Abstract
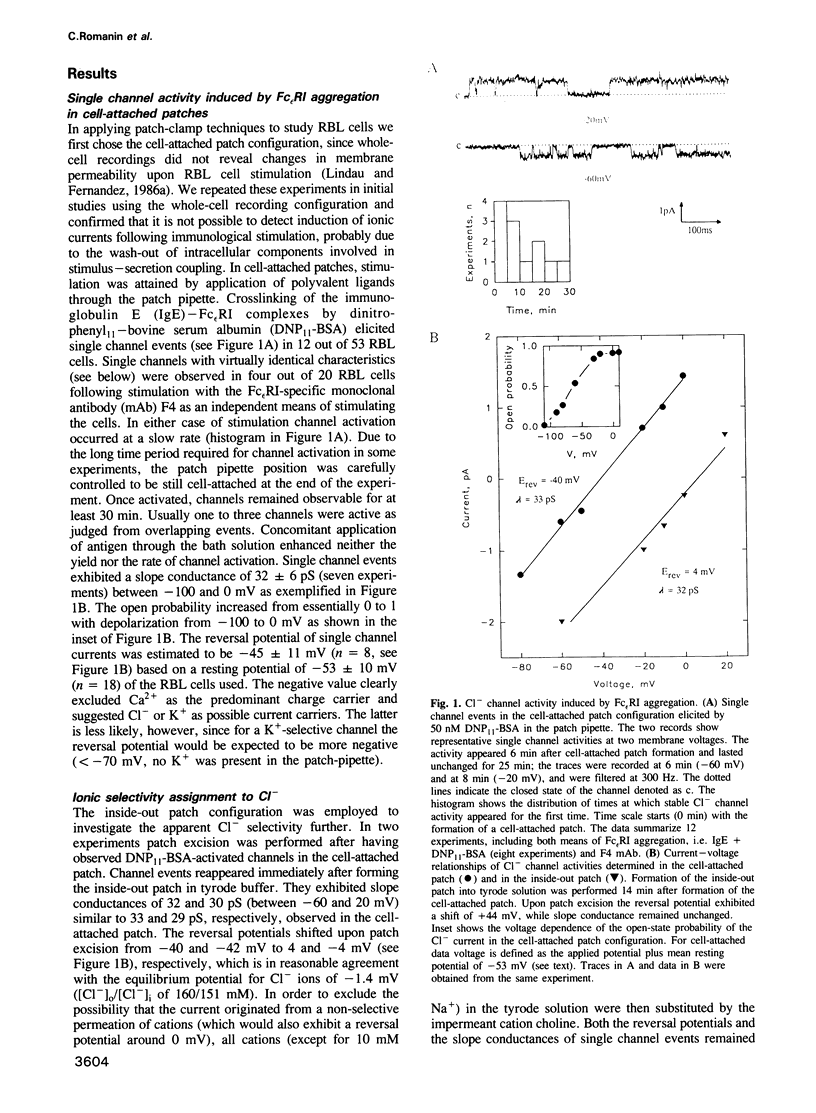
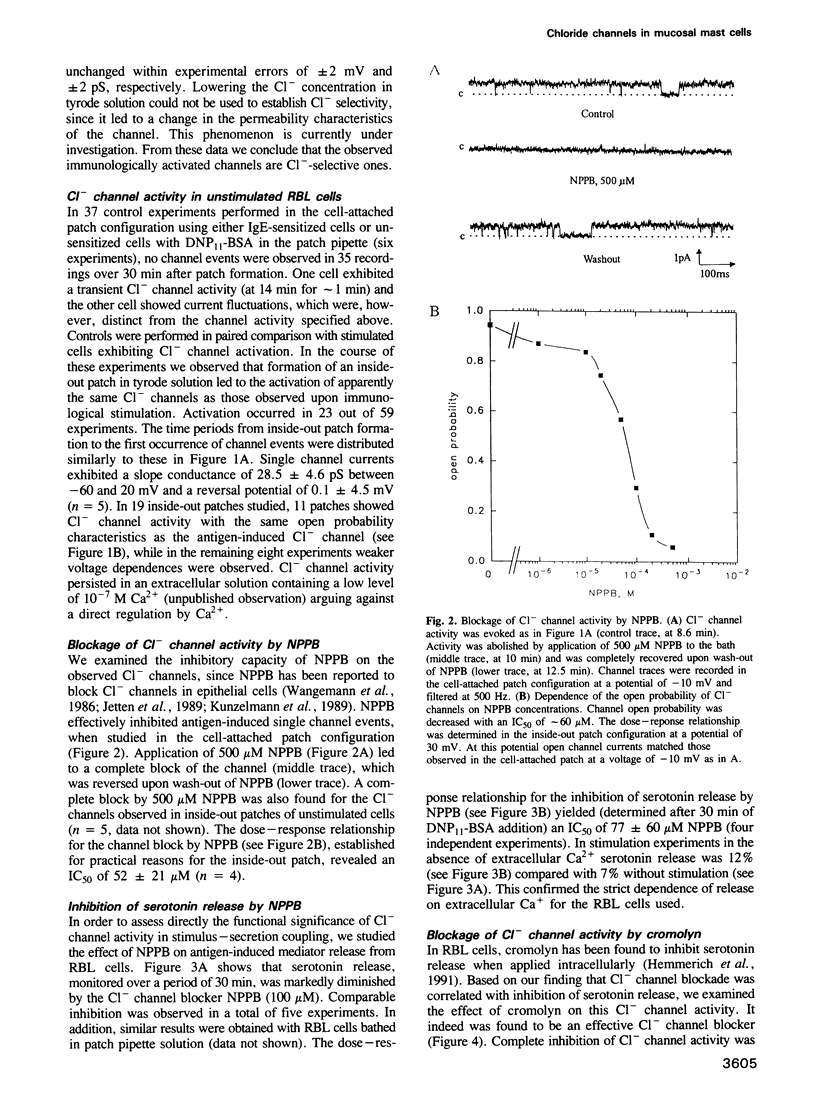
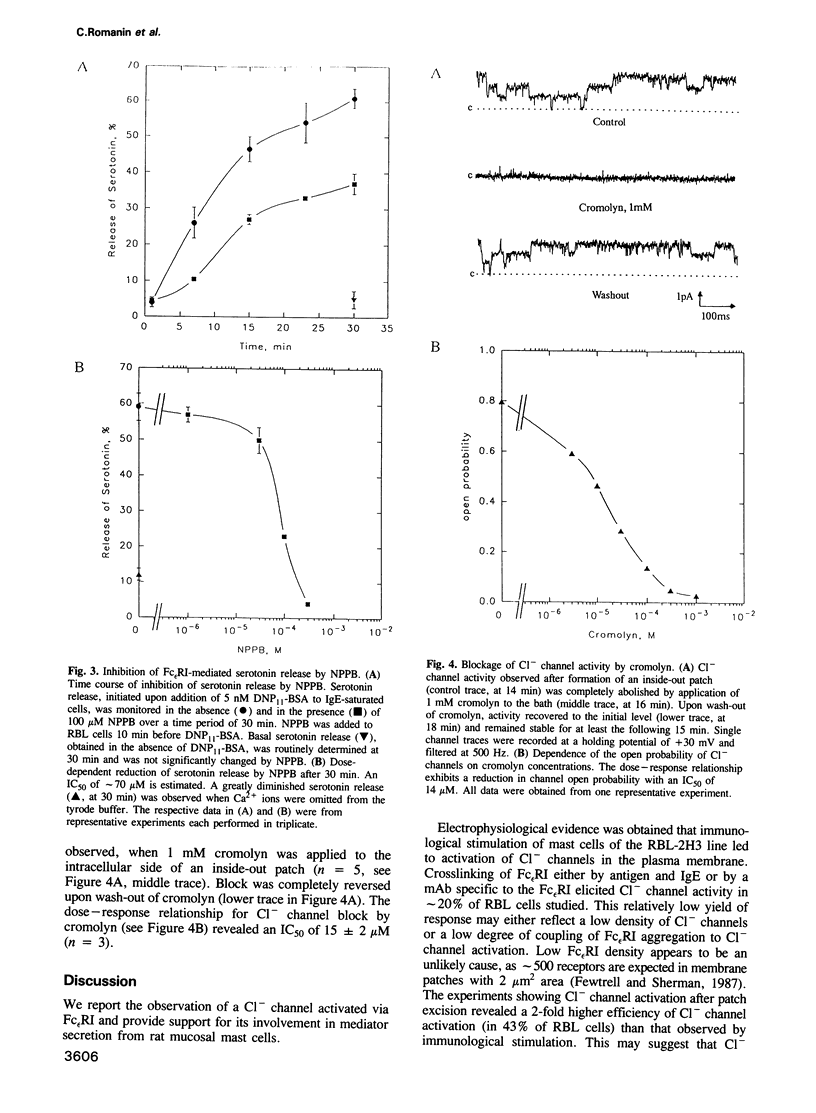
Crosslinking of type I Fc epsilon receptors (Fc epsilon RI) on the surface of basophils or mast cells initiates a cascade of processes leading to the secretion of inflammatory mediators. We report here a correlation between mediator secretion and the activation of Cl- channels in rat mucosal-type mast cells (line RBL-2H3). Stimulation of RBL cells by either IgE and antigen or by a monoclonal antibody specific for the Fc epsilon RI, resulted in the activation of Cl- ion channels as detected by the patch-clamp technique. Channel activation occurred slowly, within minutes after stimulation. The channel has a slope conductance of 32 pS at potentials between 0 and -100 mV, and an increasing open-state probability with increasing depolarization. Activation of apparently the same Cl- channels could be mimicked without stimulation by isolating inside-out membrane patches in tyrode solution. Parallel inhibition of both Cl- channel activity and mediator secretion, as monitored by serotonin release, was observed by two compounds, the Cl- channel blocker 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) and the anti-allergic drug cromolyn. NPPB inhibited both the antigen-induced Cl- current and the serotonin release, where half-maximal inhibition occurred at similar doses, at 52 microM and 77 microM, respectively. The drug cromolyn, recently found to inhibit immunologically induced mediator secretion from RBL cells upon intracellular application, also blocks Cl- channels (IC50 = 15 microM) when applied to the cytoplasmic side of an inside-out membrane patch. The observed Cl- channel activation upon immunological stimulation and the parallel inhibition of channel current and of serotonin release suggests a functional role for this Cl- channel in mediator secretion from the mast cells studied.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsumian E. L., Isersky C., Petrino M. G., Siraganian R. P. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and nonreleasing clones. Eur J Immunol. 1981 Apr;11(4):317–323. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- Beaven M. A., Moore J. P., Smith G. A., Hesketh T. R., Metcalfe J. C. The calcium signal and phosphatidylinositol breakdown in 2H3 cells. J Biol Chem. 1984 Jun 10;259(11):7137–7142. [PubMed] [Google Scholar]
- Beaven M. A., Rogers J., Moore J. P., Hesketh T. R., Smith G. A., Metcalfe J. C. The mechanism of the calcium signal and correlation with histamine release in 2H3 cells. J Biol Chem. 1984 Jun 10;259(11):7129–7136. [PubMed] [Google Scholar]
- Benhamou M., Gutkind J. S., Robbins K. C., Siraganian R. P. Tyrosine phosphorylation coupled to IgE receptor-mediated signal transduction and histamine release. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5327–5330. doi: 10.1073/pnas.87.14.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcia A., Pecht I., Hemmerich S., Ran S., Rivnay B. Calcium specificity of the antigen-induced channels in rat basophilic leukemia cells. Biochemistry. 1988 Sep 20;27(19):7499–7506. doi: 10.1021/bi00419a048. [DOI] [PubMed] [Google Scholar]
- Cox J. S., Beach J. E., Blair A. M., Clarke A. J., King J., Lee T. B., Loveday D. E., Moss G. F., Orr T. S., Ritchie J. T. Disodium cromoglycate (Intal). Adv Drug Res. 1970;5:115–196. [PubMed] [Google Scholar]
- Cox J. S. Disodium cromoglycate (FPL 670) ('Intal'): a specific inhibitor of reaginic antibody-antigen mechanisms. Nature. 1967 Dec 30;216(5122):1328–1329. doi: 10.1038/2161328a0. [DOI] [PubMed] [Google Scholar]
- Crews F. T., Morita Y., McGivney A., Hirata F., Siraganian R. P., Axelrod J. IgE-mediated histamine release in rat basophilic leukemia cells: receptor activation, phospholipid methylation, Ca2+ flux, and release of arachidonic acid. Arch Biochem Biophys. 1981 Dec;212(2):561–571. doi: 10.1016/0003-9861(81)90399-4. [DOI] [PubMed] [Google Scholar]
- Dreinhöfer J., Gögelein H., Greger R. Blocking kinetics of Cl- channels in colonic carcinoma cells (HT29) as revealed by 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB). Biochim Biophys Acta. 1988 Dec 8;946(1):135–142. doi: 10.1016/0005-2736(88)90466-x. [DOI] [PubMed] [Google Scholar]
- Fewtrell C., Sherman E. IgE receptor-activated calcium permeability pathway in rat basophilic leukemia cells: measurement of the unidirectional influx of calcium using quin2-buffered cells. Biochemistry. 1987 Nov 3;26(22):6995–7003. doi: 10.1021/bi00396a021. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Garland L. G. Cromoglycate and other antiallergic drugs: a possible mechanism of action. Br Med J. 1976 Apr 3;1(6013):820–821. doi: 10.1136/bmj.1.6013.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Hallett M. B., Mongar J. L. Site of action of the antiallergic drugs cromoglycate and doxantrazole [proceedings]. Br J Pharmacol. 1977 Mar;59(3):473P–474P. [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hemmerich S., Pecht I. Isolation and purification of an Fc epsilon receptor activated ion channel from the rat mast cell line RBL-2H3. Biochemistry. 1988 Sep 20;27(19):7488–7498. doi: 10.1021/bi00419a047. [DOI] [PubMed] [Google Scholar]
- Hemmerich S., Sijpkens D., Pecht I. A novel cell-permeable cromoglycate derivative inhibits type I Fc epsilon receptor mediated Ca2+ influx and mediator secretion in rat mucosal mast cells. Biochemistry. 1991 Feb 12;30(6):1523–1532. doi: 10.1021/bi00220a012. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Foreman J. C., Sterk A. R., Ishizaka K. Induction of calcium flux across the rat mast cell membrane by bridging IgE receptors. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5858–5862. doi: 10.1073/pnas.76.11.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Activation of mast cells for mediator release through IgE receptors. Prog Allergy. 1984;34:188–235. [PubMed] [Google Scholar]
- Ishizaka T. The Robert A. Cooke memorial lecture. Analysis of triggering events in mast cells for immunoglobulin E-mediated histamine release. J Allergy Clin Immunol. 1981 Feb;67(2):90–96. doi: 10.1016/0091-6749(81)90002-6. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Yankaskas J. R., Stutts M. J., Willumsen N. J., Boucher R. C. Persistence of abnormal chloride conductance regulation in transformed cystic fibrosis epithelia. Science. 1989 Jun 23;244(4911):1472–1475. doi: 10.1126/science.2472008. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Metzger H. Crosslinking of the receptors for immunoglobulin E depolarizes the plasma membrane of rat basophilic leukemia cells. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5744–5748. doi: 10.1073/pnas.80.18.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Okada T., Shibata T. A patch-clamp study: secretagogue-induced currents in rat peritoneal mast cells. Am J Physiol. 1989 Mar;256(3 Pt 1):C560–C568. doi: 10.1152/ajpcell.1989.256.3.C560. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K., Pavenstädt H., Greger R. Properties and regulation of chloride channels in cystic fibrosis and normal airway cells. Pflugers Arch. 1989 Nov;415(2):172–182. doi: 10.1007/BF00370589. [DOI] [PubMed] [Google Scholar]
- Labrecque G. F., Holowka D., Baird B. Antigen-triggered membrane potential changes in IgE-sensitized rat basophilic leukemia cells: evidence for a repolarizing response that is important in the stimulation of cellular degranulation. J Immunol. 1989 Jan 1;142(1):236–243. [PubMed] [Google Scholar]
- Lindau M., Fernandez J. M. A patch-clamp study of histamine-secreting cells. J Gen Physiol. 1986 Sep;88(3):349–368. doi: 10.1085/jgp.88.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M., Fernandez J. M. IgE-mediated degranulation of mast cells does not require opening of ion channels. Nature. 1986 Jan 9;319(6049):150–153. doi: 10.1038/319150a0. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Bohn J. W., Ferry E. L., Yamamoto H., Molinaro C. A., Sherman L. A., Klinman N. R., Katz D. H. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980 Jun;124(6):2728–2737. [PubMed] [Google Scholar]
- Lyall V., Croxton T. L., Armstrong W. M. Measurement of intracellular chloride activity in mouse liver slices with microelectrodes. Biochim Biophys Acta. 1987 Sep 18;903(1):56–67. doi: 10.1016/0005-2736(87)90155-6. [DOI] [PubMed] [Google Scholar]
- Matthews G., Neher E., Penner R. Chloride conductance activated by external agonists and internal messengers in rat peritoneal mast cells. J Physiol. 1989 Nov;418:131–144. doi: 10.1113/jphysiol.1989.sp017831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Neher E., Penner R. Second messenger-activated calcium influx in rat peritoneal mast cells. J Physiol. 1989 Nov;418:105–130. doi: 10.1113/jphysiol.1989.sp017830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek N., Schindler H., Schürholz T., Pecht I. The cromolyn binding protein constitutes the Ca2+ channel of basophils opening upon immunological stimulus. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6841–6845. doi: 10.1073/pnas.81.21.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGivney A., Morita Y., Crews F. T., Hirata F., Axelrod J., Siraganian R. P. Phospholipase activation in the IgE-mediated and Ca2+ ionophore A23187-induced release of histamine from rat basophilic leukemia cells. Arch Biochem Biophys. 1981 Dec;212(2):572–580. doi: 10.1016/0003-9861(81)90400-8. [DOI] [PubMed] [Google Scholar]
- Mohr F. C., Fewtrell C. Depolarization of rat basophilic leukemia cells inhibits calcium uptake and exocytosis. J Cell Biol. 1987 Mar;104(3):783–792. doi: 10.1083/jcb.104.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr F. C., Fewtrell C. IgE receptor-mediated depolarization of rat basophilic leukemia cells measured with the fluorescent probe bis-oxonol. J Immunol. 1987 Mar 1;138(5):1564–1570. [PubMed] [Google Scholar]
- Ortega E., Schweitzer-Stenner R., Pecht I. Possible orientational constraints determine secretory signals induced by aggregation of IgE receptors on mast cells. EMBO J. 1988 Dec 20;7(13):4101–4109. doi: 10.1002/j.1460-2075.1988.tb03304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce F. L., Befus A. D., Gauldie J., Bienenstock J. Mucosal mast cells. II. Effects of anti-allergic compounds on histamine secretion by isolated intestinal mast cells. J Immunol. 1982 Jun;128(6):2481–2486. [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Sagi-Eisenberg R., Pecht I. Membrane potential changes during IgE-mediated histamine release from rat basophilic leukemia cells. J Membr Biol. 1983;75(2):97–104. doi: 10.1007/BF01995629. [DOI] [PubMed] [Google Scholar]
- Sagi-Eisenberg R., Pecht I. Resolution of cellular compartments involved in membrane potential changes accompanying IgE-mediated degranulation of rat basophilic leukemia cells. EMBO J. 1984 Mar;3(3):497–500. doi: 10.1002/j.1460-2075.1984.tb01836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin D. C., Adelman S., Austen K. F., Stevens R. L., Hein A., Caulfield J. P., Woodbury R. G. Homology of the rat basophilic leukemia cell and the rat mucosal mast cell. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3871–3875. doi: 10.1073/pnas.82.11.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan T. J., Parker C. W. Possible role of arachidonic acid and its metabolites in mediator release from rat mast cells. J Immunol. 1979 Feb;122(2):431–436. [PubMed] [Google Scholar]
- Taurog J. D., Mendoza G. R., Hook W. A., Siraganian R. P., Metzger H. Noncytotoxic IgE-mediated release of histamine and serotonin from murine mastocytoma cells. J Immunol. 1977 Nov;119(5):1757–1761. [PubMed] [Google Scholar]
- Theoharides T. C., Sieghart W., Greengard P., Douglas W. W. Antiallergic drug cromolyn may inhibit histamine secretion by regulating phosphorylation of a mast cell protein. Science. 1980 Jan 4;207(4426):80–82. doi: 10.1126/science.6153130. [DOI] [PubMed] [Google Scholar]
- Wangemann P., Wittner M., Di Stefano A., Englert H. C., Lang H. J., Schlatter E., Greger R. Cl(-)-channel blockers in the thick ascending limb of the loop of Henle. Structure activity relationship. Pflugers Arch. 1986;407 (Suppl 2):S128–S141. doi: 10.1007/BF00584942. [DOI] [PubMed] [Google Scholar]



