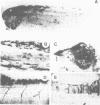
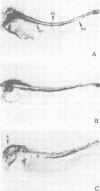
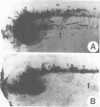
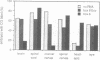
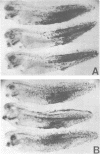
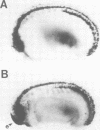
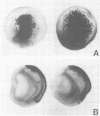
Abstract
We have injected XIHbox 6 mRNA together with the lineage tracer colloidal gold into individual dorso-anterior blastomeres of the 32-cell stage Xenopus embryo and analyzed their cell fate during embryogenesis. While the developing tadpoles appeared entirely normal, the fate of the progeny of the injected blastomere was altered. In the brain injected cells failed to differentiate terminally, as indicated by a loss of labeled cranial nerves. Differentiation of spinal nerves remained unaffected. Fate change in the CNS occurred at about the time of normal XIHbox 6 protein expression. In addition, progeny of injected blastomeres gained head epidermal fate and lost anterior notochord fate as a result of altered cell migrations during gastrulation. The results show that a homeodomain protein is capable of altering cell fate in a position-specific and cell-autonomous manner in Xenopus embryos. The experimental approach used here should be applicable to other molecules specifying cell fate.
Full text
PDF
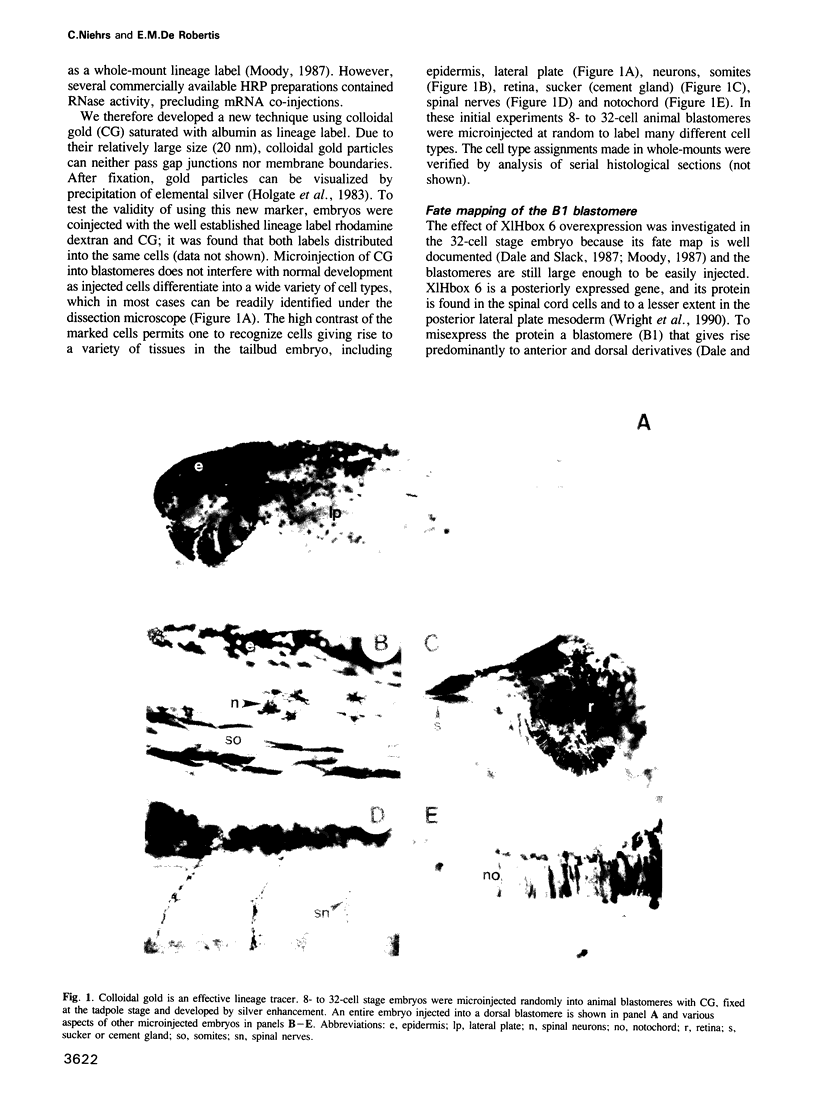



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balling R., Mutter G., Gruss P., Kessel M. Craniofacial abnormalities induced by ectopic expression of the homeobox gene Hox-1.1 in transgenic mice. Cell. 1989 Jul 28;58(2):337–347. doi: 10.1016/0092-8674(89)90848-9. [DOI] [PubMed] [Google Scholar]
- Blumberg B., Wright C. V., De Robertis E. M., Cho K. W. Organizer-specific homeobox genes in Xenopus laevis embryos. Science. 1991 Jul 12;253(5016):194–196. doi: 10.1126/science.1677215. [DOI] [PubMed] [Google Scholar]
- Busa W. B., Gimlich R. L. Lithium-induced teratogenesis in frog embryos prevented by a polyphosphoinositide cycle intermediate or a diacylglycerol analog. Dev Biol. 1989 Apr;132(2):315–324. doi: 10.1016/0012-1606(89)90228-5. [DOI] [PubMed] [Google Scholar]
- Chisaka O., Capecchi M. R. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991 Apr 11;350(6318):473–479. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- Cho K. W., De Robertis E. M. Differential activation of Xenopus homeo box genes by mesoderm-inducing growth factors and retinoic acid. Genes Dev. 1990 Nov;4(11):1910–1916. doi: 10.1101/gad.4.11.1910. [DOI] [PubMed] [Google Scholar]
- Cho K. W., Goetz J., Wright C. V., Fritz A., Hardwicke J., De Robertis E. M. Differential utilization of the same reading frame in a Xenopus homeobox gene encodes two related proteins sharing the same DNA-binding specificity. EMBO J. 1988 Jul;7(7):2139–2149. doi: 10.1002/j.1460-2075.1988.tb03053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. W., Morita E. A., Wright C. V., De Robertis E. M. Overexpression of a homeodomain protein confers axis-forming activity to uncommitted Xenopus embryonic cells. Cell. 1991 Apr 5;65(1):55–64. doi: 10.1016/0092-8674(91)90407-p. [DOI] [PubMed] [Google Scholar]
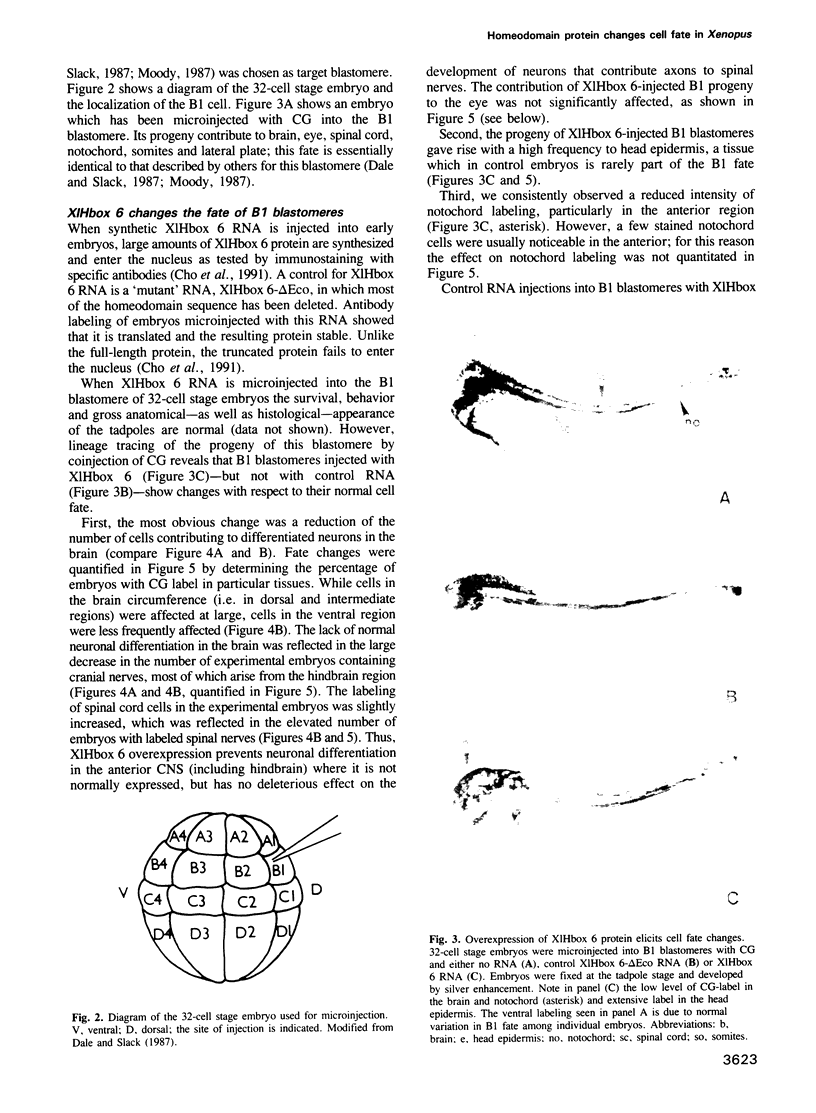
- Dale L., Slack J. M. Fate map for the 32-cell stage of Xenopus laevis. Development. 1987 Apr;99(4):527–551. doi: 10.1242/dev.99.4.527. [DOI] [PubMed] [Google Scholar]
- De Robertis E. M., Oliver G., Wright C. V. Homeobox genes and the vertebrate body plan. Sci Am. 1990 Jul;263(1):46–52. doi: 10.1038/scientificamerican0790-46. [DOI] [PubMed] [Google Scholar]
- Dent J. A., Polson A. G., Klymkowsky M. W. A whole-mount immunocytochemical analysis of the expression of the intermediate filament protein vimentin in Xenopus. Development. 1989 Jan;105(1):61–74. doi: 10.1242/dev.105.1.61. [DOI] [PubMed] [Google Scholar]
- Desplan C., Theis J., O'Farrell P. H. The sequence specificity of homeodomain-DNA interaction. Cell. 1988 Sep 23;54(7):1081–1090. doi: 10.1016/0092-8674(88)90123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring W. J. Homeo boxes in the study of development. Science. 1987 Jun 5;236(4806):1245–1252. doi: 10.1126/science.2884726. [DOI] [PubMed] [Google Scholar]
- Gimlich R. L., Braun J. Improved fluorescent compounds for tracing cell lineage. Dev Biol. 1985 Jun;109(2):509–514. doi: 10.1016/0012-1606(85)90476-2. [DOI] [PubMed] [Google Scholar]
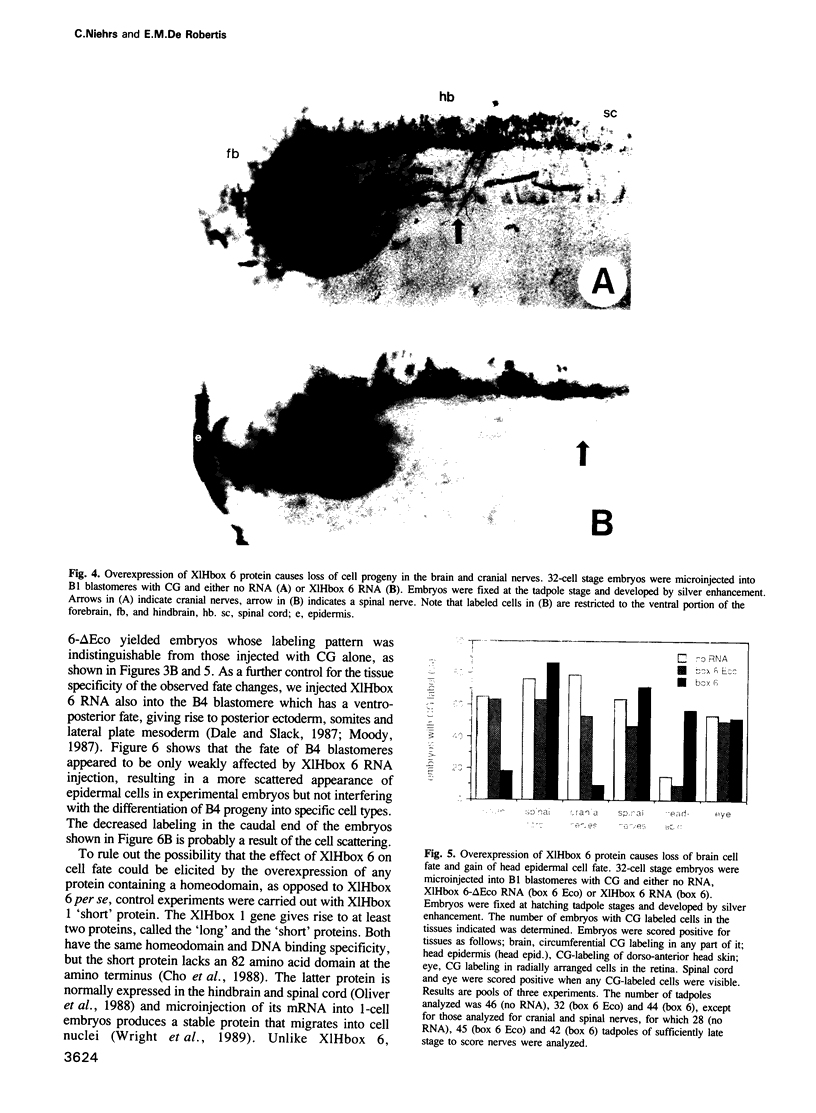
- Graham A., Papalopulu N., Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989 May 5;57(3):367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Injected nuclei in frog oocytes: fate, enlargement, and chromatin dispersal. J Embryol Exp Morphol. 1976 Dec;36(3):523–540. [PubMed] [Google Scholar]
- Harvey R. P., Melton D. A. Microinjection of synthetic Xhox-1A homeobox mRNA disrupts somite formation in developing Xenopus embryos. Cell. 1988 Jun 3;53(5):687–697. doi: 10.1016/0092-8674(88)90087-6. [DOI] [PubMed] [Google Scholar]
- Hoey T., Levine M. Divergent homeo box proteins recognize similar DNA sequences in Drosophila. Nature. 1988 Apr 28;332(6167):858–861. doi: 10.1038/332858a0. [DOI] [PubMed] [Google Scholar]
- Holgate C. S., Jackson P., Cowen P. N., Bird C. C. Immunogold-silver staining: new method of immunostaining with enhanced sensitivity. J Histochem Cytochem. 1983 Jul;31(7):938–944. doi: 10.1177/31.7.6189883. [DOI] [PubMed] [Google Scholar]
- Holt C. E., Bertsch T. W., Ellis H. M., Harris W. A. Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron. 1988 Mar;1(1):15–26. doi: 10.1016/0896-6273(88)90205-x. [DOI] [PubMed] [Google Scholar]
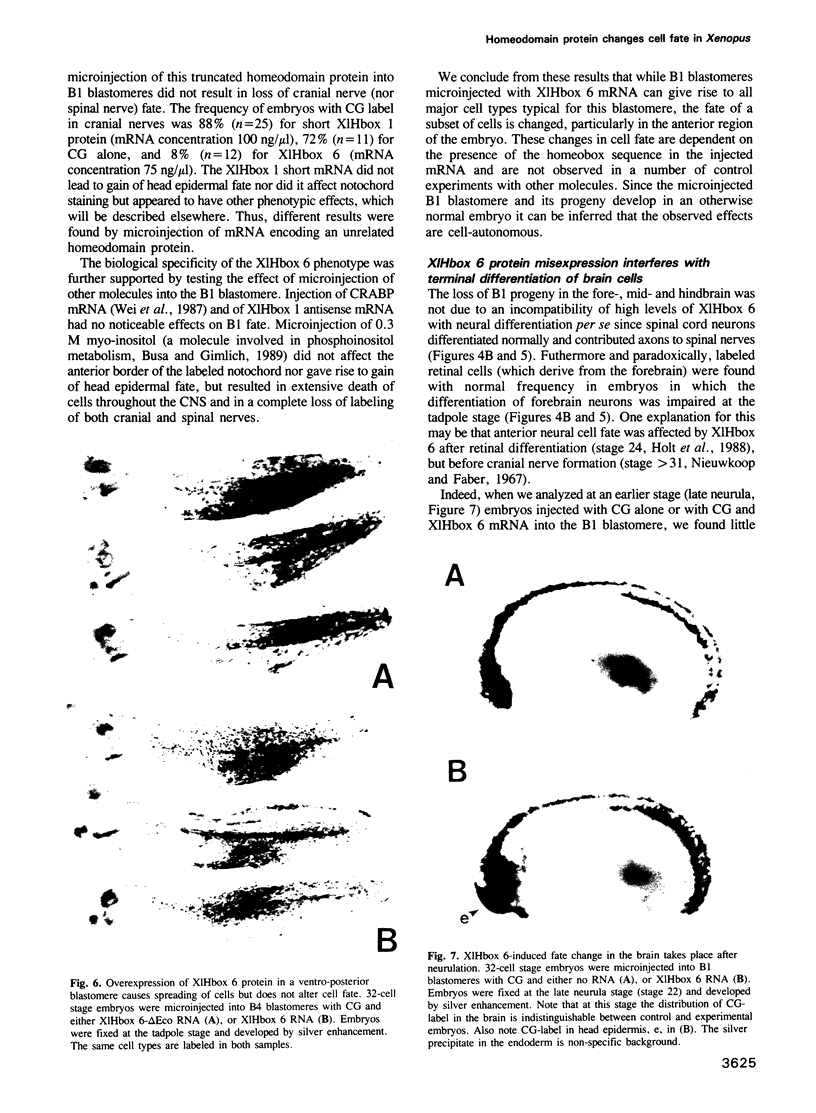
- Jacobson J. W., Hartl D. L. Coupled instability of two X-linked genes in Drosophila mauritiana: germinal and somatic mutability. Genetics. 1985 Sep;111(1):57–65. doi: 10.1093/genetics/111.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageura H. Spatial distribution of the capacity to initiate a secondary embryo in the 32-cell embryo of Xenopus laevis. Dev Biol. 1990 Dec;142(2):432–438. doi: 10.1016/0012-1606(90)90365-p. [DOI] [PubMed] [Google Scholar]
- Keller R. E. Vital dye mapping of the gastrula and neurula of Xenopus laevis. I. Prospective areas and morphogenetic movements of the superficial layer. Dev Biol. 1975 Feb;42(2):222–241. doi: 10.1016/0012-1606(75)90331-0. [DOI] [PubMed] [Google Scholar]
- Kessel M., Balling R., Gruss P. Variations of cervical vertebrae after expression of a Hox-1.1 transgene in mice. Cell. 1990 Apr 20;61(2):301–308. doi: 10.1016/0092-8674(90)90810-2. [DOI] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Murine developmental control genes. Science. 1990 Jul 27;249(4967):374–379. doi: 10.1126/science.1974085. [DOI] [PubMed] [Google Scholar]
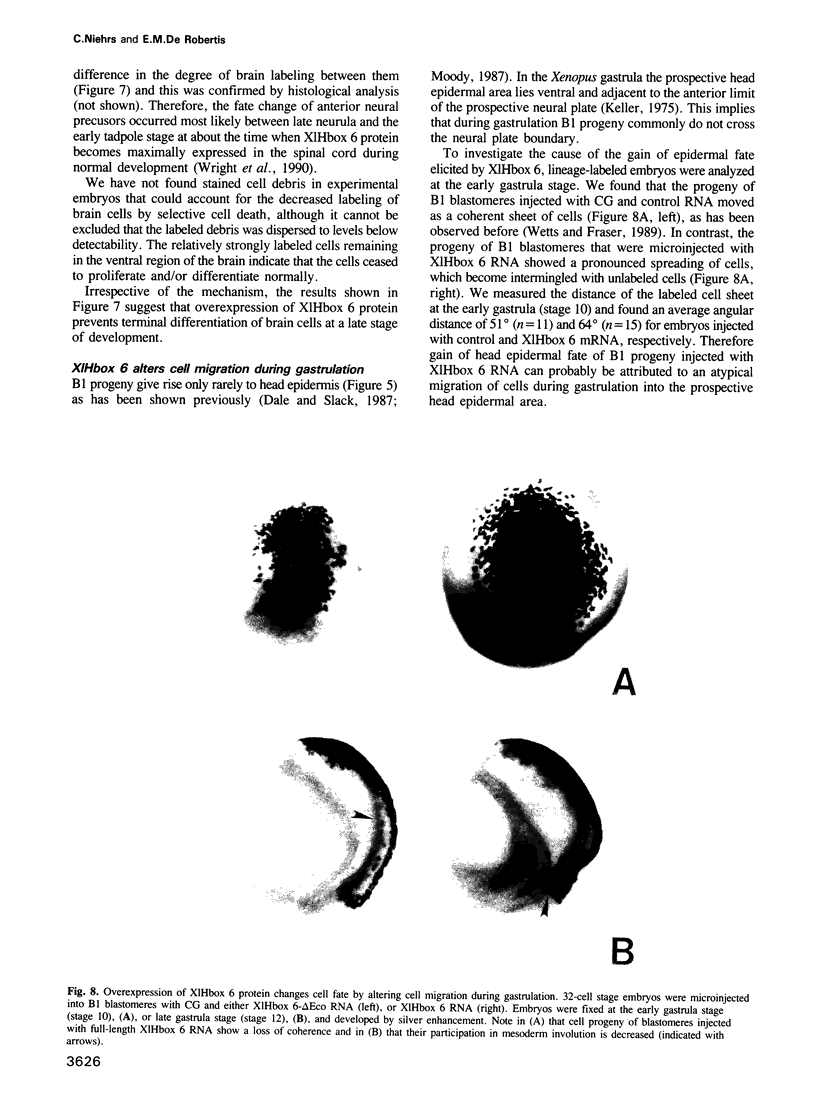
- Klein S. L., Moody S. A. Lithium changes the ectodermal fate of individual frog blastomeres because it causes ectopic neural plate formation. Development. 1989 Jul;106(3):599–610. doi: 10.1242/dev.106.3.599. [DOI] [PubMed] [Google Scholar]
- Klein S. L. The first cleavage furrow demarcates the dorsal-ventral axis in Xenopus embryos. Dev Biol. 1987 Mar;120(1):299–304. doi: 10.1016/0012-1606(87)90127-8. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody S. A. Fates of the blastomeres of the 32-cell-stage Xenopus embryo. Dev Biol. 1987 Aug;122(2):300–319. doi: 10.1016/0012-1606(87)90296-x. [DOI] [PubMed] [Google Scholar]
- Oliver G., Wright C. V., Hardwicke J., De Robertis E. M. Differential antero-posterior expression of two proteins encoded by a homeobox gene in Xenopus and mouse embryos. EMBO J. 1988 Oct;7(10):3199–3209. doi: 10.1002/j.1460-2075.1988.tb03187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa F. M. Mix.1, a homeobox mRNA inducible by mesoderm inducers, is expressed mostly in the presumptive endodermal cells of Xenopus embryos. Cell. 1989 Jun 16;57(6):965–974. doi: 10.1016/0092-8674(89)90335-8. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Melton D. A. Bimodal and graded expression of the Xenopus homeobox gene Xhox3 during embryonic development. Development. 1989 May;106(1):173–183. doi: 10.1242/dev.106.1.173. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Melton D. A. Interaction between peptide growth factors and homoeobox genes in the establishment of antero-posterior polarity in frog embryos. Nature. 1989 Sep 7;341(6237):33–38. doi: 10.1038/341033a0. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Tamkun J. W., Hartzell G. W., 3rd The structure and function of the homeodomain. Biochim Biophys Acta. 1989 Jul 28;989(1):25–48. doi: 10.1016/0304-419x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Sharpe C. R., Fritz A., De Robertis E. M., Gurdon J. B. A homeobox-containing marker of posterior neural differentiation shows the importance of predetermination in neural induction. Cell. 1987 Aug 28;50(5):749–758. doi: 10.1016/0092-8674(87)90333-3. [DOI] [PubMed] [Google Scholar]
- Wei L. N., Mertz J. R., Goodman D. S., Nguyen-Huu M. C. Cellular retinoic acid- and cellular retinol-binding proteins: complementary deoxyribonucleic acid cloning, chromosomal assignment, and tissue specific expression. Mol Endocrinol. 1987 Aug;1(8):526–534. doi: 10.1210/mend-1-8-526. [DOI] [PubMed] [Google Scholar]
- Wetts R., Fraser S. E. Slow intermixing of cells during Xenopus embryogenesis contributes to the consistency of the blastomere fate map. Development. 1989 Jan;105(1):9–15. doi: 10.1242/dev.105.1.9. [DOI] [PubMed] [Google Scholar]
- Wright C. V., Cho K. W., Hardwicke J., Collins R. H., De Robertis E. M. Interference with function of a homeobox gene in Xenopus embryos produces malformations of the anterior spinal cord. Cell. 1989 Oct 6;59(1):81–93. doi: 10.1016/0092-8674(89)90871-4. [DOI] [PubMed] [Google Scholar]
- Wright C. V., Morita E. A., Wilkin D. J., De Robertis E. M. The Xenopus XIHbox 6 homeo protein, a marker of posterior neural induction, is expressed in proliferating neurons. Development. 1990 May;109(1):225–234. doi: 10.1242/dev.109.1.225. [DOI] [PubMed] [Google Scholar]