Abstract
Attention-dependent modulation of neural activity in visual association cortex (VAC) is thought to depend on top-down modulatory control signals emanating from the prefrontal cortex (PFC). In a previous functional magnetic resonance imaging study utilizing a working memory task, we demonstrated that activity levels in scene-selective VAC (ssVAC) regions can be enhanced above or suppressed below a passive viewing baseline level depending on whether scene stimuli were attended or ignored (Gazzaley, Cooney, McEvoy, et al. 2005). Here, we use functional connectivity analysis to identify possible sources of these modulatory influences by examining how network interactions with VAC are influenced by attentional goals at the time of encoding. Our findings reveal a network of regions that exhibit strong positive correlations with a ssVAC seed during all task conditions, including foci in the left middle frontal gyrus (MFG). This PFC region is more correlated with the VAC seed when scenes were remembered and less correlated when scenes were ignored, relative to passive viewing. Moreover, the strength of MFG–VAC coupling correlates with the magnitude of attentional enhancement and suppression of VAC activity. Although our correlation analyses do not permit assessment of directionality, these findings suggest that PFC biases activity levels in VAC by adjusting the strength of functional coupling in accordance with stimulus relevance.
Keywords: attention, beta series correlation analysis, functional connectivity, functional magnetic resonance imaging, neural networks, working memory
Introduction
Working memory (WM) is the cognitive operation that underlies our ability to temporarily maintain and manipulate information that is no longer accessible in the environment in order to guide behavior (Baddeley 1986). The prefrontal cortex (PFC) has been frequently attributed a key role in the neural basis of WM, with research largely focused on its critical involvement after a stimulus is no longer present while representations are actively maintained in mind. This is supported by single-unit recording studies in nonhuman primates demonstrating persistent activity in PFC neurons during the “delay” period of WM tasks (Fuster and Alexander 1971; Kubota and Niki 1971; Funahashi et al. 1989; Wilson et al. 1993; Chafee and Goldman-Rakic 1998), as well as by functional magnetic resonance imaging (fMRI) studies in human subjects (Courtney et al. 1997; D’Esposito et al. 2000; Jha and McCarthy 2000; Postle et al. 2003). However, the PFC has also been implicated in neural processing that occurs when a stimulus is present in the environment, such as during selective attention tasks when relevant and irrelevant information compete for cognitive resources (Everling et al. 2002; Iba and Sawaguchi 2003; Pessoa et al. 2003). Thus, the PFC may serve a common role as a control region in both selective attention and WM operations, when stimuli are either present or absent (Curtis and D’Esposito 2003; Miller and D’Esposito 2005; Gazzaley and D’Esposito 2007).
Another aspect shared by selective attention and WM operations is modulation of neural activity in posterior sensory cortices. Electrophysiology and neuroimaging studies have revealed activity modulation of visual association cortex (VAC) during visual selective attention tasks when stimuli are present (Corbetta et al. 1990; Luck et al. 1997; Treue and Martinez Trujillo 1999), as well as during the delay period of visual WM tasks when stimuli are absent (Fuster 1990; Miller et al. 1993; Druzgal and D’Esposito 2001). Neural activity is enhanced in the VAC regions that encode behaviorally relevant visual stimuli (Fuster 1990; Duncan et al. 1997; Hopfinger et al. 2000; Kanwisher and Wojciulik 2000), and reciprocal suppression of activity occurs in visual regions that represent non-relevant stimuli (Duncan et al. 1997; Kastner et al. 1998; Kastner and Ungerleider 2001; Gazzaley, Cooney, McEvoy, et al. 2005). Modulation of sensory cortical activity has also been described for the auditory (Hillyard et al. 1973), olfactory (Zelano et al. 2005), and somatosensory (Seminowicz et al. 2004) systems.
It is believed that such goal-directed sensory cortical activity modulation is not an intrinsic property of sensory cortex, but rather is achieved via neural connections that subserve dynamic interactions between brain regions, or neural networks. There is accumulating evidence that suggests it is the PFC that modulates the magnitude of neural activity in distant sensory brain regions via long-range projections, a mechanism of control known as “top-down modulation.” Axonal tract-tracing studies in monkeys reveal an intricate network of reciprocal corticocortical connections between regions in the PFC and VAC (Ungerleider et al. 1989; Webster et al. 1994; Barbas 2000; Petrides and Pandya 2002), including projections from the middle frontal gyrus (MFG; Petrides and Pandya 1999; Rempel-Clower and Barbas 2000; Blatt et al. 2003). Several of these pathways have also been described in humans with postmortem dissection (Heimer 1983) and more recently in vivo with diffusion tensor magnetic resonance imaging (Makris et al. 2004). These anatomically defined networks establish the structural framework by which the PFC may exert modulatory control over VAC activity. The role of the PFC as the “top” in top-down modulation of sensory cortical activity may be the common link accounting for functional involvement of these distant regions during selective attention and WM tasks. This long-range, modulatory control process may be essential for both establishing high fidelity representations of task-relevant stimuli when they are perceived, as well as facilitating their internal maintenance when they are no longer accessible in the environment (Gazzaley and D’Esposito 2007).
It is important to note that the majority of studies supporting a role of PFC–VAC networks in these operations offer indirect evidence of functional interactions between these regions. This is because activity in these anatomically disparate brain regions are usually recorded and/or analyzed independently (Moran and Desimone 1985; Miller et al. 1993; Corbetta 1998; D’Esposito et al. 1998; Ungerleider et al. 1998). Direct evidence is rather limited, although there are 2 invasive studies in monkeys that support the PFC as a source of activity modulation on the VAC (Fuster et al. 1985; Tomita et al. 1999). Similarly, microstimulation studies of neurons in the frontal eye fields (FEF) have provided casual evidence of top-down influences on activity in VAC neurons (Moore and Armstrong 2003; Moore and Fallah 2004; Armstrong et al. 2006). Additionally in humans, electroencephalography studies on patients with PFC lesions have provided evidence of PFC-dependent top-down modulatory influences of VAC occurring in the first few hundred milliseconds of visual processing (Barcelo et al. 2000).
A noninvasive approach to evaluate interactions between brain regions with preserved structure and function is multivariate analysis of functional brain imaging data, a statistical method to generate maps of functional connectivity between regions and associate them with the cognitive processes being performed (Friston et al. 1993, 2000; McIntosh 1998; Buchel and Friston 2000; Lin et al. 2003; Penny et al. 2004; Sun et al. 2004). We have recently developed a new multivariate method to characterize functional connectivity in event-related fMRI data sets during the component stages of a multistage task, such as a delayed-recognition WM task (Rissman et al. 2004). The method, beta series correlation analysis, employs a standard general linear model (GLM) approach, as do most univariate analyses for estimating stage-specific activity (Friston et al. 1995), but adapts the model so that distinct parameter estimates (beta values) are computed for each trial and used as the dependent data in a correlation analysis. Whereas standard univariate analyses inherently treat trial-to-trial variability as noise, beta series correlation analysis explicitly measures and capitalizes on this variability. If 2 areas of the brain are functionally interacting with each other during a particular stage of WM (e.g., cue encoding), then fluctuations in the amount of activity that the 2 areas exhibit during that stage should be correlated across trials. The method can be implemented by selecting a region of interest, or “seed,” and determining the network of regions that correlate with it and how these correlations change across task conditions. Several studies have now successfully employed the beta series correlation analysis method to yield novel insights into the interregional interactions occurring during WM tasks (Gazzaley et al. 2004; Buchsbaum et al. 2005; Ranganath et al. 2005; Fiebach et al. 2006; Yoon et al. 2006). It is important to emphasize that this functional connectivity analysis method, although capable of revealing regions involved in functional networks and determining how the magnitude of connectivity varies with conditions, does not allow us to establish the directionality of interregional communication. Thus, interpretations of directionality can only be based on conclusions from other studies in the literature that have documented direct evidence of top-down influences in similar cognitive operations.
We recently characterized the brain regions that significantly correlated with a VAC seed during the maintenance period of a WM task (Gazzaley et al. 2004). This maintenance network included the dorsolateral and ventrolateral PFC, supporting the notion that coordinated functional interactions between the VAC and PFC, as well as other cortical and subcortical regions, are associated with the active maintenance of perceptual representations in WM. In the present study, we performed a comparable functional connectivity analysis using a recently published fMRI data set (Gazzaley, Cooney, McEvoy, et al. 2005) to focus on PFC–VAC networks associated with selective attention at the initial encoding stage of a WM task, when stimuli are still present. It has long been acknowledged that selective attention and WM are similar conceptually, but they have traditionally been categorized separately and studied independently. It is only recently that characterization of the mechanistic overlap between these operations has become a prominent research focus (Desimone 1996; LaBar et al. 1999; Awh and Jonides 2001; de Fockert et al. 2001). It is clear that the ability to accurately maintain information in mind depends on the quality of neural representations established when stimuli are first perceived. Given that such representations are susceptible to interference by distracting information (Miller et al. 1996), selective attention is necessary for successful WM performance by restricting the contents of capacity-limited memory to task-relevant representations (Rainer et al. 1998; Ploner et al. 2001; Vogel, McCollough Machizawa 2005). Therefore, top-down modulation mediated by PFC–VAC networks may serve an integral role at the intersection of these 2 overlapping cognitive operations.
We recently modified the classic delayed-recognition WM task to study the selective attention processes of both top-down enhancement and suppression of visual representations during the WM encoding period (Gazzaley, Cooney, McEvoy, et al. 2005). During each trial, subjects observed a sequence of 2 faces and 2 natural scenes presented in a randomized order (Fig. 1). Instructions presented at the beginning of each run informed them which stimuli were relevant and which should be ignored: 1) “Remember Faces and Ignore Scenes,” 2) “Remember Scenes and Ignore Faces,” or 3) “Passively View” faces and scenes without attempting to remember them. Across the 3 task conditions, the period during which the 4 stimuli were presented was balanced for bottom-up visual information, thus allowing us to examine the influence of goal-directed behavior on neural activity (top-down modulation). In the 2 memory conditions, the encoding of the task-relevant stimuli required selective attention, which permitted the dissociation of physiological measures of enhancement and suppression relative to the passive viewing baseline. In the fMRI component of this experiment, we used an independent functional localizer task to identify scene-selective VAC (ssVAC) regions in the parahippocampal/lingual gyrus (Gazzaley, Cooney, McEvoy, et al. 2005), an area often referred to as the parahippocampal place area (Epstein and Kanwisher 1998). We determined that blood oxygen level–dependent (BOLD) signal in ssVAC during the cue period was significantly higher in Remember Scenes trials and lower in Remember Faces trials (i.e., ignore scenes) when compared with the Passive View trials (Gazzaley, Cooney, McEvoy, et al. 2005). This finding suggested the presence of both enhancement and suppression of neural activity in the VAC relative to passive baseline.
Figure 1.
Experimental design. The 3 task conditions differ only in the instructions given at the beginning of each run, instructing the participant which, if any, stimuli they should attempt to remember over a 9-s delay, and in the response requirements. On each trial, 2 face and 2 scene stimuli were presented for 800 ms each (with a 200-ms interstimulus interval (ISI)), in a randomized sequence. In the response period of the Remember/Ignore task conditions, a face or scene stimulus was presented (corresponding to the relevant stimulus class), and subjects were required to report with a button press whether the stimulus matched one of the previously presented stimuli. In the response period of the Passive View condition, an arrow was presented, and participants were required to make a button press indicating the direction of the arrow.
In the current study, the application of the beta series correlation method to analyze this data set enables us to evaluate the network of regions that interact with ssVAC during WM encoding and assess how PFC–VAC connectivity is modulated as a function of the attentional goals of the task. Moreover, we will evaluate how individual differences in the relative strength of PFC–VAC connectivity relate to the degree of activity modulation observed in VAC.
Methods
A complete description of the experimental design and scanning protocol can be found in Gazzaley, Cooney, McEvoy, et al. (2005); the critical details are summarized below.
Subjects
Eighteen healthy subjects (8 females and 10 males; ages 19–30) took part in the study after providing informed consent. Subjects were prescreened, and none used any medication with psychoactive, cardiovascular, or homeostatic effects. All subjects had normal or corrected-to-normal vision and were right handed. Two subjects were excluded from the analysis: 1 due to the presence of data artifacts and 1 because sufficient behavioral data were not collected.
Task Design
Subjects were scanned while performing a visual delayed-recognition task under 4 different instructional conditions. Before each scanning run began, subjects were either instructed to 1) Remember Faces and Ignore Scenes, 2) Remember Scenes and Ignore Faces, or 3) Passively View both Faces and Scenes—with no attempt to remember or evaluate them. A fourth condition, in which subjects were instructed to remember all stimuli, was included in the experiment, but was not evaluated in the current analysis. These instructions were to be applied to all 10 trials occurring during that 4.5-min run. At the beginning of each trial, subjects viewed 4 sequentially presented novel grayscale images (2 faces and 2 scenes in a randomized order). Each image was presented for 800 ms, with a 200-ms blank-screen interstimulus interval. Presentation of stimuli was followed by a 9-s delay period, after which a fifth stimulus was presented for 1s. In the Remember/Ignore task conditions, a face or scene probe stimulus was presented (depending upon the condition), and subjects were required to report with a button press whether the stimulus matched one of the previously presented stimuli. In the Passive View condition, an arrow was presented, and subjects were required to make a button press indicating the direction of the arrow. Presentation of the probe stimulus was followed by a 10-s intertrial interval. Data were acquired during 12 runs (3 runs of each of the 4 task conditions), yielding a total of 30 trials per condition.
To allow us to independently localize stimulus-selective regions in each subject’s VAC, subjects performed a brief functional localizer task before beginning the main experiment. Subjects were presented with alternating 16-s blocks of face stimuli, scene stimuli, and rest periods (7 blocks of each type) and were instructed to attend to the stimuli and to indicate with a button press whenever they noticed an immediate (1-back) repeat of a stimulus.
fMRI Acquisition and Processing
Magnetic resonance data were acquired with a Varian INOVA 4T-scanner (Palo Alto, CA) equipped with a transverse electromagnetic send-and-receive radio frequency head coil. Functional data were obtained using a 2-shot T2*-weighted echo-planar imaging (EPI) sequence sensitive to BOLD contrast (time repetition [TR] = 2000 ms, time echo [TE] = 28 ms, field of view [FOV] = 22.4 cm2, matrix size = 64 × 64, in-plane resolution = 3.5 × 3.5 mm). Each functional volume consisted of eighteen 5-mm axial slices separated by a 0.5-mm interslice gap and provided nearly whole-brain coverage. Anatomical images coplanar with the EPI data were collected using a gradient-echo multislice sequence (TR = 200 ms, TE = 5 ms, FOV = 22.4 cm2, matrix size = 256 × 256). High-resolution anatomical data were acquired with an MP-FLASH 3-dimensional sequence (TR = 9 ms, TE = 5 ms, FOV= 22.4 × 22.4 × 19.8 cm, matrix size = 256 × 256 × 128). Data were corrected for between-slice timing differences using a sin c-interpolation method and were interpolated to a 1-s TR by combining each shot of half k space with the bilinear interpolation of the 2 flanking shots. Subsequent processing was performed using SPM2 software (http://www.fil.ion.ucl.ac.uk) run under Matlab 6.5 (http://www.mathworks.com). Functional data were realigned to the first volume acquired and were spatially smoothed with a 8-mm full-width half-Maximum (FWHM) Gaussian kernel.
fMRI Univariate Activity Analysis
Task-dependent changes in BOLD signal were modeled with independent regressors for each component stage (cue, delay, and probe) of each task condition. Because the cue period of each trial lasted 4 s, the regressor for this period consisted of a 4-s boxcar function. The delay period was modeled with a 2-s boxcar function whose onset was placed 4 s into the delay period to ensure that this regressor was minimally contaminated by residual hemodynamic activity from the preceding encoding period (Zarahn et al. 1997). The probe period was modeled with a 1-s boxcar function time-locked to the onset of the probe stimulus. The regressors were convolved with the canonical hemodynamic response function provided by SPM2 and entered into the modified GLM (Worsley and Friston 1995) instantiated in SPM2. Only trials with correct behavioral responses were incorporated in the analysis; trials with incorrect responses were modeled separately and excluded. The global mean signal level over all brain voxels was calculated for each time point. Within each session, a line was fit to this global mean time series. All volumes were then scaled (divided) by the piecewise linear fit, thereby normalizing the session means and removing within-session linear trends and a high-pass filter (cutoff period = 128 s) was applied to remove low-frequency artifacts from the data.
Maps of parameter estimates (β values) were computed from the GLM to assess the magnitude of activation during each task stage and condition. Individual subject activation maps were then spatially normalized into standard Montreal Neurological Institute (MNI) atlas space with SPM2, and group-level random-effects analyses were conducted. Voxels were deemed significant if they surpassed a voxel-level threshold of t > 3.29, P < 0.005 (2-tailed), and an extent threshold of 15 contiguous voxels.
fMRI Functional Connectivity Analysis
Functional connectivity analyses were conducted using the beta series correlation analysis method (Rissman et al. 2004). A new GLM design matrix was constructed in which the temporal arrangement of the covariates was identical to that used in the univariate analysis outlined above. The principal difference in this GLM was that the cue, delay, and probe stages of each individual trial were coded with a unique covariate. This resulted in a total of 360 covariates of interest being entered into the GLM (3 task stages per trial × 30 trials per condition × 4 task conditions), including the unused “Remember Both Faces and Scenes” condition. The least squares solution of the GLM yielded a unique set of 360 beta values for each voxel in the brain. For each voxel, these beta values were sorted by the task stage and condition from which they were derived to form 12 distinct beta series for that voxel. Each beta series thus reflects the voxel’s estimated activity during a particular task stage of each experimental trial of a given task condition. Only beta values from trials for which the participant produced the correct response were included in the beta series. The extent to which 2 brain regions interact during a particular task stage and condition is quantified by the degree to which their respective beta series from that stage/condition are correlated. For the purposes of the present study, we exclusively focus on the correlation maps generated from the cue period beta series, the stage when the 4 stimuli were present and task-dependent top-down modulatory signals presumably begin to exert their influence on VAC activity.
The 7 contiguous voxels in each participant’s left parahippocampal/lingual gyrus that exhibited the strongest response preference to scenes versus faces in the functional localizer task, as assessed by a t-test, were defined as that participant’s ssVAC seed. Subjects’ seed regions were identical to the regions of interest interrogated in our previous univariate analyses of this data set (Gazzaley, Cooney, McEvoy, et al. 2005, Gazzaley, Cooney, Rissman, et al. 2005). Although face-selective regions and right ssVAC regions were identified, the current study focused exclusively on the left ssVAC, which yielded the most robust measures of top-down modulation in our previous analyses. Seed correlation maps were generated by calculating the correlation of the seed’s beta series (averaged across the 7 seed voxels) with that of all brain voxels. Separate beta series, and hence separate correlation maps, were derived for each of the 4 task conditions in addition to being subdivided by task stage. Correlation magnitudes were converted into z-scores using the Fisher’s r-to-z transformation. The resulting individual subject z-maps were spatially normalized into standard MNI atlas space, using routines from SPM2. For illustrative purposes, group-level statistical maps were generated for the individual conditions using a fixed-effects analysis, in which the z-maps were averaged across subjects and liberally thresholded at z > 1.65, P < 0.05 (1-tailed). Task-dependent changes in functional connectivity were assessed using random-effects contrasts, which were thresholded at t > 3.29, P < 0.005 (2-tailed), combined with an extent threshold of 15 contiguous voxels.
Results
Univariate Data
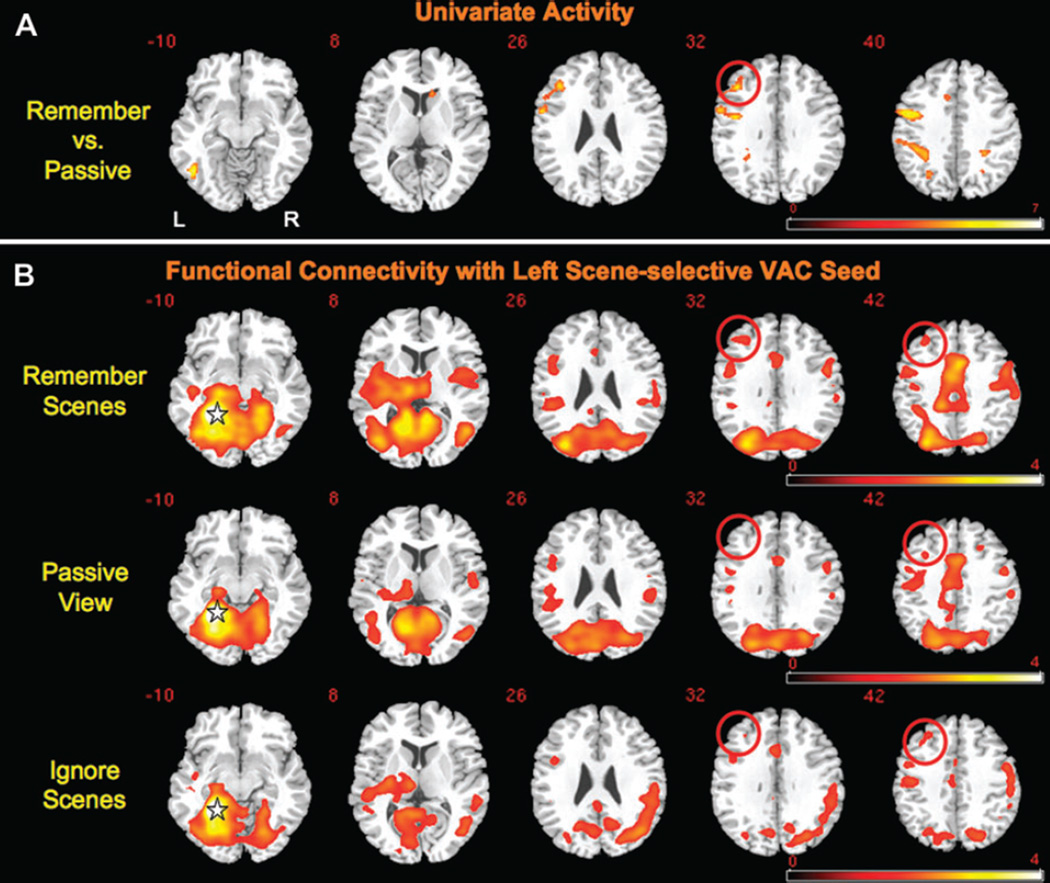
Univariate analyses were performed in the original evaluation of this data set (Gazzaley, Cooney, McEvoy, et al. 2005); however, the focus of our initial investigation was restricted to activity modulation in VAC. In the present study, to identify regions that may be involved in top-down control, we performed a whole-brain contrast of univariate activity obtained from the 4-s cue-encoding period for both the Remember Scenes/Ignore Faces and the Remember Faces/Ignore Scenes trials versus the Passive View trials (to balance this contrast, the Passive View condition was weighted twice as strongly as each of the Remember/Ignore conditions). This analysis identified a number of regions throughout the brain with greater BOLD signal during the Remember/Ignore condition than the Passive View condition (Table 1). Of particular note, this included an area of activity with a peak within the left MFG extending posteriorly into inferior frontal gyrus (MNI coordinates of peak: – 38, 32, 30; Brodmann area [BA] 45/46; Fig. 2A), a region that has been implicated in high-level executive control processes (Duncan and Owen 2000; Cabeza et al. 2002).
Table 1.
Univariate activity contrast
| Brain region | BA | x | y | z | Peak t-value |
|---|---|---|---|---|---|
| Remember Scenes and Remember Faces > Passive View | |||||
| L precentral gyrus | 6 | −29 | −2 | 59 | 9.581 |
| L intraparietal sulcus | 40/7 | −32 | 43 | 51 | 7.536 |
| L inferior occipitotemporal cortex | 37 | −46 | −60 | −11 | 6.073 |
| L parahippocampal cortex/fusiform gyrus | 30 | −22 | −30 | −23 | 5.597 |
| Medial supplementary motor area (SMA) | 6/8/32 | −2 | 13 | 54 | 5.439 |
| L MFG/inferior frontal gyrus | 45/46 | −38 | 32 | 31 | 5.282 |
| L insula | 48 | −30 | 20 | 14 | 5.058 |
| L superior temporal gyrus/temporal pole | 38 | −58 | 10 | −6 | 4.449 |
| Medial precuneus | 5 | 2 | −51 | 67 | 4.286 |
| R intraparietal sulcus | 40/7 | 22 | −66 | 61 | 7.431 |
| R precentral gyrus | 6 | 29 | −5 | 52 | 5.859 |
| R parahippocampal cortex | 30 | −20 | −32 | −23 | 5.319 |
| R caudate | — | 14 | 22 | 8 | 4.131 |
| R superior frontal gyrus | 6 | 32 | 2 | 66 | 3.605 |
Note: L, left; R, right.
Figure 2.
Group-level statistical maps of the univariate activation and left ssVAC seed correlation data. (A) A random-effects contrast reveals regions exhibiting greater univariate activity during the 2 Remember/Ignore conditions than the Passive View condition. An activation cluster in the left MFG is encircled for emphasis. Regions exhibiting negative activation in this contrast (i.e., greater activity during the Passive View condition) are not displayed. Activations from selected slices are shown overlaid on an MNI template brain and displayed in neurological convention (left 5 left). The MNI z-coordinate of each slice is shown in yellow in the upper left-hand corner. The color scale indicates the magnitude of the t-values. (B) Group-averaged correlation z-maps reveal the network of regions exhibiting functional connectivity with the left ssVAC seed during each of the 3 task conditions. The approximate location of the ssVAC seed is indicated with a star. The MFG correlates with the ssVAC seed in all conditions (red circles), but its correlation is most extensive in the Remember Scenes condition. The color scale indicates the magnitude of the z values.
Functional Connectivity Data
To evaluate interregional network interactions, beta series correlation analysis was performed on the cue period data of each participant using the left ssVAC region as a seed. Qualitative evaluation of the group connectivity maps for each of the 3 task conditions (Remember Scenes, Remember Faces, and Passive View) revealed that in addition to the anticipated high connectivity across visuospatial processing areas (occipital and parietal cortices) there was also a focus of high connectivity in the left MFG (Fig. 2B). This left MFG connectivity cluster was most extensive in the Remember Scenes condition, where it overlapped with the left MFG area of high univariate activity noted in the previous section.
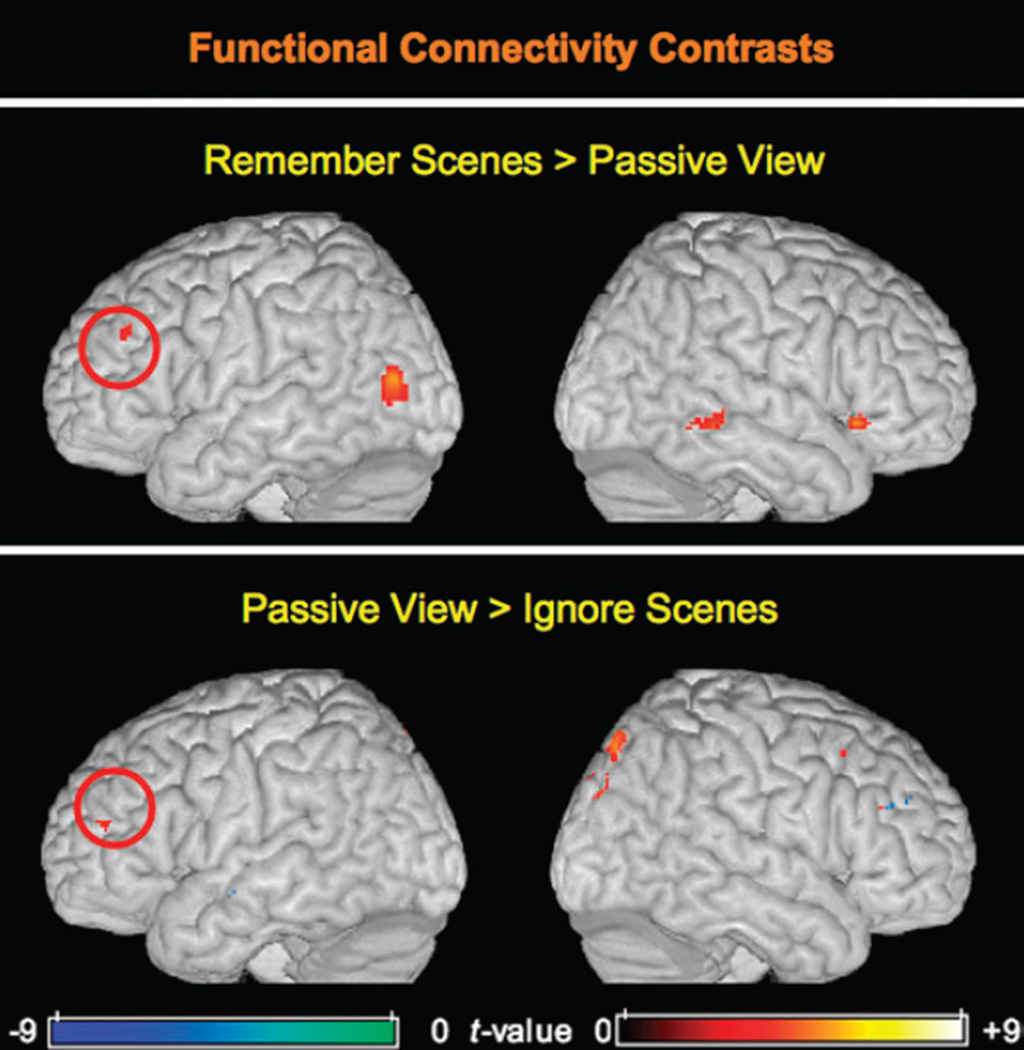
To directly compare networks associated with enhancement and suppression of relevant and irrelevant information, respectively, we contrasted the group ssVAC connectivity maps from the Remember Scenes/Ignore Faces (enhancement) and Remember Faces/Ignore Scenes (suppression) task conditions with the connectivity map from the Passive View task. These mapwise contrasts revealed that there were positive correlations in all 3 conditions, which varied in strength with instruction. Of note, there was greater left ssVAC–left MFG connectivity when subjects were attempting to remember scenes and less when they were attempting to ignore scenes relative to when they were passively viewing scenes (Fig. 3). Although these clusters are not overlapping at this threshold, they are located near each other within the left MFG (peak Remember Scenes > Passive View MNI coordinates: –44, 38, 32; peak Passive View > Remember Faces MNI coordinates: –30, 48, 20; Euclidean distance between peaks: 21 mm). Table 2 lists other brain regions exhibiting significant connectivity differences in these contrasts.
Figure 3.
Random-effects functional connectivity contrasts. The top panel depicts regions exhibiting significantly greater functional connectivity with the ssVAC seed in the Remember Scenes condition relative to the Passive View baseline condition. A significant cluster in the left MFG is encircled. The bottom panel depicts a contrast between the Passive View condition and the Ignore Scenes (Remember Faces) condition. A left MFG cluster exhibiting significantly greater connectivity with the ssVAC seed in the Passive View condition than in the Ignore Scenes condition is encircled. Maps are displayed on a surface-rendered MNI template brain.
Table 2.
Functional connectivity contrasts
| Brain region | BA | x | y | z | Peak t-value |
|---|---|---|---|---|---|
| Remember Scenes > Passive View | |||||
| L middle occipital cortex | 19 | −34 | −77 | 12 | 5.44 |
| L fusiform/parahippocampal cortex | 37 | −35 | −39 | −10 | 5.01 |
| L inferior temporal cortex | 20 | −45 | 38 | 32 | 3.99 |
| L MFG | 45/46 | −44 | 38 | 32 | 3.99 |
| L anterior hippocampus/amygdala | 20 | −22 | −12 | −10 | 3.61 |
| Medial midbrain | — | 1 | −12 | −16 | 4.54 |
| R inferior orbitofrontal cortex | 38/47 | 50 | 22 | −6 | 5.44 |
| R middle temporal cortex | 21 | 66 | −38 | −8 | 4.74 |
| R caudate | — | 11 | 2 | 12 | 4.19 |
| R thalamus | — | 6 | −13 | 9 | 3.89 |
| R insula | 48 | 49 | −9 | 2 | 3.64 |
| Passive View > Remember Scenes | |||||
| R anterior cingulate cortex | 32 | 3 | 50 | 12 | 4.48 |
| Ignore scenes (Remember Faces) > Passive view | |||||
| R inferior frontal gyrus | 45 | 56 | 38 | 26 | 4.62 |
| L superior temporal gyrus | 21/48 | −46 | −8 | −8 | 4.02 |
| L parahippocampal cortex/hippocampus | 20/36 | −26 | 14 | −23 | 3.98 |
| Passive View > Ignore scenes (Remember Faces) | |||||
| L pre-SMA | 32/6 | −13 | 16 | 49 | 5.67 |
| L postcentral sulcus | 3/40 | −24 | −40 | 53 | 4.13 |
| L insula | 48 | −26 | 26 | 12 | 3.92 |
| L MFG | 46 | −30 | 48 | 20 | 3.85 |
| Medial cuneus | 19 | 4 | −85 | 36 | 5.03 |
| R inferior frontal suclus | 48 | 28 | 25 | 26 | 5.96 |
| R superior parietal cortex | 7 | 31 | −78 | 53 | 5.17 |
| R pre-SMA | 6 | 8 | 16 | 50 | 4.68 |
| R middle cingulate/SMA | 6 | 14 | −5 | 46 | 4.19 |
| R inferior frontal sulcus | 48 | 36 | 32 | 25 | 4.07 |
| R middle/superior occipital cortex | 19 | 38 | −86 | 31 | 3.81 |
| R rolandic operculum/insula | 48 | 42 | −8 | 19 | 3.64 |
| R MFG/superior frontal sulcus | 8/9 | 30 | 17 | 50 | 3.49 |
Note: L, left; R, right.
The pattern of left ssVAC–left MFG correlations across the 3 task conditions mimics the pattern we recently reported for the univariate activity in left ssVAC (Gazzaley, Cooney, McEvoy, et al. 2005), such that the magnitudes of the correlations increased or decreased relative to Passive View depending on the task instructions. This raised the possibility that connectivity between these regions may be directly related to VAC modulation, a finding that would offer stronger evidence of a role of the left MFG as a source of the top-down modulatory signal. To evaluate this, we investigated if the magnitude of the functional connectivity between these 2 regions correlated with the univariate activity level in the VAC region across subjects. Across-participant correlation analysis revealed that the left MFG voxel exhibiting the greatest Remember Scenes versus Passive View connectivity with the left ssVAC seed showed a significant positive correlation with the left ssVAC seed enhancement index (Remember Scenes minus Passive View; r = 0.556, P = 0.012 1-tailed), but no correlation with the suppression index (r = − 0.111, P > 0.1). Comparably, the left MFG voxel exhibiting the greatest Passive View versus Remember Faces connectivity with the left ssVAC seed showed a significant positive correlation with the left ssVAC seed suppression index (Passive View minus Remember Faces; r = 0.441, P = 0.044 1-tailed), but no correlation with the enhancement index (r = 0.199, P > 0.1). Thus, the degree of connectivity between the left MFG and the stimulus-selective VAC correlates with the level of activity modulation in the VAC.
To determine whether there was a relationship between MFG univariate activity and VAC modulation, we performed an across-subject correlation analysis using individual subject univariate activity measures from the left MFG. For this analysis, we used the left MFG voxel exhibiting the strongest univariate activity difference in the contrast between the 2 conditions requiring selective attention (Remember Scenes and Remember Faces) versus the Passive View condition (viewable at the group level in Fig. 2A). Activity in this voxel failed to show a significant correlation with the left ssVAC enhancement index (r = 0.284, P > 0.1) or suppression index (r = 0.055, P > 0.1). Therefore, only functional connectivity between the MFG and VAC correlates with VAC activity modulation and not the magnitude of MFG activity in isolation.
Discussion
The view that the PFC is a brain region critical for multiple cognitive control processes has been entrenched in the scientific literature since the mid twentieth century when studies were performed using sophisticated neuropsychological tests on patients with PFC lesions (Milner 1963; Luria 1966; Benton 1968). These findings from human studies paralleled the landmark contributions of Jacobsen (1935) 30 years earlier, which demonstrated that monkeys with bilateral PFC lesions were impaired on WM delayed-response tasks. More recent efforts by cognitive neuroscientists have attempted to explore the mechanistic role of the PFC in control processes; this has largely employed single-unit recordings of PFC neurons in experimental animals and functional neuroimaging studies in healthy human subjects utilizing univariate statistical data analyses.
One of the most influential theories of cognitive/executive control is Baddeley’s model of WM (Baddeley 1986). Based on behavioral studies of healthy adults, Baddeley proposed that WM involves a central executive system that actively controls the distribution of limited attentional resources and coordinates the processing of information within verbal and spatial memory buffers. It has been proposed that the PFC is the core of such a central executive system, and processing information in distant brain regions based on task goals is mediated via long-range connections between the PFC and other regions (top-down modulation). There are many researchers who have attributed such an operational role to the PFC: Contingent Encoding (Mesulam 2002), Dynamic Filtering (Shimamura 1997), Adaptive Encoding (Duncan 2001), Gazzaley and D’Esposito (2007), Petrides (1994), Knight et al. (1999), and Miyashita (2004), as well as those who advocate a hybrid of an operational model and a contribution by the PFC to the representation of information (Goldman-Rakic 1998; Miller and Cohen 2001).
Traditionally, most functional neuroimaging studies have utilized univariate analyses, permitting only the assessment of activity within a given brain region in isolation. However, there has been an increasingly frequent application of multivariate analyses to neuroimaging data. Several groups, including our own, have begun to study functional interactions between the PFC and other cortical regions during cognitive control operations, such as attention to action (Rowe et al. 2002), visual WM (McIntosh et al. 1996; Cooney et al. 2005), and visual imagery (Mechelli et al. 2004). In the current study, we have extended this approach to study PFC–VAC functional connectivity during a visual WM task requiring selective object-based attention to sequentially presented relevant and irrelevant visual stimuli.
Univariate Data
Univariate analysis of the data set was performed first and replicated a common finding in the literature. A region of the lateral PFC, specifically the left MFG, was more active (i.e., showed greater BOLD signal) in the WM-encoding period when the task required stimuli to be remembered/ignored than passively viewed. This finding constitutes further evidence of a PFC role in cognitive control operations. However, the interpretation of this result is under the same limitations as those of most previous studies; univariate analysis does not permit us to evaluate the relationship between PFC and VAC activity and thus does not inform us of the PFC role in top-down modulation. Furthermore, due to limitations in the temporal resolution of fMRI, we are unable to generate independent BOLD signal maps while subjects attempt to either remember or ignore stimuli because the 4 individual cue stimuli were only spaced 200 ms apart from one another. Thus, with univariate analysis it is not possible to differentially assess PFC involvement in top-down enhancement and suppression of relevant and irrelevant information. To address both of these limitations, we utilized functional connectivity analysis with a ssVAC seed to explore how the task relevancy of the scene stimuli altered the way PFC regions were functionally connected with posterior scene-processing regions.
Connectivity Data
Beta series correlation analysis (Rissman et al. 2004) was used to characterize functional connectivity in this data set. We chose this analysis technique over other multivariate approaches because it is specifically designed to dissociate functional networks corresponding to distinct stages of a cognitive task. Thus, interregional interactions associated with selective attention processes at the time of encoding (the focus of this study) could be dissociated from those occurring during the delay period (Gazzaley et al. 2004). This functional connectivity technique also had the advantage over other effective connectivity methods, such as structural equation modeling (McIntosh 1999) and dynamic causal modeling (Mechelli et al. 2003), in that a structural model did not have to be prespecified, but rather the data could be explored in a hypothesis-driven, but unconstrained manner. The limitation, however, is that directionality cannot be determined by these data alone.
The following discussion focuses on interpretations of the data that may be reached when the connectivity analysis are assumed to reflect the influence of the PFC on VAC activity. This interpretation is based on extrapolating from the evidence in the literature that PFC neurons influence activity in the VAC in a top-down manner (Fuster et al. 1985; Tomita et al. 1999; Barcelo et al. 2000). Alternatively, it is possible that the observed correlation between the VAC and the PFC is reflective of bottom-up processing, and these results reveal connectivity involved in the transfer of information to the PFC for effective memory storage during the delay period. This possibility aside, the beta series correlation data suggest 3 aspects of the PFC–VAC network and top-down modulatory control.
Similar PFC–VAC networks are present for all task conditions, with similar areas of the left MFG exhibiting high connectivity with the left ssVAC in all 3 tasks: remembering, ignoring, and passively viewing scenes. Furthermore, left MFG–left ssVAC correlations were positive for all tasks. These 2 pieces of data together suggest that enhancement and suppression of VAC activity reflects varying levels of excitatory modulatory influences from the PFC, rather than excitatory and inhibitory modulatory influences.
The degree of connectivity is associated with different instructions (Remember > Passive and Passive > Ignore). Although all MFG–ssVAC correlations were positive, there were differences in the degree of connectivity, such that MFG–ssVAC connectivity was greater when scenes were being remembered and lower when scenes were being ignored, both relative to passively viewing scenes. This finding suggests the possibility that a mechanism of VAC activity enhancement and suppression may involve regulation of the degree of coupling between a source of control (left MFG) and a site of modulation (left ssVAC).
PFC–VAC connectivity predicts the magnitude of VAC activity modulation. The degree that MFG–ssVAC connectivity was greater or less than Passive View connectivity when subjects were remembering or ignoring scenes correlates with the magnitude of activity modulation in the ssVAC. This offers further evidence that an interaction between the PFC–VAC is involved in top-down modulation. It is important to note that a similar analysis with univariate activity from the left MFG did not yield a significant correlation with ssVAC modulation indices, supporting the case for the use of functional connectivity analysis to gain new insights into neural processes.
The connectivity data presented in the current study suggest that the left MFG is involved in the top-down control of left VAC activity modulation. This lateral PFC region also revealed greater univariate activity in the Remember/Ignore tasks than the Passive View task. The MFG has been shown to be robustly active in functional neuroimaging data sets utilizing numerous cognitive tasks, including episodic memory encoding and retrieval, WM encoding, maintenance and retrieval, and selective attention (Duncan and Owen 2000; Frith 2000; Cabeza et al. 2002; Ranganath et al. 2003). We propose that the role of this region in top-modulatory control of sensory cortical activity may be the basis for its association with such a diverse collection of cognitive operations. Supporting this assertion, the left MFG was also a node of the WM maintenance network that we identified using beta series correlation analysis and VAC seeds (Gazzaley et al. 2004). This suggests that similar source regions of top-down modulation in the PFC are involved in both stimulus-present and stimulus-absent modulation. Although the current analysis identified the left MFG as a putative source of top-down control signals, we do not wish to make strong claims about the hemispheric specificity of PFC involvement because this analysis focused exclusively on correlations with the left scene-selective region, the most robustly modulated VAC region.
These data further suggest that the MFG influences activity magnitude in the VAC for both relevant and irrelevant information by varying the level of top-down excitatory control, as reflected by attention-dependent changes in the degree of positive functional connectivity between the regions. The data lead us to this interpretation over the alternative possibility that excitatory influences mediate enhancement and inhibitory influences mediate suppression. If a PFC region was influencing VAC activity via inhibitory influences, we would expect to observe negative correlations between these regions when the task required suppression of the visual stimuli (i.e., the more PFC activity on a given Remember Faces/Ignore Scenes trial, the less ssVAC activity on that trial). This was not the case; the correlations were positive, and thus suggest the presence of an excitatory influence even when stimuli were ignored.
However, we do not wish to rule out a potential role for top-down inhibitory signaling to sensory cortical regions representing task-irrelevant information. The fact that these correlation maps do not reveal regions exhibiting negative correlations with seed regions may be because our method is not optimized to detect negative coupling between regions. This may be the result of a global noise component that biases all brain voxels toward weak positive correlations. Task-dependent positive and negative correlations may ride on top of this weak global effect, and negative correlations may not be strong enough to overcome the positive bias. Our finding that PFC control is mediated by levels of excitation is at odds with some evidence of an inhibitory pathway from PFC that regulates the flow of sensory information via thalamic relay nuclei. It was observed that cooling the PFC in cats resulted in increased amplitudes of evoked electrophysiological responses recorded from the primary cortex for all sensory modalities (Skinner and Yingling 1977). Conversely, stimulation of specific regions of the thalamus that surround the sensory relay thalamic nuclei (i.e., nucleus reticularis thalami) resulted in modality-specific suppression of activity in primary sensory cortex (Yingling and Skinner 1977). There is further evidence in humans that the PFC exhibits inhibitory control over distant cortical regions. For example, event-related potential (ERP) studies revealed that auditory-evoked (Knight et al. 1989) and somatosensory-evoked (Yamaguchi and Knight 1990) responses are increased in patients with focal PFC damage, suggesting an inhibitory influence of the PFC on sensory activity in these regions. Further research will be necessary to place the findings of the current study in the context of evidence of PFC inhibitory control.
If the PFC does control distant sensory activity by varying levels of excitatory influences based on task goals, this does not necessarily imply that the process of top-down suppression is passive and merely a consequence of limited attentional resources being allocated elsewhere. On the contrary, the process of suppression may be active and require cognitive resources in order to withdraw excitatory influences and thus suppress irrelevant information. We have recently obtained data to this effect by manipulating the cognitive demands of this task and evaluating its effect on VAC modulation measures (Rissman et al. 2006). Specifically, subjects performed a verbal WM task concurrently with the visual selective attention WM task described in the current study. At the beginning of each trial, subjects were presented auditorily with 6 digits to memorize. On half of the trials, the digit sequence was random (high load), and on the other half, the digit sequence was “1,2,3,4,5,6” (low load). After hearing the digits, the subjects then performed the same face/scene WM task reported in the present study. Preliminary results revealed that high digit load results in increased ssVAC activity relative to low digit load when subjects were ignoring scenes. Thus, increasing cognitive load with an overlapping task disrupts the ability of subjects to suppress irrelevant information, suggesting that suppression is an actively mediated control process.
On a related note, if enhancement and suppression reflect different levels of excitatory modulatory influences, it does not imply that these top-down control processes are dependent on the same mechanisms. In a recent study, using this WM paradigm to study top-down modulation in normal aging, we demonstrated that enhancement and suppression processes are dissociable. We compared left ssVAC modulation indices between young subject data used in the current study and data from a population of healthy older individuals (60–77 years of age) and revealed an age-related decrease in top-down modulation attributable to a selective decline in suppression with no significant difference in enhancement (Gazzaley, Cooney, Rissman, et al. 2005). Thus, enhancement and suppression, although potentially the result of varying levels of positive influence from the same PFC subregion, may still be mechanistically dissociable.
As already mentioned, an assumption present throughout our discussion is that the direction of modulatory influence is top-down, from PFC to VAC. However, the data we present here, although revealing task-specific relationships between these regions, are correlational and thus cannot inform interpretations of causality. The optimal experimental design to directly assess the direction of information flow involves disruption of PFC and physiological recordings of distant brain regions while subjects are engaged in a control task. There have been several studies that have implemented a lesion/physiology design in experimental animals. These studies support the conclusion that top-down modulation, utilizing both enhancement and suppression, is a mechanism of PFC control over neural activity in sensory cortices. In addition to the research of Skinner and Yingling already discussed, studies in monkeys revealed PFC mediated top-down modulation during a WM task by coupling single-cell recordings and cortical cooling (Fuster et al. 1985). This experiment showed that PFC cooling results in both augmentation and diminution of spontaneous and task-specific activity in inferotemporal neurons during the encoding (stimulus-present modulation) and delay period (stimulus-absent modulation) of a visual delayed-response task, suggesting the presence of both enhancing and suppressive PFC influences. Furthermore, cooling was accompanied by WM performance deficits, thus establishing a link between PFC mediated top-down modulation and cognition. These findings are complemented by the elegant callosal lesion/physiology study of Tomita et al. (1999), which revealed that top-down enhancement signals from the PFC to inferior temporal cortex during visual memory recall are by corticocortical projections and that this modulatory influence was necessary for successful memory recall (Miyashita 2004). Recent lesion/physiology studies in rodents have also revealed the presence of modulatory PFC influences on the activity of hippocampal place cells (Kyd and Bilkey 2003) and perirhinal neurons during a spatial delayed-response task (Zironi et al. 2001). Similarly, microstimulation studies of neurons in the FEF revealed direct influences on activity in VAC neurons (Moore and Armstrong 2003; Moore and Fallah 2004; Armstrong et al. 2006).
The present results extend our knowledge of the role of the PFC in cognitive control operations by more firmly establishing its involvement in top-down modulation of VAC activity based on task goals. Moreover, given the nature of the experimental paradigm, it offers additional evidence of a PFC role at the crossroads of selective attention and WM. Further empirical research is needed to advance our understanding of the precise mechanisms of top-down enhancement and suppression, as well as place these modulatory control mechanisms within the framework of PFC functional architecture and associated neural networks. One exciting new development is the use of transcranial magnetic stimulation to induce transient cortical disruptions in the PFC, whereas activity in distant brain regions is recorded using Position emission tomography (PET) (Mottaghy et al. 2000; Paus et al. 2001), fMRI (Ruff et al. 2006), or ERP (Evers et al. 2001) during task performance. By coupling multivariate analytical approaches, as presented in the current study, with lesion/physiology methodology in both experimental animals and human research subjects, future research will continue to elucidate the precise nature of the PFC role in top-down modulation of sensory cortex activity.
Acknowledgments
Funding
National Institutes of Health (AG025221 to A.G.; MH63901, NS40813 to M.D.).
We thank Brian Miller for helpful discussions and suggestions.
Footnotes
Conflict of Interest: None declared.
References
- Armstrong KM, Fitzgerald JK, Moore T. Changes in visual receptive fields with microstimulation of frontal cortex. Neuron. 2006;50(5):791–798. doi: 10.1016/j.neuron.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn Sci. 2001;5(3):119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Oxford: Oxford University Press; 1986. [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull. 2000;52(5):319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Suwazono S, Knight RT. Prefrontal modulation of visual processing in humans. Nat Neurosci. 2000;3(4):399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- Benton A. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;28:171–179. [Google Scholar]
- Blatt GJ, Pandya DN, Rosene DL. Parcellation of cortical afferents to three distinct sectors in the parahippocampal gyrus of the rhesus monkey: an anatomical and neurophysiological study. J Comp Neurol. 2003;466(2):161–179. doi: 10.1002/cne.10866. [DOI] [PubMed] [Google Scholar]
- Buchel C, Friston K. Assessing interactions among neuronal systems using functional neuroimaging. Neural Netw. 2000;13(8–9):871–882. doi: 10.1016/s0893-6080(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Olsen RK, Koch PF, Kohn P, Kippenhan JS, Berman KF. Reading, hearing, and the planum temporale. Neuroimage. 2005;24(2):444–454. doi: 10.1016/j.neuroimage.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage. 2002;16(2):317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol. 1998;79(6):2919–2940. doi: 10.1152/jn.1998.79.6.2919. [DOI] [PubMed] [Google Scholar]
- Cooney JW, Gazzaley A, D’Esposito M. Frontal lobe strokes impair top-down modulation of visual processing: fMRI evidence. Soc Neurosci Abstr. 2005 Online. [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc Natl Acad Sci USA. 1998;95(3):831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Attentional modulation of neural processing of shape, color, and velocity in humans. Science. 1990;248(4962):1556–1559. doi: 10.1126/science.2360050. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386(6625):608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7(9):415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- de Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291(5509):1803–1806. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 1998;7(1):1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp Brain Res. 2000;133(1):3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci USA. 1996;93(24):13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzgal TJ, D’Esposito M. Activity in fusiform face area modulated as a function of working memory load. Brain Res Cogn Brain Res. 2001;10(3):355–364. doi: 10.1016/s0926-6410(00)00056-2. [DOI] [PubMed] [Google Scholar]
- Duncan J. An adaptive coding model of neural function in the prefrontal cortex. Nat Rev Neurosci. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- Duncan J, Humphreys G, Ward R. Competitive brain activity in visual attention. Curr Opin Neurobiol. 1997;7(2):255–261. doi: 10.1016/s0959-4388(97)80014-1. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23(10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392(6676):598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Everling S, Tinsley CJ, Gaffan D, Duncan J. Filtering of neural signals by focused attention in the monkey prefrontal cortex. Nat Neurosci. 2002;5(7):671–676. doi: 10.1038/nn874. [DOI] [PubMed] [Google Scholar]
- Evers S, Bockermann I, Nyhuis PW. The impact of transcranial magnetic stimulation on cognitive processing: an event-related potential study. Neuroreport. 2001;12(13):2915–2918. doi: 10.1097/00001756-200109170-00032. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Rissman J, D’Esposito M. Modulation of inferotemporal cortex activation during verbal working memory maintenance. Neuron. 2006;51(2):251–261. doi: 10.1016/j.neuron.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Phillips J, Chawla D, Buchel C. Nonlinear PCA: characterizing interactions between modes of brain activity. Phil Trans R Soc B. 2000;355(1393):135–146. doi: 10.1098/rstb.2000.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13(1):5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Frith CD. The role of dorsolateral prefrontal cortex in the selection of action as revealed by functional imaging. In: Monsell S, Driver J, editors. Control of cognitive processes. 18th ed. Cambridge (MA): MIT Press; 2000. pp. 549–566. [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61(2):331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Inferotemporal units in selective visual attention and short-term memory. J Neurophysiol. 1990;64(3):681–697. doi: 10.1152/jn.1990.64.3.681. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173(997):652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Bauer RH, Jervey JP. Functional interactions between inferotemporal and prefrontal cortex in a cognitive task. Brain Res. 1985;330(2):299–307. doi: 10.1016/0006-8993(85)90689-4. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, McEvoy K, Knight RT, D’Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci. 2005;17(3):507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8(10):1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, D’Esposito M. Unifying prefrontal cortex function: executive control, neural networks and top-down modulation. In: Cummings J, Miller B, editors. The human frontal lobes. 2nd ed. New York: The Guildford Press; 2007. [Google Scholar]
- Gazzaley A, Rissman J, Desposito M. Functional connectivity during working memory maintenance. Cogn Affect Behav Neurosci. 2004;4(4):580–599. doi: 10.3758/cabn.4.4.580. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and central executive. In: Roberts AC, Robbins TW, Weiskrantz L, editors. The prefrontal cortex: executive and cognitive functions. Oxford: Oxford Univeristy Press; 1998. pp. 87–102. [Google Scholar]
- Heimer L. The human brain and spinal cord: functional neuroanatomy and dissection guide. New York: Springer Verlag; 1983. [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182(4108):177–179. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3(3):284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Iba M, Sawaguchi T. Involvement of the dorsolateral prefrontal cortex of monkeys in visuospatial target selection. J Neurophysiol. 2003;89(1):587–599. doi: 10.1152/jn.00148.2002. [DOI] [PubMed] [Google Scholar]
- Jacobsen CF. Functions of frontal association areas in primates. Arch Neurol Psychiatry. 1935;33:558–560. [Google Scholar]
- Jha AP, McCarthy G. The influence of memory load upon delay-interval activity in a working-memory task: an event-related functional MRI study. J Cogn Neurosci. 2000;12(Suppl 2):90–105. doi: 10.1162/089892900564091. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Wojciulik E. Visual attention: insights from brain imaging. Nat Rev Neurosci. 2000;1(2):91–100. doi: 10.1038/35039043. [DOI] [PubMed] [Google Scholar]
- Kastner S, De Weerd P, Desimone R, Ungerleider LG. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science. 1998;282(5386):108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. The neural basis of biased competition in human visual cortex. Neuropsychologia. 2001;39(12):1263–1276. doi: 10.1016/s0028-3932(01)00116-6. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods DL. Prefrontal cortex gating of auditory transmission in humans. Brain Res. 1989;504(2):338–342. doi: 10.1016/0006-8993(89)91381-4. [DOI] [PubMed] [Google Scholar]
- Knight RT, Staines WR, Swick D, Chao LL. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychol (Amst) 1999;101(2–3):159–178. doi: 10.1016/s0001-6918(99)00004-9. [DOI] [PubMed] [Google Scholar]
- Kubota K, Niki H. Prefrontal cortical unit activity and delayed alternation performance in monkeys. J Neurophysiol. 1971;34(3):337–347. doi: 10.1152/jn.1971.34.3.337. [DOI] [PubMed] [Google Scholar]
- Kyd RJ, Bilkey DK. Prefrontal cortex lesions modify the spatial properties of hippocampal place cells. Cereb Cortex. 2003;13(5):444–451. doi: 10.1093/cercor/13.5.444. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam M. Neuroanatomic overlap of working memory and spatial attention networks: a functional MRI comparison within subjects. Neuroimage. 1999;10(6):695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Lin FH, McIntosh AR, Agnew JA, Eden GF, Zeffiro TA, Belliveau JW. Multivariate analysis of neuronal interactions in the generalized partial least squares framework: simulations and empirical studies. Neuroimage. 2003;20(2):625–642. doi: 10.1016/S1053-8119(03)00333-1. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77(1):24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Luria AR. Human brain and psychological processes. New York: Harper & Row; 1966. [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Understanding neural interactions in learning and memory using functional neuroimaging. Ann N Y Acad Sci. 1998;855:556–571. doi: 10.1111/j.1749-6632.1998.tb10625.x. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Mapping cognition to the brain through neural interactions. Memory. 1999;7(5–6):523–548. doi: 10.1080/096582199387733. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Grady CL, Haxby JV, Ungerleider LG, Horwitz B. Changes in limbic and prefrontal functional interactions in a working memory task for faces. Cereb Cortex. 1996;6(4):571–584. doi: 10.1093/cercor/6.4.571. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ishai A. Where bottom-up meets top-down: neuronal interactions during perception and imagery. Cereb Cortex. 2004;14(11):1256–1265. doi: 10.1093/cercor/bhh087. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Noppeney U, Friston KJ. A dynamic causal modeling study on category effects: bottom-up or top-down mediation? J Cogn Neurosci. 2003;15(7):925–934. doi: 10.1162/089892903770007317. [DOI] [PubMed] [Google Scholar]
- Mesulam M. The human frontal lobes: transcending the default mode through contingent encoding. In: Stuss D, Knight RT, editors. Principles of frontal lobe function. Oxford: Oxford University Press; 2002. [Google Scholar]
- Miller BT, D’Esposito M. Searching for “the top” in top-down control. Neuron. 2005;48(4):535–538. doi: 10.1016/j.neuron.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16(16):5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J Neurosci. 1993;13(4):1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. Effects of different brain regions on card sorting. Arch Neurol. 1963;9:90–100. [Google Scholar]
- Miyashita Y. Cognitive memory: cellular and network machineries and their top-down control. Science. 2004;306(5695):435–440. doi: 10.1126/science.1101864. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421(6921):370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. J Neurophysiol. 2004;91(1):152–162. doi: 10.1152/jn.00741.2002. [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229(4715):782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM, Krause BJ, Kemna LJ, Topper R, Tellmann L, Beu M, Pascual-Leone A, Muller-Gartner HW. Modulation of the neuronal circuitry subserving working memory in healthy human subjects by repetitive transcranial magnetic stimulation. Neurosci Lett. 2000;280(3):167–170. doi: 10.1016/s0304-3940(00)00798-9. [DOI] [PubMed] [Google Scholar]
- Paus T, Castro-Alamancos MA, Petrides M. Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur J Neurosci. 2001;14(8):1405–1411. doi: 10.1046/j.0953-816x.2001.01757.x. [DOI] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ. Comparing dynamic causal models. Neuroimage. 2004;22(3):1157–1172. doi: 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: from modulation of sensory processing to top-down control. J Neurosci. 2003;23(10):3990–3998. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Frontal lobes and working memory: evidence from investigations of the effects of cortical excisions in nonhuman primates. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Amsterdam: Elsevier Science B.V.; 1994. pp. 59–84. [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11(3):1011–1136. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16(2):291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Ploner CJ, Ostendorf F, Brandt SA, Gaymard BM, Rivaud-Pechoux S, Ploner M, Villringer A, Pierrot-Deseilligny C. Behavioural relevance modulates access to spatial working memory in humans. Eur J Neurosci. 2001;13(2):357–363. [PubMed] [Google Scholar]
- Postle BR, Druzgal TJ, D’Esposito M. Seeking the neural substrates of visual working memory storage. Cortex. 2003;39(4–5):927–946. doi: 10.1016/s0010-9452(08)70871-2. [DOI] [PubMed] [Google Scholar]
- Rainer G, Asaad WF, Miller EK. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature. 1998;393(6685):577–579. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Heller A, Cohen MX, Brozinsky CJ, Rissman J. Functional connectivity with the hippocampus during successful memory formation. Hippocampus. 2005;15(8):997–1005. doi: 10.1002/hipo.20141. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, D’Esposito M. Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia. 2003;41(3):378–389. doi: 10.1016/s0028-3932(02)00169-0. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H. The laminar pattern of connections between prefrontal and anterior temporal cortices in the rhesus monkey is related to cortical structure and function. Cereb Cortex. 2000;10(9):851–865. doi: 10.1093/cercor/10.9.851. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23(2):752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Evidence for a capacity-limited working memory control mechanism in the posterior frontolateral cortex. Soc Neurosci Abstr. 2006 Online. [Google Scholar]
- Rowe J, Friston K, Frackowiak R, Passingham R. Attention to action: specific modulation of corticocortical interactions in humans. Neuroimage. 2002;17(2):988. [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16(15):1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mikulis DJ, Davis KD. Cognitive modulation of pain-related brain responses depends on behavioral strategy. Pain. 2004;112(1–2):48–58. doi: 10.1016/j.pain.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Shimamura AP. The role of the prefrontal cortex in dynamic filtering. Psychobiology. 1997;28(2):207–218. [Google Scholar]
- Skinner J, Yingling C. Central gating mechanisms that regulate event-related potentials and behavior. In: Desmedt J, editor. Progress in clinical neurophysiology. Basel (Switzerland): S Karger; 1977. pp. 30–69. [Google Scholar]
- Sun FT, Miller LM, D’Esposito M. Measuring interregional functional connectivity using coherence and partial coherence analyses of fMRI data. Neuroimage. 2004;21(2):647–658. doi: 10.1016/j.neuroimage.2003.09.056. [DOI] [PubMed] [Google Scholar]
- Tomita H, Ohbayashi M, Nakahara K, Hasegawa I, Miyashita Y. Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature. 1999;401(6754):699–703. doi: 10.1038/44372. [DOI] [PubMed] [Google Scholar]
- Treue S, Martinez Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399(6736):575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Courtney SM, Haxby JV. A neural system for human visual working memory. Proc Natl Acad Sci USA. 1998;95(3):883–890. doi: 10.1073/pnas.95.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Gaffan D, Pelak VS. Projections from inferior temporal cortex to prefrontal cortex via the uncinate fascicle in rhesus monkeys. Exp Brain Res. 1989;76(3):473–484. doi: 10.1007/BF00248903. [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Bachevalier J, Ungerleider LG. Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cereb Cortex. 1994;4(5):470–483. doi: 10.1093/cercor/4.5.470. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260(5116):1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT. Gating of somatosensory input by human prefrontal cortex. Brain Res. 1990;521(1–2):281–288. doi: 10.1016/0006-8993(90)91553-s. [DOI] [PubMed] [Google Scholar]
- Yingling C, Skinner J. Gating of thalamic input to cerebral cortex by nucleus reticularis thalami. In: Desmedt J, editor. Progress in clinical neurophysiology. Basel (Switzerland): S Karger; 1977. pp. 70–96. [Google Scholar]
- Yoon JH, Curtis CE, D’Esposito M. Differential effects of distraction during working memory on delay-period activity in the prefrontal cortex and the visual association cortex. Neuroimage. 2006;29(4):1117–1126. doi: 10.1016/j.neuroimage.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D’Esposito M. A trial-based experimental design for fMRI. Neuroimage. 1997;6(2):122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]
- Zelano C, Bensafi M, Porter J, Mainland J, Johnson B, Bremner E, Telles C, Khan R, Sobel N. Attentional modulation in human primary olfactory cortex. Nat Neurosci. 2005;8(1):114–120. doi: 10.1038/nn1368. [DOI] [PubMed] [Google Scholar]
- Zironi I, Iacovelli P, Aicardi G, Liu P, Bilkey DK. Prefrontal cortex lesions augment the location-related firing properties of area TE/perirhinal cortex neurons in a working memory task. Cereb Cortex. 2001;11(11):1093–1100. doi: 10.1093/cercor/11.11.1093. [DOI] [PubMed] [Google Scholar]