037//0021-843x.101.4.617. [DOI] [PubMed] [Google Scholar]