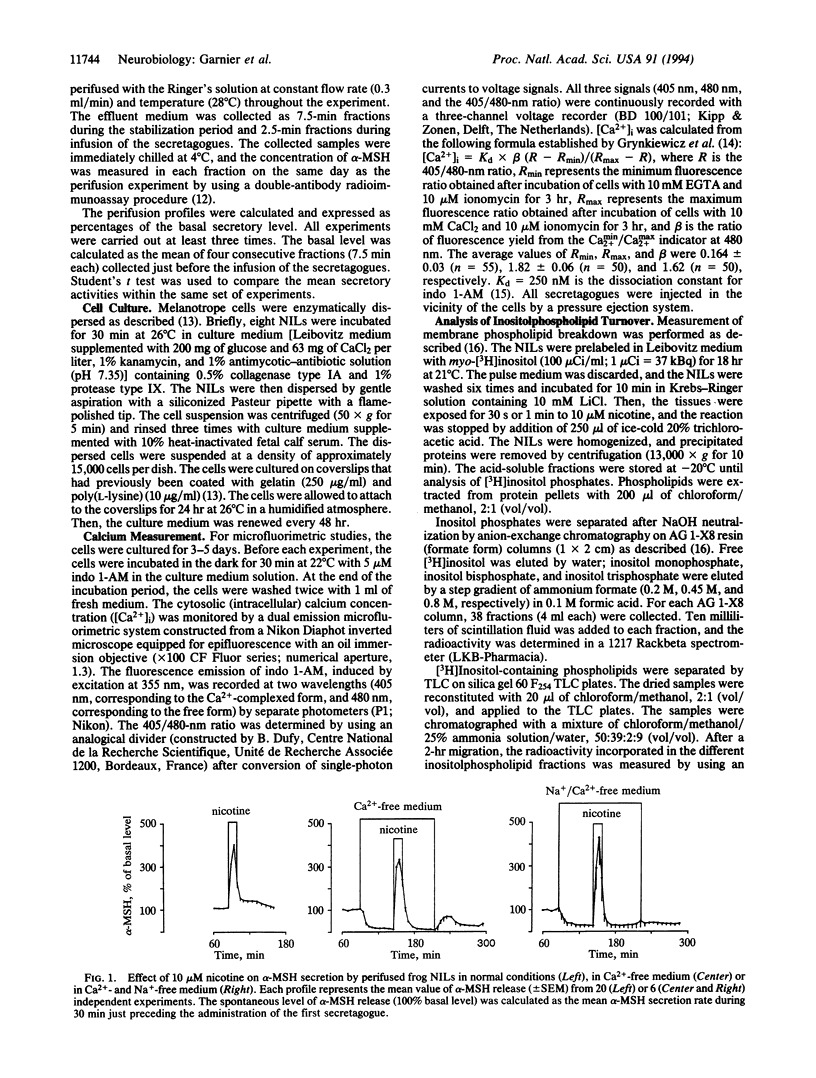
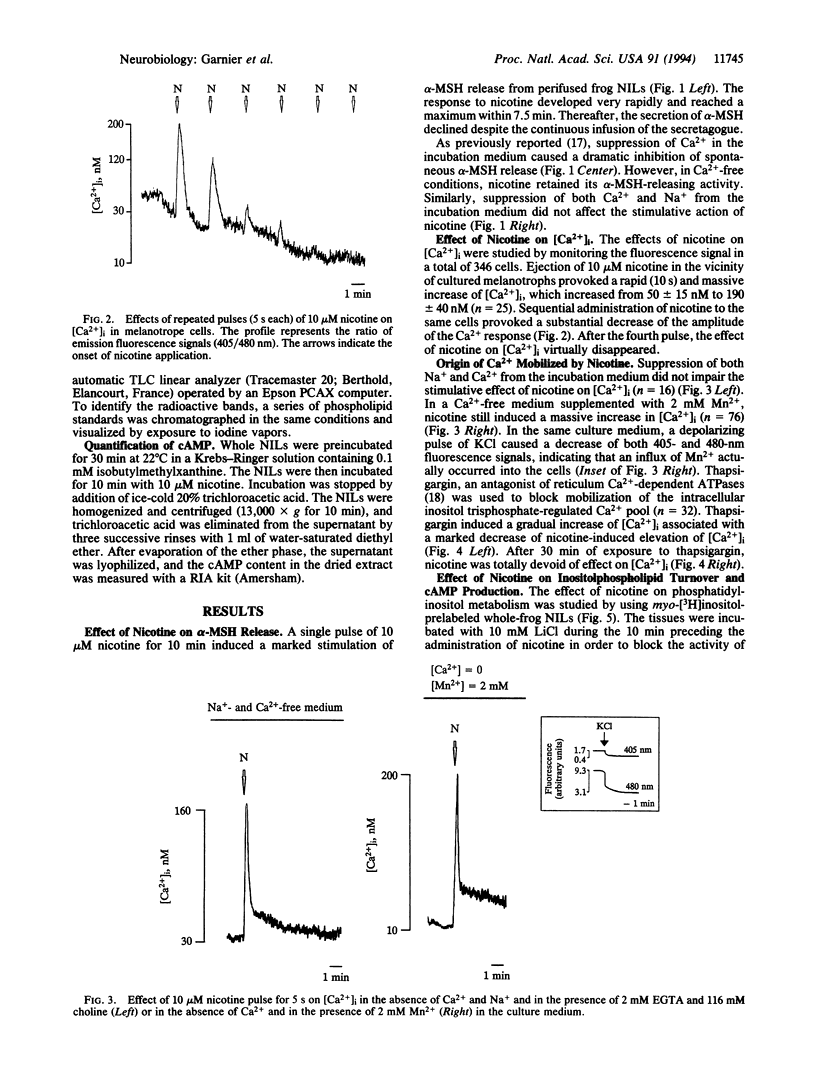
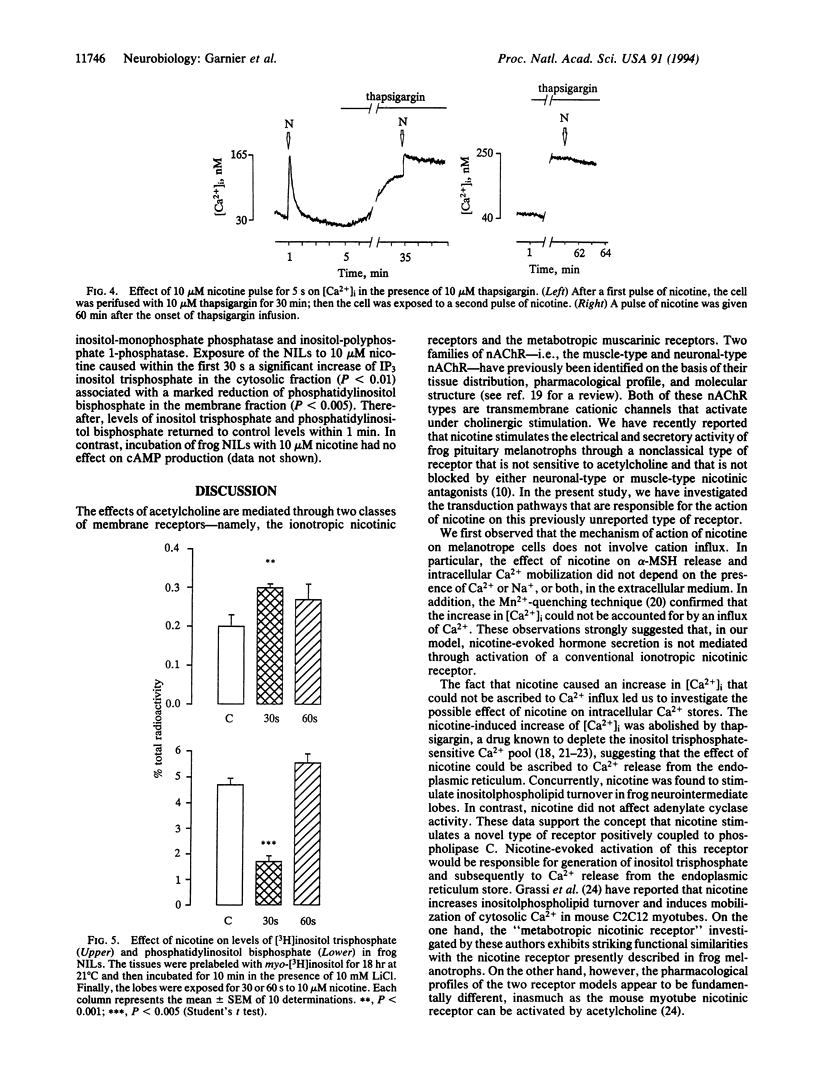
Abstract
Classical nicotinic receptors are neurotransmitter-gated channels that, upon activation by acetylcholine, induce the opening of an intrinsic cationic channel. We have recently observed that, in frog pituitary melanotrophs, nicotine stimulates alpha-melanocyte-stimulating hormone (alpha-MSH) release through a noncholinergic mechanism. In the study reported here, we investigated the intracellular events that mediate the response of frog melanotrophs to nicotine. Nicotine was capable of stimulating alpha-MSH release in the absence of Ca2+ and/or Na+ in the extracellular medium. A short pulse of nicotine induced a rapid and transient increase of cytosolic free Ca2+ concentration ([Ca2+]i). The effect of nicotine on Ca2+ mobilization was not affected in the absence of Na+ and Ca2+ in the extracellular medium, indicating that the nicotine-evoked increase in [Ca2+]i did not result from Na+ or Ca2+ influx. Nicotine induced both an increase in inositol trisphosphate and a reduction in phosphaditylinositol bisphosphate concentrations but did not affect cAMP production. The present results indicate that nicotine-induced stimulation of alpha-MSH release in frog melanotrophs can be explained by activation of inositolphospholipid breakdown and mobilization of inositol triphosphate-dependent intracellular Ca2+ pools. These data provide evidence for the existence of an unusual type of noncholinergic nicotine receptor positively coupled to phospholipase C.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abood L. G., Reynolds D. T., Bidlack J. M. Stereospecific 3H-nicotine binding to intact and solubilized rat brain membranes and evidence for its noncholinergic nature. Life Sci. 1980 Oct 6;27(14):1307–1314. doi: 10.1016/0024-3205(80)90224-6. [DOI] [PubMed] [Google Scholar]
- Anand R., Conroy W. G., Schoepfer R., Whiting P., Lindstrom J. Neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes have a pentameric quaternary structure. J Biol Chem. 1991 Jun 15;266(17):11192–11198. [PubMed] [Google Scholar]
- Andersen A. C., Tonon M. C., Pelletier G., Conlon J. M., Fasolo A., Vaudry H. Neuropeptides in the amphibian brain. Int Rev Cytol. 1992;138:89-210, 315-26. doi: 10.1016/s0074-7696(08)61588-0. [DOI] [PubMed] [Google Scholar]
- Chartrel N., Conlon J. M., Danger J. M., Fournier A., Tonon M. C., Vaudry H. Characterization of melanotropin-release-inhibiting factor (melanostatin) from frog brain: homology with human neuropeptide Y. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3862–3866. doi: 10.1073/pnas.88.9.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrues L., Tonon M. C., Vaudry H. Thyrotrophin-releasing hormone stimulates polyphosphoinositide metabolism in the frog neurointermediate lobe. J Mol Endocrinol. 1990 Oct;5(2):129–136. doi: 10.1677/jme.0.0050129. [DOI] [PubMed] [Google Scholar]
- Grassi F., Giovannelli A., Fucile S., Eusebi F. Activation of the nicotinic acetylcholine receptor mobilizes calcium from caffeine-insensitive stores in C2C12 mouse myotubes. Pflugers Arch. 1993 Mar;422(6):591–598. doi: 10.1007/BF00374007. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hajnóczky G., Várnai P., Holló Z., Christensen S. B., Balla T., Enyedi P., Spät A. Thapsigargin-induced increase in cytoplasmic Ca2+ concentration and aldosterone production in rat adrenal glomerulosa cells: interaction with potassium and angiotensin-II. Endocrinology. 1991 May;128(5):2639–2644. doi: 10.1210/endo-128-5-2639. [DOI] [PubMed] [Google Scholar]
- Hallam T. J., Rink T. J. Agonists stimulate divalent cation channels in the plasma membrane of human platelets. FEBS Lett. 1985 Jul 8;186(2):175–179. doi: 10.1016/0014-5793(85)80703-1. [DOI] [PubMed] [Google Scholar]
- Hill J. A., Jr, Zoli M., Bourgeois J. P., Changeux J. P. Immunocytochemical localization of a neuronal nicotinic receptor: the beta 2-subunit. J Neurosci. 1993 Apr;13(4):1551–1568. doi: 10.1523/JNEUROSCI.13-04-01551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamacz M., Hindelang C., Tonon M. C., Vaudry H., Stoeckel M. E. Three distinct thyrotropin-releasing hormone-immunoreactive axonal systems project in the median eminence-pituitary complex of the frog Rana ridibunda. Immunocytochemical evidence for co-localization of thyrotropin-releasing hormone and mesotocin in fibers innervating pars intermedia cells. Neuroscience. 1989;32(2):451–462. doi: 10.1016/0306-4522(89)90093-6. [DOI] [PubMed] [Google Scholar]
- Lamacz M., Netchitailo P., Tonon M. C., Feuilloley M., Ling N., Pelletier G., Vaudry H. Atrial natriuretic factor (ANF) stimulates the release of alpha-MSH from frog neurointermediate lobes in vitro. Interaction with dopamine, GABA and neuropeptide Y. Life Sci. 1987 May 11;40(19):1853–1857. doi: 10.1016/0024-3205(87)90042-7. [DOI] [PubMed] [Google Scholar]
- Lamacz M., Tonon M. C., Louiset E., Cazin L., Strosberg D., Vaudry H. Acetylcholine stimulates alpha-melanocyte-stimulating hormone release from frog pituitary melanotrophs through activation of muscarinic and nicotinic receptors. Endocrinology. 1989 Aug;125(2):707–714. doi: 10.1210/endo-125-2-707. [DOI] [PubMed] [Google Scholar]
- Lamacz M., Tonon M. C., Louiset E., Desrues L., Cazin L., Guy J., Pelletier G., Vaudry H. Role of calcium in thyrotrophin-releasing hormone-stimulated release of melanocyte-stimulating hormone from frog neurointermediate lobe. J Mol Endocrinol. 1988 Sep;1(2):131–139. doi: 10.1677/jme.0.0010131. [DOI] [PubMed] [Google Scholar]
- Law G. J., Pachter J. A., Thastrup O., Hanley M. R., Dannies P. S. Thapsigargin, but not caffeine, blocks the ability of thyrotropin-releasing hormone to release Ca2+ from an intracellular store in GH4C1 pituitary cells. Biochem J. 1990 Apr 15;267(2):359–364. doi: 10.1042/bj2670359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J., Schoepfer R., Whiting P. Molecular studies of the neuronal nicotinic acetylcholine receptor family. Mol Neurobiol. 1987 Winter;1(4):281–337. doi: 10.1007/BF02935740. [DOI] [PubMed] [Google Scholar]
- Louiset E., Cazin L., Duval O., Lamacz M., Tonon M. C., Vaudry H. Effect of acetylcholine on the electrical and secretory activities of frog pituitary melanotrophs. Brain Res. 1990 Nov 19;533(2):300–308. doi: 10.1016/0006-8993(90)91353-i. [DOI] [PubMed] [Google Scholar]
- Louiset E., Cazin L., Lamacz M., Tonon M. C., Vaudry H. Patch-clamp study of the ionic currents underlying action potentials in cultured frog pituitary melanotrophs. Neuroendocrinology. 1988 Nov;48(5):507–515. doi: 10.1159/000125057. [DOI] [PubMed] [Google Scholar]
- Léna C., Changeux J. P. Allosteric modulations of the nicotinic acetylcholine receptor. Trends Neurosci. 1993 May;16(5):181–186. doi: 10.1016/0166-2236(93)90150-k. [DOI] [PubMed] [Google Scholar]
- Mollard P., Guerineau N., Audin J., Dufy B. Measurement of CA2+ transients using simultaneous dual-emission microspectrofluorimetry and electrophysiology in individual pituitary cells. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1045–1052. doi: 10.1016/0006-291x(89)91775-0. [DOI] [PubMed] [Google Scholar]
- Stroud R. M., McCarthy M. P., Shuster M. Nicotinic acetylcholine receptor superfamily of ligand-gated ion channels. Biochemistry. 1990 Dec 18;29(50):11009–11023. doi: 10.1021/bi00502a001. [DOI] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonon M. C., Adjeroud S., Lamacz M., Louiset E., Danger J. M., Desrues L., Cazin L., Nicolas P., Vaudry H. Central-type benzodiazepines and the octadecaneuropeptide modulate the effects of GABA on the release of alpha-melanocyte-stimulating hormone from frog neurointermediate lobe in vitro. Neuroscience. 1989;31(2):485–493. doi: 10.1016/0306-4522(89)90391-6. [DOI] [PubMed] [Google Scholar]
- Tonon M. C., Leroux P., Stoeckel M. E., Jegou S., Pelletier G., Vaudry H. Catecholaminergic control of alpha-melanocyte-stimulating hormone (alpha MSH) release by frog neurointermediate lobe in vitro: evidence for direct stimulation of alpha MSH release by thyrotropin-releasing hormone. Endocrinology. 1983 Jan;112(1):133–141. doi: 10.1210/endo-112-1-133. [DOI] [PubMed] [Google Scholar]
- Vaudry H., Tonon M. C., Delarue C., Vaillant R., Kraicer J. Biological and radioimmunological evidence for melanocyte stimulating hormones (MSH) of extrapituitary origin in the rat brain. Neuroendocrinology. 1978;27(1-2):9–24. doi: 10.1159/000122796. [DOI] [PubMed] [Google Scholar]
- Whiting P., Lindstrom J. Purification and characterization of a nicotinic acetylcholine receptor from rat brain. Proc Natl Acad Sci U S A. 1987 Jan;84(2):595–599. doi: 10.1073/pnas.84.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. W., Feltz P. Nicotinic acetylcholine receptors in porcine hypophyseal intermediate lobe cells. J Physiol. 1990 Mar;422:83–101. doi: 10.1113/jphysiol.1990.sp017974. [DOI] [PMC free article] [PubMed] [Google Scholar]


