Abstract
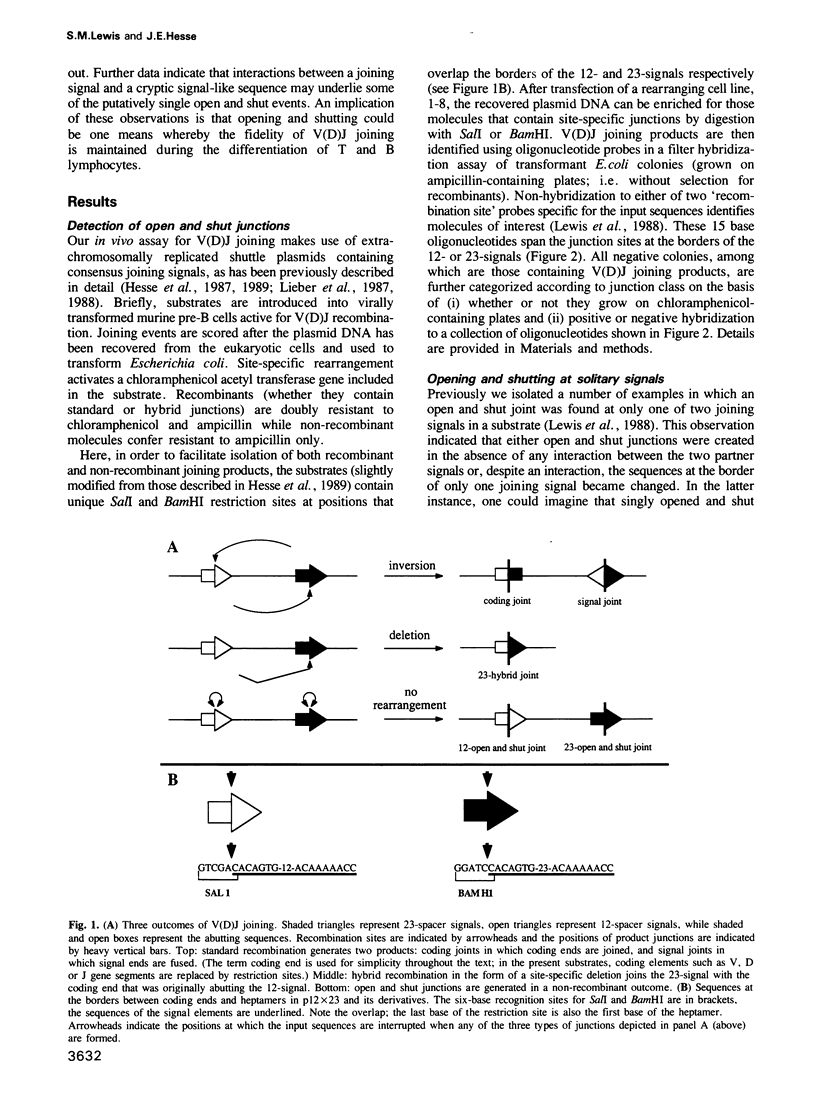
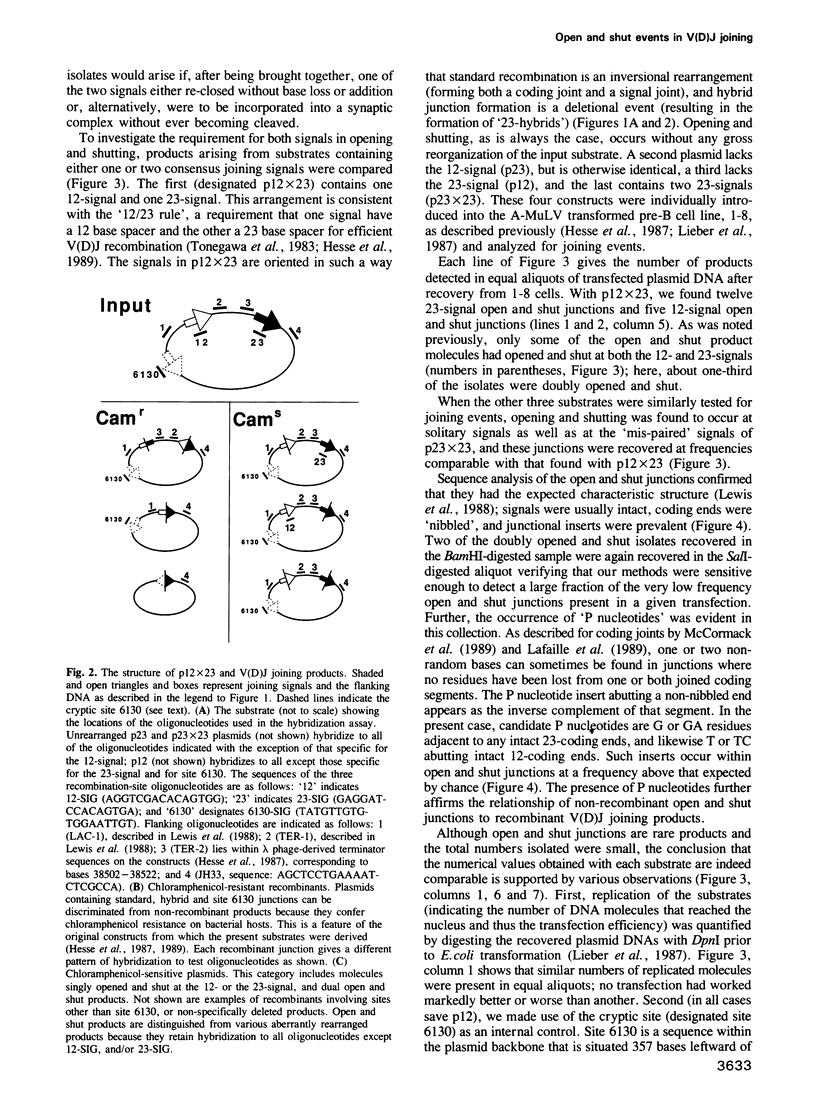
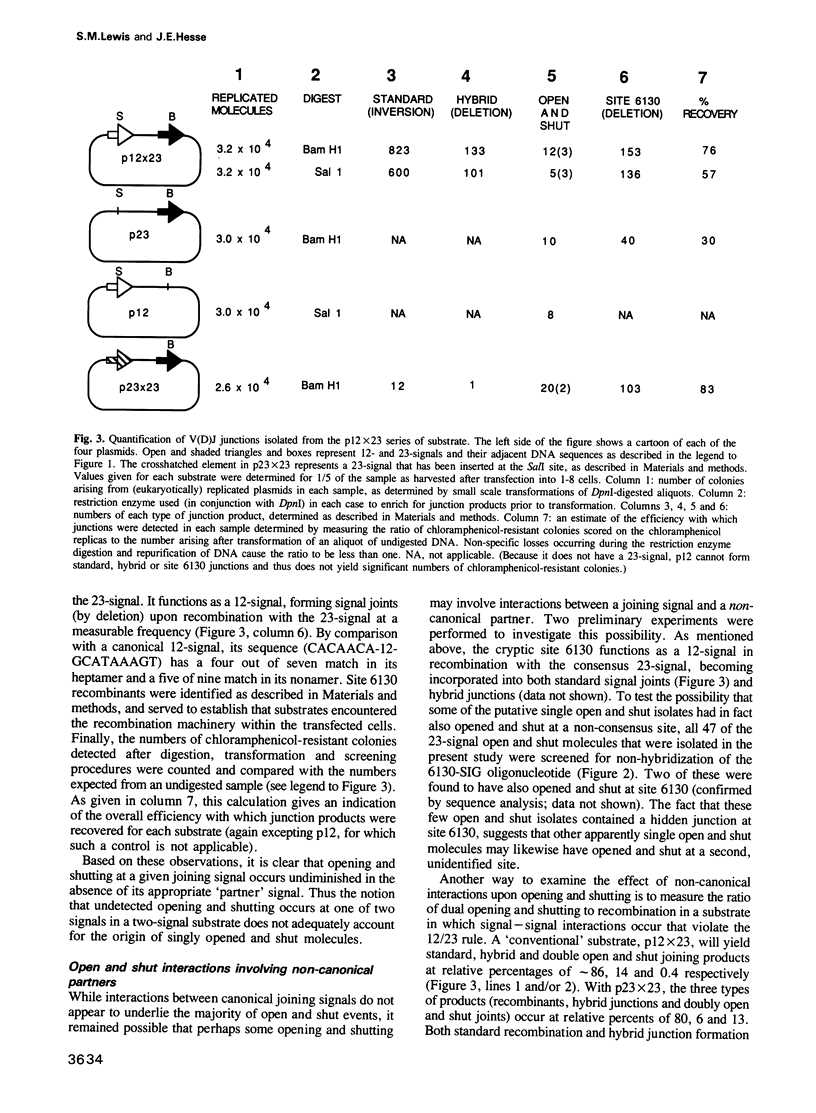
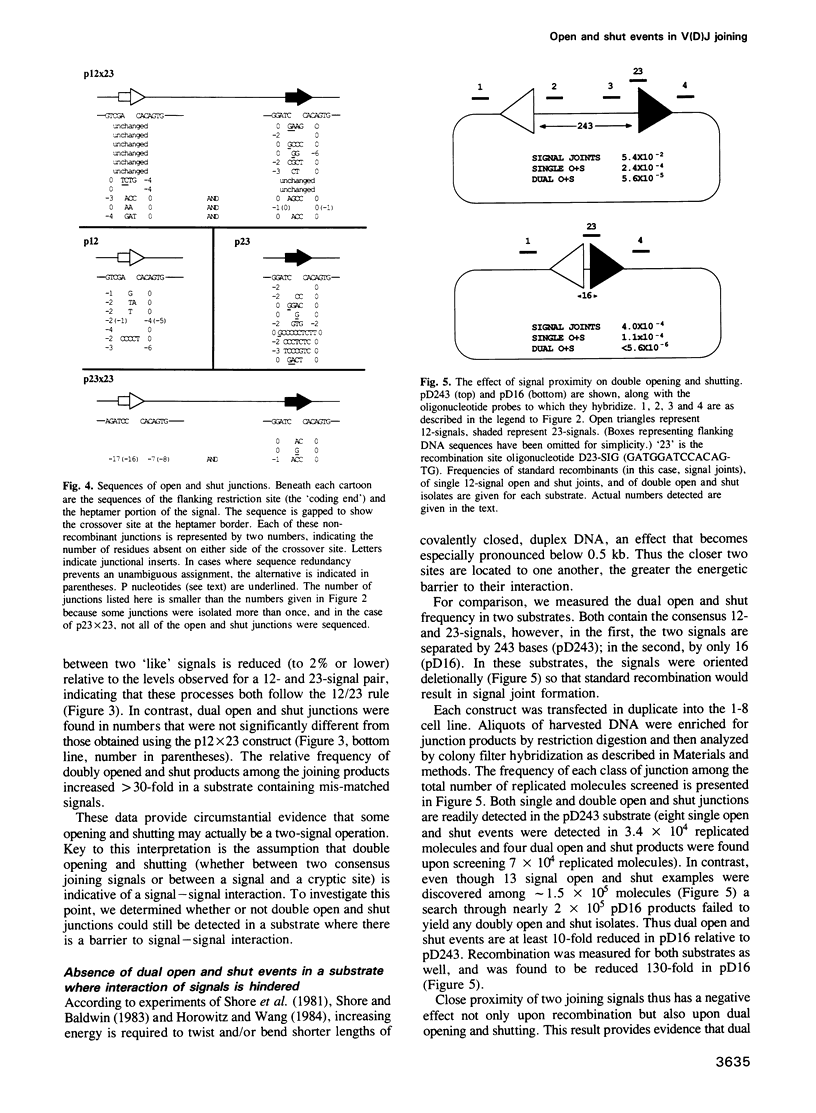
Open and shut junctions are rare (V(D)J joining products in which site-specific recognition, cleavage and re-ligation of joining signals has been uncoupled from recombination. Here, we investigate the relationship of opening and shutting to recombination in two ways. First, we have tested a series of substrates containing one or two joining signals in an in vivo assay. Opening and shutting can be readily observed in substrates that have only one consensus joining signal. Thus, unlike recombination, the majority of open and shut events do not require interactions between two canonical joining signals. Next we examined two-signal substrates to investigate the effect of signal proximity on the frequency of dual open and shut events. These experiments indicate that at least some of the time opening and shutting can be a two-signal transaction. Together these results point to two mechanistically related, but distinct origins for open and shut joining events. In one case, cutting and closing may occur without interaction between two signals. In the other, we suggest that interaction of a canonical signal with 'cryptic' signal-like elements whose sequence is extensively diverged from canonical signals, may bias the V(D)J recombination machinery towards opening and shutting rather than recombination. Open and shut operations could in this way provide a means whereby mistakes in target recognition by the V(D)J recombination machinery produce a non-recombinant outcome, avoiding deleterious chromosomal rearrangements in lymphoid tissues.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aplan P. D., Lombardi D. P., Ginsberg A. M., Cossman J., Bertness V. L., Kirsch I. R. Disruption of the human SCL locus by "illegitimate" V-(D)-J recombinase activity. Science. 1990 Dec 7;250(4986):1426–1429. doi: 10.1126/science.2255914. [DOI] [PubMed] [Google Scholar]
- Brown L., Cheng J. T., Chen Q., Siciliano M. J., Crist W., Buchanan G., Baer R. Site-specific recombination of the tal-1 gene is a common occurrence in human T cell leukemia. EMBO J. 1990 Oct;9(10):3343–3351. doi: 10.1002/j.1460-2075.1990.tb07535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig N. L. The mechanism of conservative site-specific recombination. Annu Rev Genet. 1988;22:77–105. doi: 10.1146/annurev.ge.22.120188.000453. [DOI] [PubMed] [Google Scholar]
- Hendrickson E. A., Liu V. F., Weaver D. T. Strand breaks without DNA rearrangement in V (D)J recombination. Mol Cell Biol. 1991 Jun;11(6):3155–3162. doi: 10.1128/mcb.11.6.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Gellert M., Mizuuchi K. Extrachromosomal DNA substrates in pre-B cells undergo inversion or deletion at immunoglobulin V-(D)-J joining signals. Cell. 1987 Jun 19;49(6):775–783. doi: 10.1016/0092-8674(87)90615-5. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Mizuuchi K., Gellert M. V(D)J recombination: a functional definition of the joining signals. Genes Dev. 1989 Jul;3(7):1053–1061. doi: 10.1101/gad.3.7.1053. [DOI] [PubMed] [Google Scholar]
- Horowitz D. S., Wang J. C. Torsional rigidity of DNA and length dependence of the free energy of DNA supercoiling. J Mol Biol. 1984 Feb 15;173(1):75–91. doi: 10.1016/0022-2836(84)90404-2. [DOI] [PubMed] [Google Scholar]
- Lafaille J. J., DeCloux A., Bonneville M., Takagaki Y., Tonegawa S. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989 Dec 1;59(5):859–870. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- Lewis S. M., Hesse J. E., Mizuuchi K., Gellert M. Novel strand exchanges in V(D)J recombination. Cell. 1988 Dec 23;55(6):1099–1107. doi: 10.1016/0092-8674(88)90254-1. [DOI] [PubMed] [Google Scholar]
- Lewis S., Gellert M. The mechanism of antigen receptor gene assembly. Cell. 1989 Nov 17;59(4):585–588. doi: 10.1016/0092-8674(89)90002-0. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Lewis S., Bosma G. C., Rosenberg N., Mizuuchi K., Bosma M. J., Gellert M. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988 Oct 7;55(1):7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Mizuuchi K., Gellert M. Developmental stage specificity of the lymphoid V(D)J recombination activity. Genes Dev. 1987 Oct;1(8):751–761. doi: 10.1101/gad.1.8.751. [DOI] [PubMed] [Google Scholar]
- McCormack W. T., Tjoelker L. W., Carlson L. M., Petryniak B., Barth C. F., Humphries E. H., Thompson C. B. Chicken IgL gene rearrangement involves deletion of a circular episome and addition of single nonrandom nucleotides to both coding segments. Cell. 1989 Mar 10;56(5):785–791. doi: 10.1016/0092-8674(89)90683-1. [DOI] [PubMed] [Google Scholar]
- Morzycka-Wroblewska E., Lee F. E., Desiderio S. V. Unusual immunoglobulin gene rearrangement leads to replacement of recombinational signal sequences. Science. 1988 Oct 14;242(4876):261–263. doi: 10.1126/science.3140378. [DOI] [PubMed] [Google Scholar]
- Roth M. E., Lacy M. J., McNeil L. K., Kranz D. M. Correction: selection of variable-joining region combinations in the T cell receptor. Science. 1989 Sep 8;245(4922):1032–1032. doi: 10.1126/science.2528208. [DOI] [PubMed] [Google Scholar]
- Roth M. E., Lacy M. J., McNeil L. K., Kranz D. M. Selection of variable-joining region combinations in the alpha chain of the T cell receptor. Science. 1988 Sep 9;241(4871):1354–1358. doi: 10.1126/science.2970673. [DOI] [PubMed] [Google Scholar]
- Shore D., Baldwin R. L. Energetics of DNA twisting. I. Relation between twist and cyclization probability. J Mol Biol. 1983 Nov 15;170(4):957–981. doi: 10.1016/s0022-2836(83)80198-3. [DOI] [PubMed] [Google Scholar]
- Shore D., Langowski J., Baldwin R. L. DNA flexibility studied by covalent closure of short fragments into circles. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4833–4837. doi: 10.1073/pnas.78.8.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]



