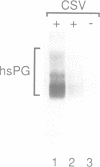Abstract
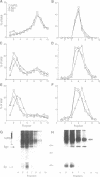
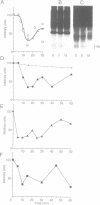
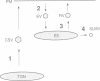
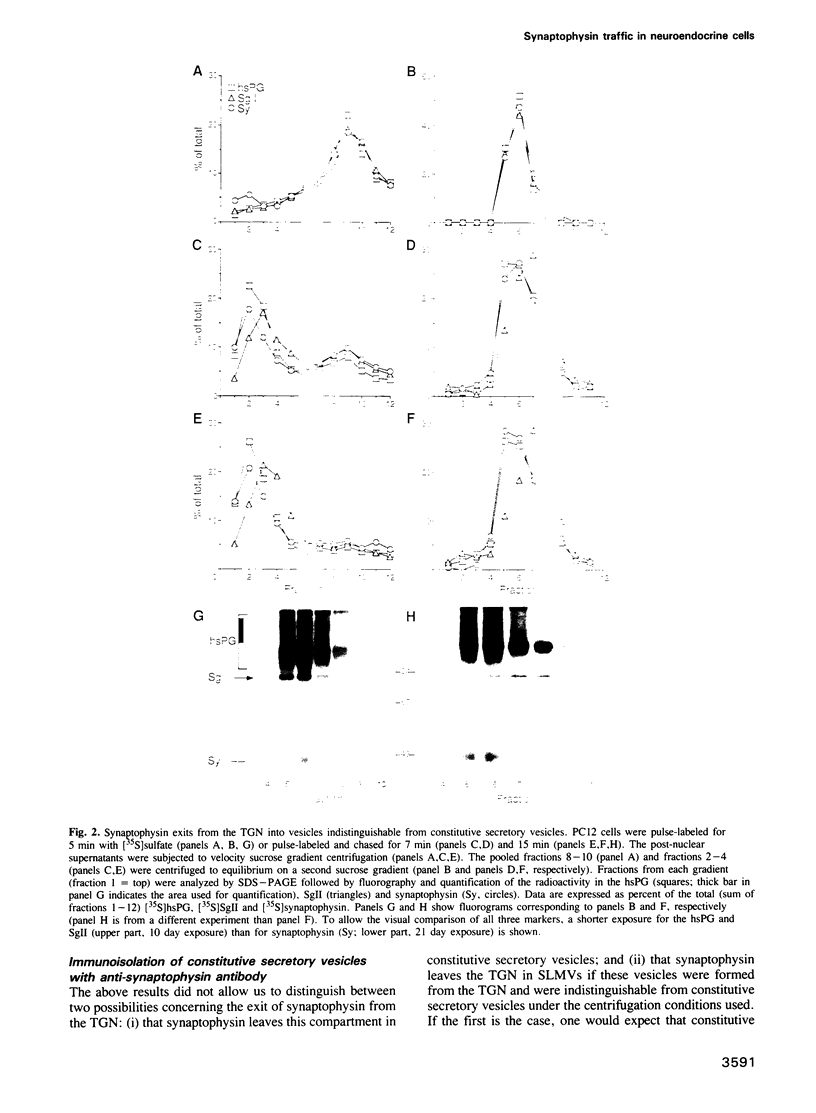
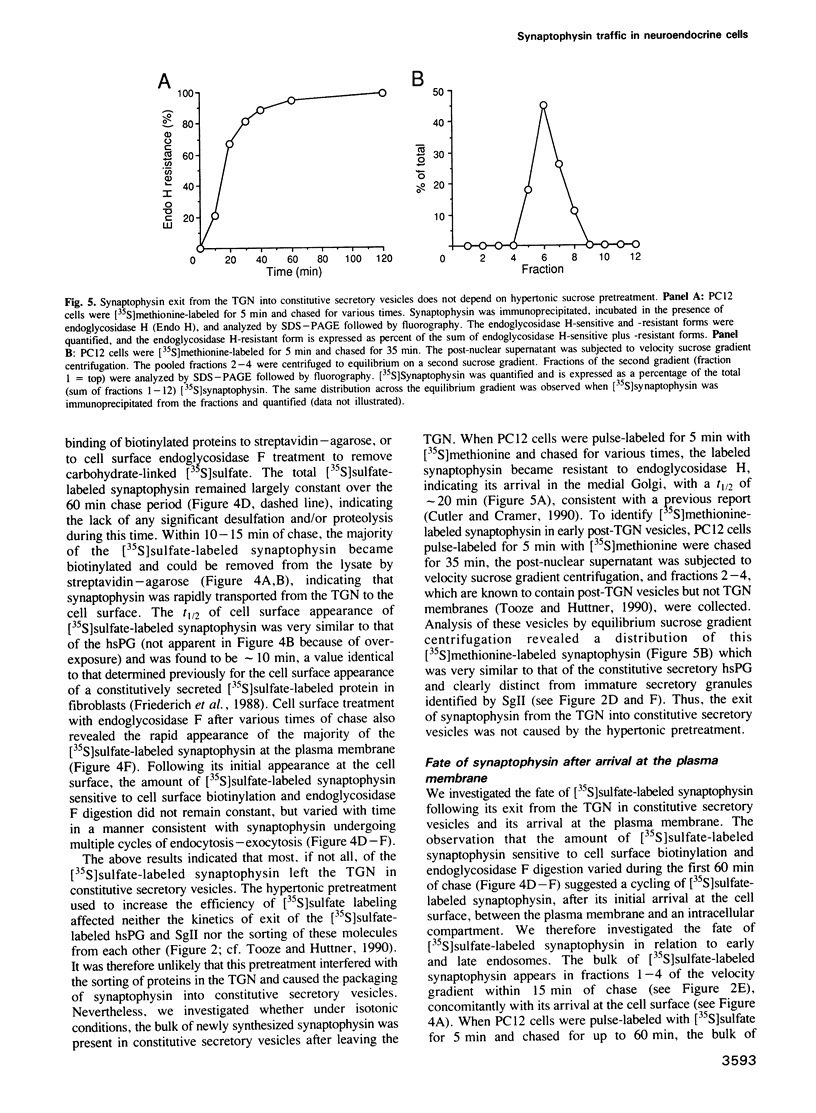
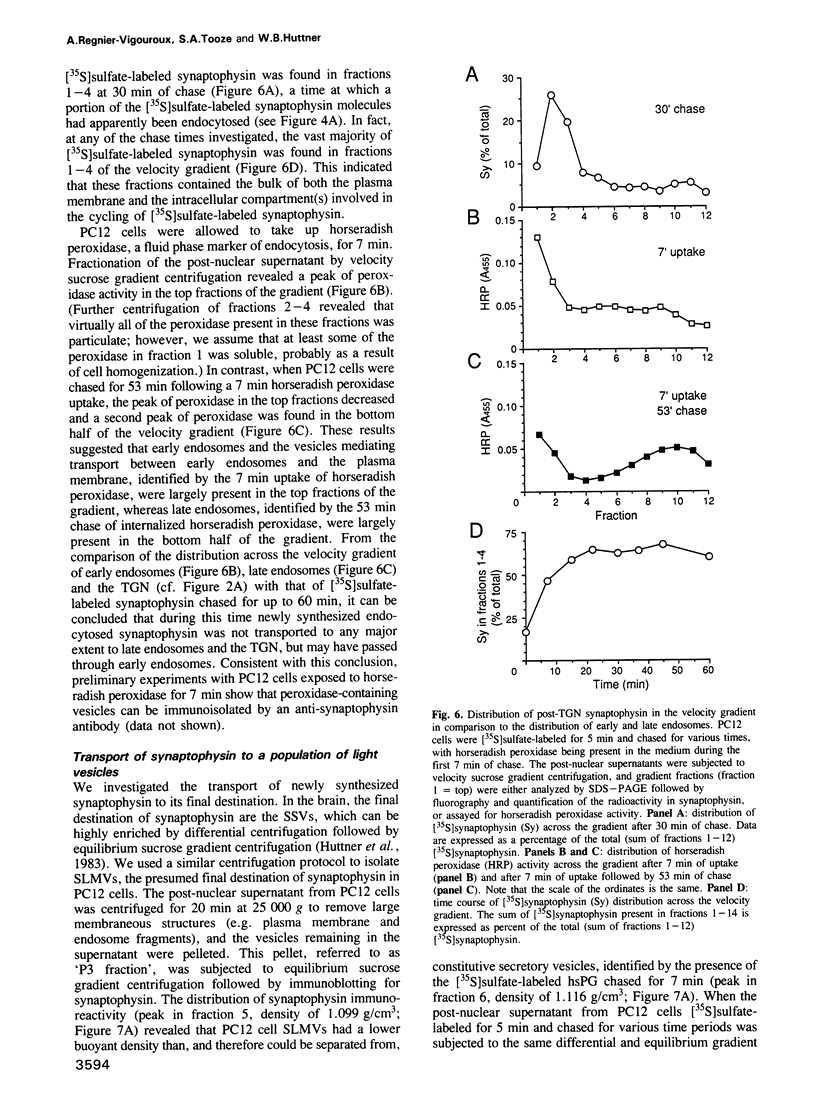
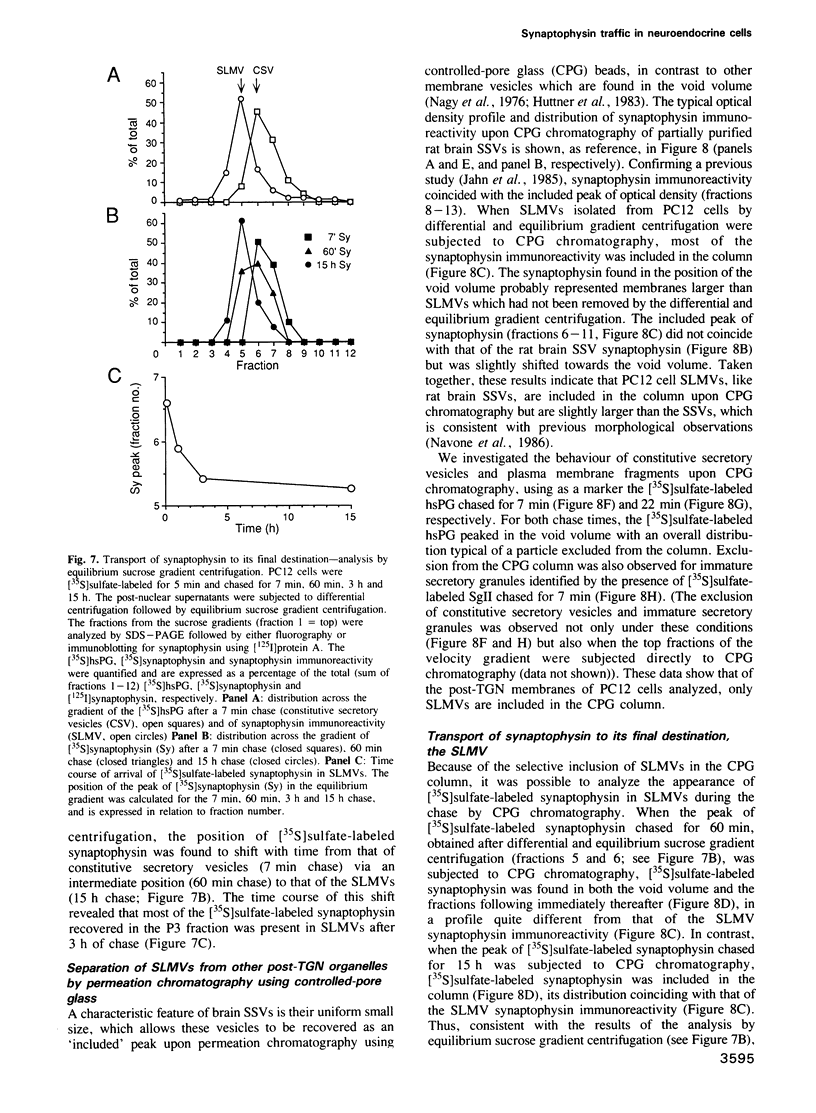
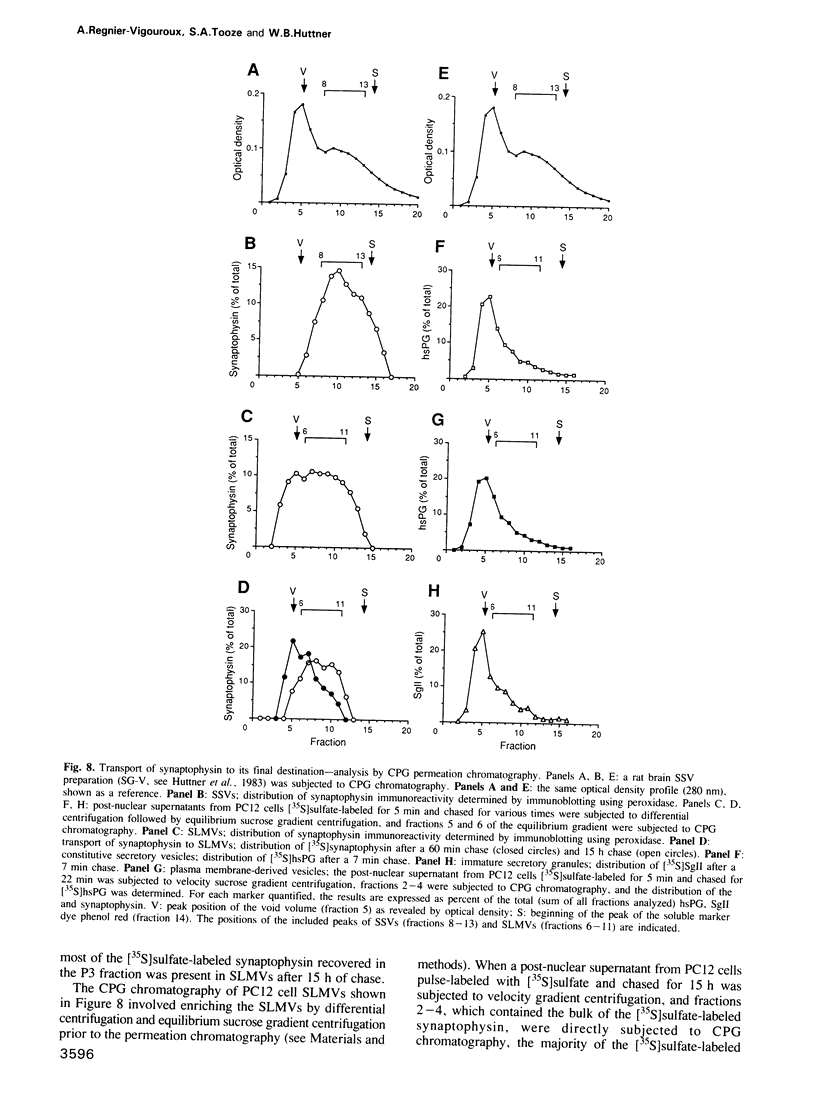
The biogenesis of synaptic-like microvesicles (SLMVs) in neuroendocrine cells was investigated by studying the traffic of newly synthesized synaptophysin to SLMVs in PC12 cells. Synaptophysin was found to be sulfated, which facilitated the determination of its exit route from the trans-Golgi network (TGN). Virtually all [35S]sulfate-labeled synaptophysin was found to leave the TGN in vesicles which were indistinguishable from constitutive secretory vesicles but distinct from immature secretory granules and SLMVs. [35S]sulfate-labeled synaptophysin was rapidly transported from the TGN to the cell surface, with a t1/2 of approximately 10 min in resting cells. After arrival at the cell surface, [35S]sulfate-labeled synaptophysin cycled for at least 1 h between the plasma membrane and an intracellular compartment likely to be the early endosome. Up to approximately 40% of the [35S]sulfate-labeled synaptophysin eventually (after 3 h and later) reached SLMVs, which could be distinguished from the other post-TGN compartments by their lower buoyant density in a sucrose gradient and their selective inclusion upon permeation chromatography using a controlled-pore glass column. Our results suggest that newly synthesized membrane proteins of SLMVs in neuroendocrine cells, and possibly of small synaptic vesicles in neurons, reach these organelles via the TGN----plasma membrane----early endosome.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Huttner W. B. Tyrosine sulfation is a trans-Golgi-specific protein modification. J Cell Biol. 1987 Dec;105(6 Pt 1):2655–2664. doi: 10.1083/jcb.105.6.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley K. M., Floor E., Kelly R. B. Cloning and sequence analysis of cDNA encoding p38, a major synaptic vesicle protein. J Cell Biol. 1987 Dec;105(6 Pt 1):2447–2456. doi: 10.1083/jcb.105.6.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge M. B. Initial endocytosis of perioxidase or ferritin by growth cones of cultured nerve cells. J Neurocytol. 1977 Aug;6(4):407–439. doi: 10.1007/BF01178226. [DOI] [PubMed] [Google Scholar]
- Burgess T. L., Kelly R. B. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Clift-O'Grady L., Linstedt A. D., Lowe A. W., Grote E., Kelly R. B. Biogenesis of synaptic vesicle-like structures in a pheochromocytoma cell line PC-12. J Cell Biol. 1990 May;110(5):1693–1703. doi: 10.1083/jcb.110.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collawn J. F., Stangel M., Kuhn L. A., Esekogwu V., Jing S. Q., Trowbridge I. S., Tainer J. A. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990 Nov 30;63(5):1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- Cutler D. F., Cramer L. P. Sorting during transport to the surface of PC12 cells: divergence of synaptic vesicle and secretory granule proteins. J Cell Biol. 1990 Mar;110(3):721–730. doi: 10.1083/jcb.110.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daukas G., Zigmond S. H. Inhibition of receptor-mediated but not fluid-phase endocytosis in polymorphonuclear leukocytes. J Cell Biol. 1985 Nov;101(5 Pt 1):1673–1679. doi: 10.1083/jcb.101.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. G., Lehrman M. A., Russell D. W., Anderson R. G., Brown M. S., Goldstein J. L. The J.D. mutation in familial hypercholesterolemia: amino acid substitution in cytoplasmic domain impedes internalization of LDL receptors. Cell. 1986 Apr 11;45(1):15–24. doi: 10.1016/0092-8674(86)90533-7. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Jahn R. Pathways to regulated exocytosis in neurons. Annu Rev Physiol. 1990;52:625–645. doi: 10.1146/annurev.ph.52.030190.003205. [DOI] [PubMed] [Google Scholar]
- Dotti C. G., Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990 Jul 13;62(1):63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- Friederich E., Fritz H. J., Huttner W. B. Inhibition of tyrosine sulfation in the trans-Golgi retards the transport of a constitutively secreted protein to the cell surface. J Cell Biol. 1988 Nov;107(5):1655–1667. doi: 10.1083/jcb.107.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel J. P., Chavrier P., Zerial M., Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991 Mar 8;64(5):915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Howell K. E. Immuno-isolation of vesicles using antigenic sites either located on the cytoplasmic or the exoplasmic domain of an implanted viral protein. A quantitative analysis. Eur J Cell Biol. 1985 Sep;38(2):312–321. [PubMed] [Google Scholar]
- Heuser J. E., Anderson R. G. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989 Feb;108(2):389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B. Determination and occurrence of tyrosine O-sulfate in proteins. Methods Enzymol. 1984;107:200–223. doi: 10.1016/0076-6879(84)07013-0. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Schiebler W., Greengard P., De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983 May;96(5):1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Ouimet C., Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetzko A., Zimmermann H., Volknandt W. Intraneuronal distribution of a synaptic vesicle membrane protein: antibody binding sites at axonal membrane compartments and trans-Golgi network and accumulation at nodes of Ranvier. Neuroscience. 1989;32(1):65–77. doi: 10.1016/0306-4522(89)90108-5. [DOI] [PubMed] [Google Scholar]
- Johnston P. A., Cameron P. L., Stukenbrok H., Jahn R., De Camilli P., Südhof T. C. Synaptophysin is targeted to similar microvesicles in CHO and PC12 cells. EMBO J. 1989 Oct;8(10):2863–2872. doi: 10.1002/j.1460-2075.1989.tb08434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B. The cell biology of the nerve terminal. Neuron. 1988 Aug;1(6):431–438. doi: 10.1016/0896-6273(88)90174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J. H., Lohmander L. S., Hascall V. C. Studies on the biosynthesis of cartilage proteoglycan in a model system of cultured chondrocytes from the Swarm rat chondrosarcoma. J Cell Biochem. 1984;26(4):261–278. doi: 10.1002/jcb.240260406. [DOI] [PubMed] [Google Scholar]
- Lee R. W., Huttner W. B. Tyrosine-O-sulfated proteins of PC12 pheochromocytoma cells and their sulfation by a tyrosylprotein sulfotransferase. J Biol Chem. 1983 Sep 25;258(18):11326–11334. [PubMed] [Google Scholar]
- Leube R. E., Kaiser P., Seiter A., Zimbelmann R., Franke W. W., Rehm H., Knaus P., Prior P., Betz H., Reinke H. Synaptophysin: molecular organization and mRNA expression as determined from cloned cDNA. EMBO J. 1987 Nov;6(11):3261–3268. doi: 10.1002/j.1460-2075.1987.tb02644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leube R. E., Wiedenmann B., Franke W. W. Topogenesis and sorting of synaptophysin: synthesis of a synaptic vesicle protein from a gene transfected into nonneuroendocrine cells. Cell. 1989 Nov 3;59(3):433–446. doi: 10.1016/0092-8674(89)90028-7. [DOI] [PubMed] [Google Scholar]
- Lowe A. W., Madeddu L., Kelly R. B. Endocrine secretory granules and neuronal synaptic vesicles have three integral membrane proteins in common. J Cell Biol. 1988 Jan;106(1):51–59. doi: 10.1083/jcb.106.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. G., Moore H. P. Reconstitution of constitutive secretion using semi-intact cells: regulation by GTP but not calcium. J Cell Biol. 1991 Jan;112(1):39–54. doi: 10.1083/jcb.112.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A., Baker R. R., Morris S. J., Whittaker V. P. The preparation and characterization of synaptic vesicles of high purity. Brain Res. 1976 Jun 11;109(2):285–309. doi: 10.1016/0006-8993(76)90531-x. [DOI] [PubMed] [Google Scholar]
- Navone F., Jahn R., Di Gioia G., Stukenbrok H., Greengard P., De Camilli P. Protein p38: an integral membrane protein specific for small vesicles of neurons and neuroendocrine cells. J Cell Biol. 1986 Dec;103(6 Pt 1):2511–2527. doi: 10.1083/jcb.103.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Louvard D., Perrelet A. Clathrin-immunoreactive sites in the Golgi apparatus are concentrated at the trans pole in polypeptide hormone-secreting cells. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5385–5389. doi: 10.1073/pnas.82.16.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P., Weiss U., Pepperkok R., Ansorge W., Niehrs C., Stelzer E. H., Huttner W. B. An antibody against secretogranin I (chromogranin B) is packaged into secretory granules. J Cell Biol. 1989 Jul;109(1):17–34. doi: 10.1083/jcb.109.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T. C., Jahn R. Proteins of synaptic vesicles involved in exocytosis and membrane recycling. Neuron. 1991 May;6(5):665–677. doi: 10.1016/0896-6273(91)90165-v. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Lottspeich F., Greengard P., Mehl E., Jahn R. A synaptic vesicle protein with a novel cytoplasmic domain and four transmembrane regions. Science. 1987 Nov 20;238(4830):1142–1144. doi: 10.1126/science.3120313. [DOI] [PubMed] [Google Scholar]
- Tixier-Vidal A., Faivre-Bauman A., Picart R., Wiedenmann B. Immunoelectron microscopic localization of synaptophysin in a Golgi subcompartment of developing hypothalamic neurons. Neuroscience. 1988 Sep;26(3):847–861. doi: 10.1016/0306-4522(88)90104-2. [DOI] [PubMed] [Google Scholar]
- Tooze J., Tooze S. A. Clathrin-coated vesicular transport of secretory proteins during the formation of ACTH-containing secretory granules in AtT20 cells. J Cell Biol. 1986 Sep;103(3):839–850. doi: 10.1083/jcb.103.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze S. A., Huttner W. B. Cell-free protein sorting to the regulated and constitutive secretory pathways. Cell. 1990 Mar 9;60(5):837–847. doi: 10.1016/0092-8674(90)90097-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble W. S., Linial M., Scheller R. H. Cellular and molecular biology of the presynaptic nerve terminal. Annu Rev Neurosci. 1991;14:93–122. doi: 10.1146/annurev.ne.14.030191.000521. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Ishikawa H. The movement of membranous organelles in axons. Electron microscopic identification of anterogradely and retrogradely transported organelles. J Cell Biol. 1980 Mar;84(3):513–530. doi: 10.1083/jcb.84.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985 Jul;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Huttner W. B. Synaptophysin and chromogranins/secretogranins--widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;58(2):95–121. doi: 10.1007/BF02890062. [DOI] [PubMed] [Google Scholar]