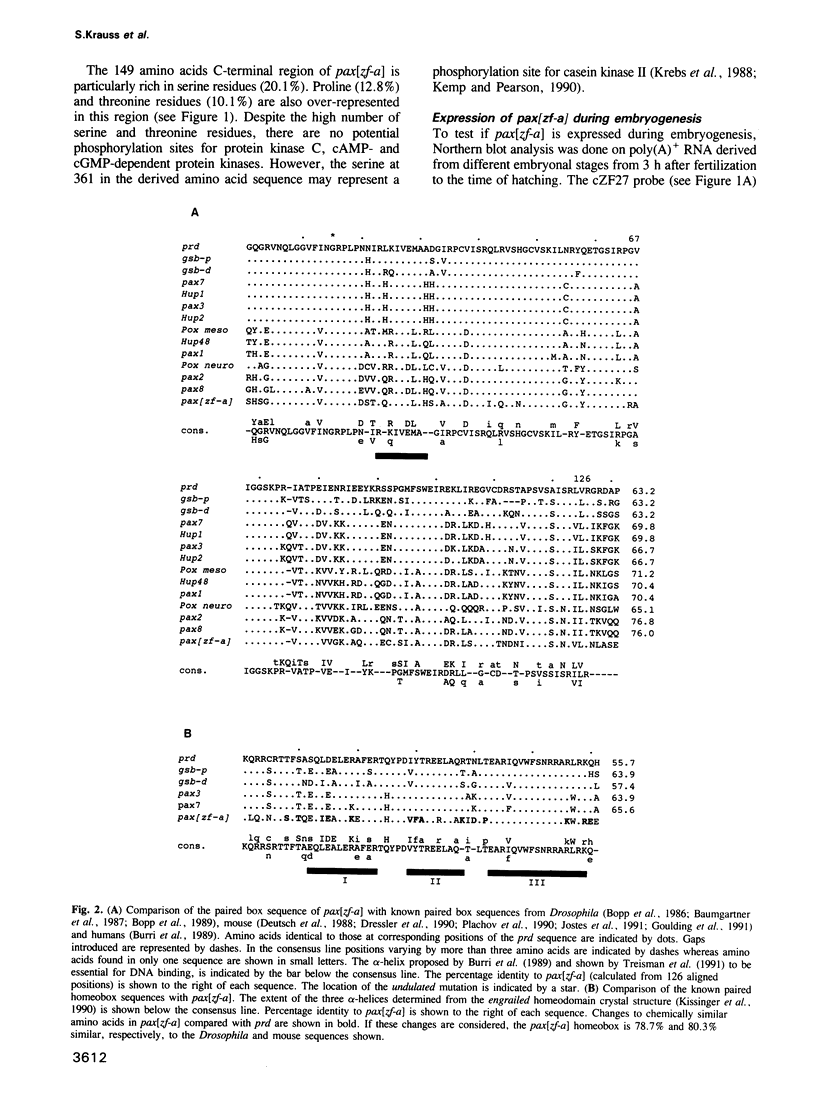
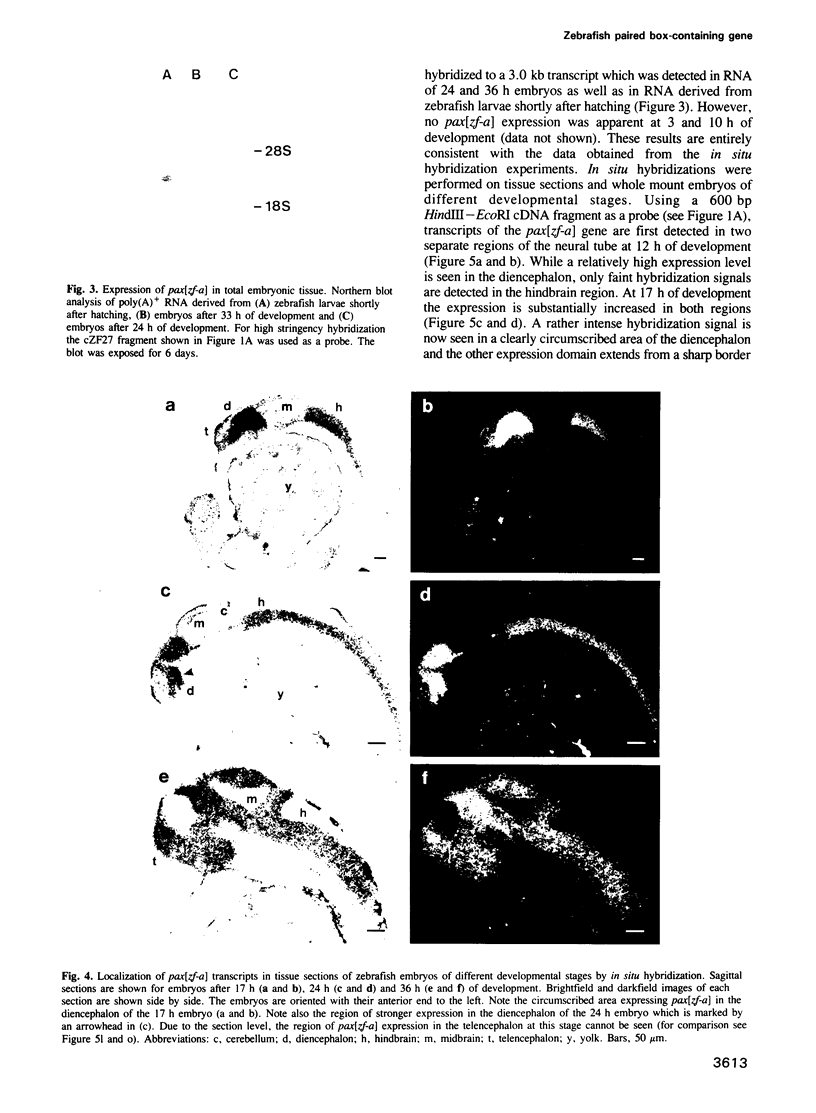
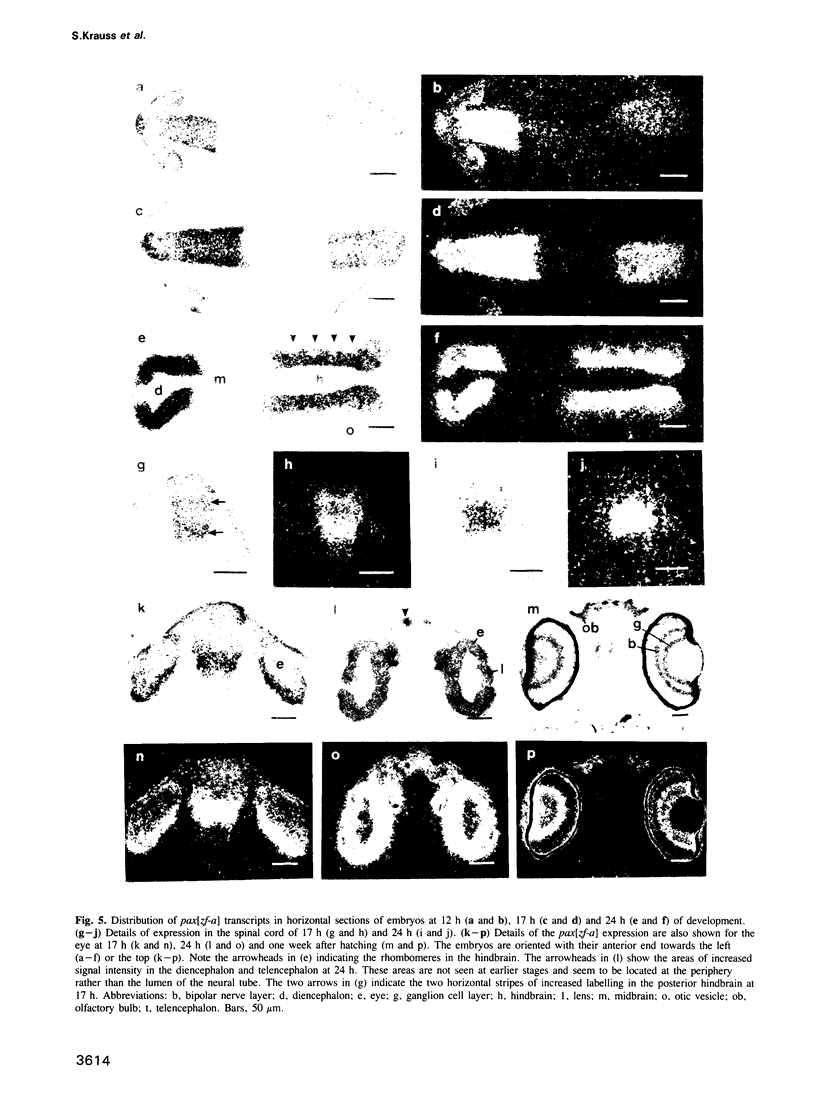
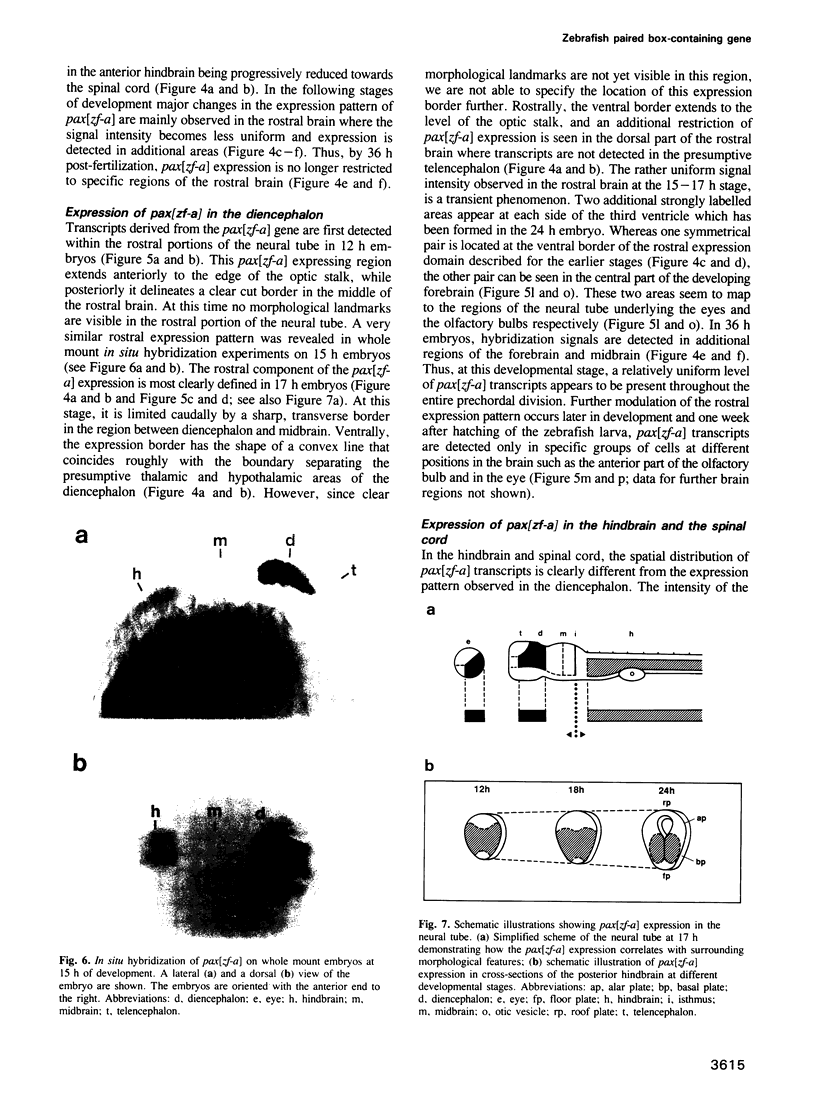
Abstract
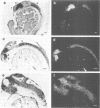
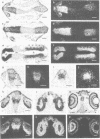
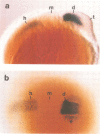
Murine and human sequences homologous to the paired box of the Drosophila segmentation gene paired have been reported previously. Here we describe a zebrafish (Brachydanio rerio) paired box-containing clone, pax[zf-a], which is clearly distinct from reported vertebrate Pax genes. The putative protein encoded by pax[zf-a] contains a paired box and a paired-type homeobox separated by a glycine-rich, acidic linker and a carboxy-terminal end which is remarkably rich in serine, threonine and proline residues. By in situ hybridization to embryonic tissue sections and whole mount embryos, pax[zf-a] transcripts were found within restricted regions of the central nervous system and the eye. In contrast to the murine Pax genes recently characterized, pax[zf-a] is not expressed in the segmented mesoderm. At the 17 h stage, pax[zf-a] expression is detected in a defined area of the diencephalon which circumscribes the presumptive thalamus. This suggests an involvement of pax[zf-a] in pattern formation in the rostral brain. The pax[zf-a] gene is also expressed throughout the hindbrain and spinal cord. This hybridization signal is restricted to a longitudinal column which includes the basal plate. Later in development, at 36 h post-fertilization, pax[zf-a] transcripts are no longer restricted to a specific region of the diencephalon, but are distributed over the entire developing brain.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGQUIST H., KALLEN B. Notes on the early histogenesis and morphogenesis of the central nervous system in vertebrates. J Comp Neurol. 1954 Jun;100(3):627–659. doi: 10.1002/cne.901000308. [DOI] [PubMed] [Google Scholar]
- Balling R., Deutsch U., Gruss P. undulated, a mutation affecting the development of the mouse skeleton, has a point mutation in the paired box of Pax 1. Cell. 1988 Nov 4;55(3):531–535. doi: 10.1016/0092-8674(88)90039-6. [DOI] [PubMed] [Google Scholar]
- Baumgartner S., Bopp D., Burri M., Noll M. Structure of two genes at the gooseberry locus related to the paired gene and their spatial expression during Drosophila embryogenesis. Genes Dev. 1987 Dec;1(10):1247–1267. doi: 10.1101/gad.1.10.1247. [DOI] [PubMed] [Google Scholar]
- Bopp D., Burri M., Baumgartner S., Frigerio G., Noll M. Conservation of a large protein domain in the segmentation gene paired and in functionally related genes of Drosophila. Cell. 1986 Dec 26;47(6):1033–1040. doi: 10.1016/0092-8674(86)90818-4. [DOI] [PubMed] [Google Scholar]
- Bopp D., Jamet E., Baumgartner S., Burri M., Noll M. Isolation of two tissue-specific Drosophila paired box genes, Pox meso and Pox neuro. EMBO J. 1989 Nov;8(11):3447–3457. doi: 10.1002/j.1460-2075.1989.tb08509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri M., Tromvoukis Y., Bopp D., Frigerio G., Noll M. Conservation of the paired domain in metazoans and its structure in three isolated human genes. EMBO J. 1989 Apr;8(4):1183–1190. doi: 10.1002/j.1460-2075.1989.tb03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. B. Zebra patterns in fly embryos: activation of stripes or repression of interstripes? Cell. 1990 Jan 12;60(1):9–16. doi: 10.1016/0092-8674(90)90711-m. [DOI] [PubMed] [Google Scholar]
- Courey A. J., Holtzman D. A., Jackson S. P., Tjian R. Synergistic activation by the glutamine-rich domains of human transcription factor Sp1. Cell. 1989 Dec 1;59(5):827–836. doi: 10.1016/0092-8674(89)90606-5. [DOI] [PubMed] [Google Scholar]
- Deutsch U., Dressler G. R., Gruss P. Pax 1, a member of a paired box homologous murine gene family, is expressed in segmented structures during development. Cell. 1988 May 20;53(4):617–625. doi: 10.1016/0092-8674(88)90577-6. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler G. R., Deutsch U., Chowdhury K., Nornes H. O., Gruss P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development. 1990 Aug;109(4):787–795. doi: 10.1242/dev.109.4.787. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Frasch M., Levine M. Complementary patterns of even-skipped and fushi tarazu expression involve their differential regulation by a common set of segmentation genes in Drosophila. Genes Dev. 1987 Nov;1(9):981–995. doi: 10.1101/gad.1.9.981. [DOI] [PubMed] [Google Scholar]
- Frigerio G., Burri M., Bopp D., Baumgartner S., Noll M. Structure of the segmentation gene paired and the Drosophila PRD gene set as part of a gene network. Cell. 1986 Dec 5;47(5):735–746. doi: 10.1016/0092-8674(86)90516-7. [DOI] [PubMed] [Google Scholar]
- Gehring W. J. Homeo boxes in the study of development. Science. 1987 Jun 5;236(4806):1245–1252. doi: 10.1126/science.2884726. [DOI] [PubMed] [Google Scholar]
- Goulding M. D., Chalepakis G., Deutsch U., Erselius J. R., Gruss P. Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J. 1991 May;10(5):1135–1147. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989 May 5;57(3):367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Han K., Levine M. S., Manley J. L. Synergistic activation and repression of transcription by Drosophila homeobox proteins. Cell. 1989 Feb 24;56(4):573–583. doi: 10.1016/0092-8674(89)90580-1. [DOI] [PubMed] [Google Scholar]
- Hanneman E., Trevarrow B., Metcalfe W. K., Kimmel C. B., Westerfield M. Segmental pattern of development of the hindbrain and spinal cord of the zebrafish embryo. Development. 1988 May;103(1):49–58. doi: 10.1242/dev.103.1.49. [DOI] [PubMed] [Google Scholar]
- Hoey T., Levine M. Divergent homeo box proteins recognize similar DNA sequences in Drosophila. Nature. 1988 Apr 28;332(6167):858–861. doi: 10.1038/332858a0. [DOI] [PubMed] [Google Scholar]
- Ingham P. W. The molecular genetics of embryonic pattern formation in Drosophila. Nature. 1988 Sep 1;335(6185):25–34. doi: 10.1038/335025a0. [DOI] [PubMed] [Google Scholar]
- Jacobson M. Clonal analysis and cell lineages of the vertebrate central nervous system. Annu Rev Neurosci. 1985;8:71–102. doi: 10.1146/annurev.ne.08.030185.000443. [DOI] [PubMed] [Google Scholar]
- Jostes B., Walther C., Gruss P. The murine paired box gene, Pax7, is expressed specifically during the development of the nervous and muscular system. Mech Dev. 1990 Dec;33(1):27–37. doi: 10.1016/0925-4773(90)90132-6. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990 Sep;15(9):342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Murine developmental control genes. Science. 1990 Jul 27;249(4967):374–379. doi: 10.1126/science.1974085. [DOI] [PubMed] [Google Scholar]
- Keyser A. The development of the diencephalon of the Chinese hamster. An investigation of the validity of the criteria of subdivision of the brain. Acta Anat Suppl (Basel) 1972;59:1–178. [PubMed] [Google Scholar]
- Kissinger C. R., Liu B. S., Martin-Blanco E., Kornberg T. B., Pabo C. O. Crystal structure of an engrailed homeodomain-DNA complex at 2.8 A resolution: a framework for understanding homeodomain-DNA interactions. Cell. 1990 Nov 2;63(3):579–590. doi: 10.1016/0092-8674(90)90453-l. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Krauss S., Johansen T., Korzh V., Fjose A. Expression pattern of zebrafish pax genes suggests a role in early brain regionalization. Nature. 1991 Sep 19;353(6341):267–270. doi: 10.1038/353267a0. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Eisenman R. N., Kuenzel E. A., Litchfield D. W., Lozeman F. J., Lüscher B., Sommercorn J. Casein kinase II as a potentially important enzyme concerned with signal transduction. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):77–84. doi: 10.1101/sqb.1988.053.01.012. [DOI] [PubMed] [Google Scholar]
- Layer P. G., Rommel S., Bülthoff H., Hengstenberg R. Independent spatial waves of biochemical differentiation along the surface of chicken brain as revealed by the sequential expression of acetylcholinesterase. Cell Tissue Res. 1988 Mar;251(3):587–595. doi: 10.1007/BF00214007. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Przybyla A. E., Chirgwin J. M. Isolation of RNA using guanidinium salts. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- Malicki J., Schughart K., McGinnis W. Mouse Hox-2.2 specifies thoracic segmental identity in Drosophila embryos and larvae. Cell. 1990 Nov 30;63(5):961–967. doi: 10.1016/0092-8674(90)90499-5. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Garber R. L., Wirz J., Kuroiwa A., Gehring W. J. A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell. 1984 Jun;37(2):403–408. doi: 10.1016/0092-8674(84)90370-2. [DOI] [PubMed] [Google Scholar]
- Mermod N., O'Neill E. A., Kelly T. J., Tjian R. The proline-rich transcriptional activator of CTF/NF-I is distinct from the replication and DNA binding domain. Cell. 1989 Aug 25;58(4):741–753. doi: 10.1016/0092-8674(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Moitoso de Vargas L., Pargellis C. A., Hasan N. M., Bushman E. W., Landy A. Autonomous DNA binding domains of lambda integrase recognize two different sequence families. Cell. 1988 Sep 23;54(7):923–929. doi: 10.1016/0092-8674(88)90107-9. [DOI] [PubMed] [Google Scholar]
- Murphy P., Davidson D. R., Hill R. E. Segment-specific expression of a homoeobox-containing gene in the mouse hindbrain. Nature. 1989 Sep 14;341(6238):156–159. doi: 10.1038/341156a0. [DOI] [PubMed] [Google Scholar]
- Nornes H. O., Dressler G. R., Knapik E. W., Deutsch U., Gruss P. Spatially and temporally restricted expression of Pax2 during murine neurogenesis. Development. 1990 Aug;109(4):797–809. doi: 10.1242/dev.109.4.797. [DOI] [PubMed] [Google Scholar]
- Patel N. H., Schafer B., Goodman C. S., Holmgren R. The role of segment polarity genes during Drosophila neurogenesis. Genes Dev. 1989 Jun;3(6):890–904. doi: 10.1101/gad.3.6.890. [DOI] [PubMed] [Google Scholar]
- Plachov D., Chowdhury K., Walther C., Simon D., Guenet J. L., Gruss P. Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development. 1990 Oct;110(2):643–651. doi: 10.1242/dev.110.2.643. [DOI] [PubMed] [Google Scholar]
- Price M., Lemaistre M., Pischetola M., Di Lauro R., Duboule D. A mouse gene related to Distal-less shows a restricted expression in the developing forebrain. Nature. 1991 Jun 27;351(6329):748–751. doi: 10.1038/351748a0. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Roberts B. Nuclear location signal-mediated protein transport. Biochim Biophys Acta. 1989 Aug 14;1008(3):263–280. doi: 10.1016/0167-4781(89)90016-x. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Tamkun J. W., Hartzell G. W., 3rd The structure and function of the homeodomain. Biochim Biophys Acta. 1989 Jul 28;989(1):25–48. doi: 10.1016/0304-419x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Treisman J., Gönczy P., Vashishtha M., Harris E., Desplan C. A single amino acid can determine the DNA binding specificity of homeodomain proteins. Cell. 1989 Nov 3;59(3):553–562. doi: 10.1016/0092-8674(89)90038-x. [DOI] [PubMed] [Google Scholar]
- Trevarrow B., Marks D. L., Kimmel C. B. Organization of hindbrain segments in the zebrafish embryo. Neuron. 1990 May;4(5):669–679. doi: 10.1016/0896-6273(90)90194-k. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bailes J. A., McMahon A. P. Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell. 1987 Jul 3;50(1):79–88. doi: 10.1016/0092-8674(87)90664-7. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Cook M., Boncinelli E., Krumlauf R. Segmental expression of Hox-2 homoeobox-containing genes in the developing mouse hindbrain. Nature. 1989 Oct 5;341(6241):405–409. doi: 10.1038/341405a0. [DOI] [PubMed] [Google Scholar]
- Wilson S. W., Ross L. S., Parrett T., Easter S. S., Jr The development of a simple scaffold of axon tracts in the brain of the embryonic zebrafish, Brachydanio rerio. Development. 1990 Jan;108(1):121–145. doi: 10.1242/dev.108.1.121. [DOI] [PubMed] [Google Scholar]
- Xiao J. H., Davidson I., Matthes H., Garnier J. M., Chambon P. Cloning, expression, and transcriptional properties of the human enhancer factor TEF-1. Cell. 1991 May 17;65(4):551–568. doi: 10.1016/0092-8674(91)90088-g. [DOI] [PubMed] [Google Scholar]