Abstract
Obese women exhibit decreased fertility, high miscarriage rates and dysfunctional corpus luteum (CL), but molecular mechanisms are poorly defined. We hypothesized that weight gain induces alterations in CL gene expression. RNA sequencing was used to identify changes in the CL transcriptome in the vervet monkey (Chlorocebus aethiops) during weight gain. 10 months of high-fat, high-fructose diet (HFHF) resulted in a 20% weight gain for HFHF animals vs. 2% for controls (p = 0.03) and a 66% increase in percent fat mass for HFHF group. Ovulation was confirmed at baseline and after intervention in all animals. CL were collected on luteal day 7–9 based on follicular phase estradiol peak. 432 mRNAs and 9 miRNAs were differentially expressed in response to HFHF diet. Specifically, miR-28, miR-26, and let-7b previously shown to inhibit sex steroid production in human granulosa cells, were up-regulated. Using integrated miRNA and gene expression analysis, we demonstrated changes in 52 coordinately regulated mRNA targets corresponding to opposite changes in miRNA. Specifically, 2 targets of miR-28 and 10 targets of miR-26 were down-regulated, including genes linked to follicular development, steroidogenesis, granulosa cell proliferation and survival. To the best of our knowledge, this is the first report of dietary-induced responses of the ovulating ovary to developing adiposity. The observed HFHF diet-induced changes were consistent with development of a dysfunctional CL and provide new mechanistic insights for decreased sex steroid production characteristic of obese women. MiRNAs may represent novel biomarkers of obesity-related subfertility and potential new avenues for therapeutic intervention.
Introduction
Over 300 million adult women are classified as obese worldwide [1]. Female obesity is frequently associated with ovulatory and menstrual dysfunction [2], increased congenital anomalies [3], as well as iatrogenic [4] and spontaneous preterm birth [5]. Adiposity exerts a harmful impact on reproductive function even if ovulatory capacity is preserved. Obese women with spontaneous and regular ovulation demonstrate increased pregnancy loss [6, 7], longer time to pregnancy [8], and decreased fertility [9]. Maternal obesity is also an independent risk factor for fetal origin of adult disease, including obesity and metabolic disorders in offspring [10]. Mechanisms for obesity-linked reproductive dysfunction may hold the key to avoiding its transgenerational impact and have considerable public health implications.
Obese women exhibit a state of relative hypogonadotropic hypogonadism [11–14]. Both pituitary and ovarian markers are affected as evidenced by reduced output of luteinizing hormone (LH) and luteal progesterone [13, 15]. In a large-scale evaluation of daily hormone patterns from ovulatory cycles, obese women exhibited greater than 50% decrease in luteal progesterone output and a 30% reduction in LH, when compared to their normal weight counterparts [16]. This implies that the ovary undergoes additional dysfunction in excess of that occurring at the hypothalamus and pituitary, yet molecular mechanisms remain unclear.
MicroRNAs (miRNAs) are non-coding RNAs that function post-transcriptionally [17] and are estimated to regulate up to 30% of all protein-coding genes [18]. In 2008, impairment of miRNA processing was demonstrated as a cause of corpus luteum (CL) insufficiency and infertility in mice [19]. Several more recent studies have shown that miRNA-mediated control of mRNA transcripts is critical for ovarian function [20–22]. Because of the diversity of miRNA targeting, identification of functionally important mRNA targets is crucial for recognizing tissue-specific roles for miRNAs [23]. Several miRNAs regulate ovarian sex steroid synthesis in vitro [24]. The role of miRNAs in mediating effects of adiposity on CL function has not been explored.
The vervet monkey (Chlorocebus aethiops sabaeus) is an Old World, nonhuman primate of the same subfamily as macaques. Adult females have an average intermenstrual interval of 30 days and exhibit cyclic hormonal changes that mimic women [25–28]. In a fully pedigreed and genotyped vervet research colony (VRC) of over 400 animals [29], vervets developed obesity and its associated metabolic profile in a manner very similar to humans [30]. Thus, the VRC represents a source of a potentially highly translatable primate model to elucidate the impact of obesity on various target organs, including the reproductive axis. We hypothesized that there are alterations in vervet CL gene expression that occur due to adiposity. MiRNA and mRNA differential expression patterns were compared between monkeys receiving either a high-fat, high-fructose (HFHF) or control diets.
We have documented changes in the CL gene expression in response to diet-induced weight gain. To the best of our knowledge, this is the first report of dietary-induced responses of the ovulating ovary to developing adiposity. Use of a menstrual non-human primate species allowed us to apply invasive investigative tools that cannot be practically used in humans. Thus, our work bridges a knowledge gap by addressing potential underlying molecular mechanisms for effects of obesity on ovarian function.
Materials and Methods
Animal Handling, Morphometrics and Urine collection
Ten adult female vervet monkeys (Chlorocebus aethiops sabaeus) were selected randomly from the middle of distribution of body mass from the Vervet Research Colony at the Wake Forest Primate Center (WFRC, Winston-Salem, NC). Monkeys were pair-housed at Wake Forest School of Medicine Primate Center / Center for Comparative Medicine and Research/ Friedberg Campus (Winston-Salem, NC). Monkeys were housed indoors in a climate controlled, temperature and humidity monitored room/building. The caging was USDA approved, steel Quad cages constructed of mesh flooring, removable dividers to allow horizontal movement between the two cages for each pair of monkeys, and a pan underneath to collect excrement and other waste. Monkeys were exposed to artificial lighting in a 12 hour, light-dark cycle from 6am to 6pm, with additional ambient light via windows in the hallway external to their housing room. Monkeys had ad-libitum access to water through water lixits and were fed 120kcal of experimental diet /kg of body weight once per day, as detailed in SI Materials and Methods. In addition, feeding and foraging opportunities were provided 3–4 times per week (fresh fruits & vegetables, popcorn, sunflower seeds). For environmental enrichment, all cages were equipped with perches inside and hanging mirrors and puzzle feeders on the outside of the cage. The WFRC Friedberg Campus is an AAALAC-accredited facility and all housing is AAALAC-and FDA approved. All monkeys were within the normal weight range for this species and were sexually mature with a mean age of 6.8 years (Table 1) as detailed further in SI Materials and Methods. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by the WFRC Animal Care and Use Committee. All policies and procedures were done in compliance with state and federal laws, and regulations and guidelines established by the WFRC Animal Care and Use Committee (Protocol number A10-093). Wake Forest University is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC: Assurance number A3391-01). No adverse events occurred. After the study, all animals were examined and released back into the colony.
Table 1. Biometric and Metabolic Characteristics Before and After Dietary Intervention*.
| Variable | Control Diet (n = 4) | HFHF Diet (n = 6) | |
|---|---|---|---|
| Age (years) | Baseline | 7.8 (1.2) | 6.2 (0.9) |
| Body weight (kg) | Baseline | 5.1 (0.5) 0.72 | 4.4 (0.2) 0.72 |
| 10 months | 5.3 (0.4) 0.72 | 5.2 (0.2) 0.02 | |
| Percent change | 2.4 (4.1) | 19.6 (7.0) 0.03 | |
| Waist circumference (cm) | Baseline | 28.9 (2.4) 0.72 | 25.4 (1.1) |
| 10 months | 29.0 (1.3) 0.93 | 30.0 (1.2) 0.03 | |
| Percent change | 1.9 (6.0) | 19.0 (6.6) 0.20 | |
| Total Body Fat Mass (g) | Baseline | 999.4 (179.4) 0. | 682.6 (83.2) |
| 10 months | 1160.4 (129.3) 0.27 | 1310.8 (183.6) 0.02 | |
| Percent change | 23.1 (17.1) | 105.5 (40.5) 0.07 | |
| Percentage Body Fat | Baseline | 19.1 (2.2) 0.72 | 15.1 (1.3) |
| 10 months | 21.5 (1.0) 0.39 | 24.3 (2.7) 0.02 | |
| Percent change | 16.7(12.0) | 65.9 (22.3)0.11 | |
| Triglycerides (mg/dL) | Baseline | 51.3 (3.9) 0.72 | 33.8 (1.6) |
| 10 months | 36.0 (4.5) 0.09 | 68.0 (25.7) 0.03 | |
| Percent change | -27.6 (12.1) | 104.2 (78.5) 0.01 | |
| Total cholesterol (mg/dL) | Baseline | 168.3 (6.1) 0.72 | 151.2 (10.0) |
| 10 months | 163.0 (7.7) 0.67 | 158.2 (11.1) 0.42 | |
| Percent change | -2.5 (6.8) | 5.1 (4.8) 0.39 | |
| Total Adiponectin (ng/mL) | Baseline | 49,602 (4931) 0.72 | 57,548 (5568) 0.72 |
| 10 months | 46,445 (6179) 0.68 | 46,166 (6841) 0.13 | |
| Percent change | -4.4 (14.6) | -19.3 (11.8) 0.44 | |
Values indicate mean (standard error of mean). HFHF, High fat high fructose
* Superscripts are P values for within the group comparisons for 10 month values (vs. baseline) and for between the group comparisons for percent change values (control vs. HFHF diet)
Serum and Urine Analytes and Assays
The urinary excretion of sex steroids closely corresponds to the serum concentrations of the parent hormones [31]. Urine was assayed for estrone conjugates (E1c) and pregnanediol glucuronide (Pdg) using previously described methods [32], as detailed further in SI Materials and Methods (S2 Table).
Luteectomy
In order to time the luteal phase, serum estradiol levels were measured daily from menstrual cycle day 7 during the luteectomy cycle. The first day of low serum estradiol (defined as values less than 100 pg/ml [33]) after the midcycle estradiol peak was denoted day 1 of the luteal phase [27]. Luteectomies were performed twice on all monkeys, at baseline and after dietary intervention. A laparotomy approach was used to collect CL tissue on luteal day 7–9 as detailed further in SI Materials and Methods (S1 Fig).
Dietary Intervention
At baseline, the monkeys were fed a commercial non-human primate diet (Purina Monkey Chow) once daily in the afternoon. After completion of the baseline procedures, monkeys were randomly assigned to either adipogenic HFHF diet (n = 6), similar to that used for previous studies in baboons [34] and cynomolgus monkeys [35] or a control diet (n = 4) (Table 2) as detailed further in SI Materials and Methods (S1 Table).
Table 2. Composition of Control and High Fat High Fructose (HFHF) Experimental Diets.
| Diet | Caloric Density | Protein (% Kcal) | Carbohydrate (% Kcal) | Simple sugars (% Kcal) | Fat (% Kcal) | Fiber (% of diet) |
|---|---|---|---|---|---|---|
| Control | 2.3 † | 17.6 | 57 | 11 | 26 | 12.8 |
| HFHF | 2.7 † | 17.1 | 45 | 30 | 38 | 10.5 |
| Drink | 0.60* | 0 | 100 | 100 | 0 | 0 |
Monkeys were fed 120 Kcal of diet / kg of body weight per day plus 10% to account for waste. Simple sugars were derived from sucrose (3% for both diets) and high fructose corn syrup (HFCS; 2.4% for control and 10% for HFHF). In addition to the diet, monkeys in the HFHF group were given daily access to a Kool-Aid drink containing 15ml of HFCS / 100ml of water, providing 150–250 additional Kcal per day.
† Kcal/g of diet
* Kcal/ml
RNA isolation, Library Preparation, and Sequencing
CL total RNA was extracted using TRIzol method followed by purification using MirVana RNA Isolation Kit (Ambion) as detailed further in SI Materials and Methods.
Computational Analysis and Quality Control. mRNA profiling
All sequence reads were trimmed to an overall quality score of Q15 [36], and any sequences that were trimmed to less than 75bp were subsequently removed, as detailed further in SI Materials and Methods (S4 Table).
miRNA profiling
As the miRNA sequence reads are shorter than the 50bp reads generated, the first step in quality assessment of the miRNA reads was to identify the reverse complement of the reverse sequencing adapter. As no vervet monkey-specific miRNA databases exist, it was necessary to take an agnostic approach to find all small RNAs that might be differentially expressed. Thus reads that passed quality filtering were analyzed with a custom Python script to identify unique sequences, and produce a normalized read count using DESeq normalization [37] as detailed further in SI Materials and Methods (S5 Table).
Genome Annotation
Transcript sequencing output was mapped with the current vervet genome assembly (Chlorocebus_sabeus 1.0). We utilized the Vervet Genome Sequencing Project [38] as detailed further in SI Materials and Methods.
Target Gene Prediction and Integrated Analysis
The selected miRNAs that were differentially expressed were further analyzed to identify the networks and pathways targets as detailed further in SI Materials and Methods.
Results
Acquisition of Adiposity Following 10 Months of HFHF Diet
Animals were given either HFHF or control diet (Table 1, S1 Table) after baseline studies were completed (S2 Table). Monkeys in the intervention group gained fat mass and body weight, increased their waist circumference, and developed a significant increase in serum triglycerides (Table 2). On average, dietary intervention resulted in a 20% weight gain for HFHF-fed monkeys vs. 2% in controls (p = 0.03). There was a 66% increase in percent body fat for HFHF group vs. 17% for control. While fasting glucose and total cholesterol were not significantly affected, the reduction in total adiponectin (-19%) did approach statistical significance for the experimental group (p = 0.13). Assessment of reproductive hormones showed no differences between the two groups with respect to menses or serum AMH. All animals were ovulatory before and after intervention, based on assays of sex steroid metabolites from serial urine collections. Indices of estrogen excretion and luteal phase progesterone excretion were similar before and after intervention (S2 Table). This implied that our model is best suited to assess early, pre-clinical CL response to de novo adiposity.
To evaluate changes in CL gene expression that mediate adiposity-related reduction in reproductive fitness, we performed luteectomy at baseline and after the dietary intervention. For each analyzed cycle, lifespan of CL was timed by daily serum estradiol (E2) in the follicular phase to document midcycle E2 surge and subsequent E2 drop corresponding to LH surge [39]. All luteectomy procedures were conducted on luteal day 7–9 as this corresponds to a mid-stage, fully functioning CL based on dynamic transcript changes during CL developmental phases in the rhesus macaque [33]. RNA sequencing was conducted on the obtained CL tissue by paired assessments of the same animal. Joint genomic profiling of mRNA and miRNA was done to evaluate the initial adaptive changes of the ovulating ovary to weight gain.
mRNA Expression Changes with Adiposity, Weight Gain and Fat Mass Gain
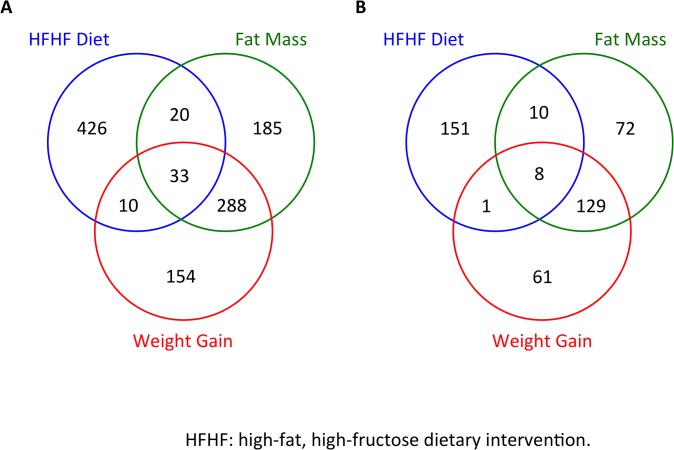
Using RNA sequencing, 61.8 to 101.7 million total single-paired end reads per sample were received and 48.6 to 88.1 million reads were mappable to the draft vervet genome [18]. Approximately 1100 mRNA exhibited significant changes in response (p<0.05, FDR<0.15) to the HFHF diet within the CL or correlated with increases in body weight and/or fat mass (Fig 1A). Of these, 432 sequences were identified and annotated by homology to the human genome Fig 1B). Analysis of the transcriptome in each category (diet, weight gain and fat mass gain) identified subsets of differentially expressed genes (DEG). As expected, the majority of genes correlating with weight gain overlapped with those associated with increased fat mass and/or diet allocation. However, we also observed specific, mutually exclusive, subsets of genes responsive to dietary intervention, fat mass or weight gain only (S3 Table).
Fig 1. Venn Diagrams for Total Differentially Expressed Genes by Diet, Weight Gain and Fat Mass.
A. all vervet mRNAs. B. all mRNAs that were annotated to human genes. (p,0.05, FDR<0.15).
Observed Changes in miRNA Gene Expression were Consistent with Development of Dysfunctional CL
Sequencing of the small RNA fraction identified 50 miRNAs, based upon homology to their human counterparts, of which 9 were differentially expressed (p<0.05, FDR<0.15) in response to HFHF diet (Table 3). These included members of the Let 7 family, miR-26a and miR-143, which are among most abundant miRNAs found in mouse, bovine, sheep and human ovaries [40–43]. Notably, several miRNAs induced in response to the HFHF diet were consistent with the development of dysfunctional CL. Specifically, Let-7b and miR -28 have been shown to inhibit progesterone and testosterone production in human granulosa cells (GC), while miR-26a and miR-28 suppress estrogen secretion [44–46]. Similarly, expression of let-7b, miR-26a, miR-28 and miR-143 were previously associated with decreased proliferation of GC, while let7b and miR-26a were found to promote GC apoptosis[45–47]. Additionally, we identified small nucleolar RNAs, splicing factors and several sequences, present in the vervet and other primate genomes which lack a human homolog; these may represent novel species specific miRs [48]. Several tRNA-derived fragments (tRFs) [49, 50], which are postulated to play a role in gene silencing mechanisms by interacting with canonical miR pathways [51, 52], also exhibited changes in abundance in response to the HFHF diet.
Table 3. Differentially Expressed Corpus Luteum miRNAs after High Fat High Fructose Diet.
| miRNA | Fold Change | P |
|---|---|---|
| let7b-5p | 56 | 0.002 |
| let7e-5p | 17,320 | 0.016 |
| 26a-5p | 61,129 | 0.008 |
| 28-3p | 4,381 | 0.008 |
| 143-5p | 166,866 | 0.0001 |
| 186-5p | 298 | 0.004 |
| 7193-5p | 145,501 | 0.017 |
| 193b-3p | -15,448 | 0.017 |
| 486-5p | -25,555 | 0.015 |
Integrated miRNA and mRNA Analysis
We used Ingenuity software to identify concordant changes in miRNAs and mRNAs. This approach evaluated an increase in any miRNA that was reflected by a corresponding reduction in its predicted target mRNA and vice versa. Among the 432 mRNAs differentially expressed in the vervet CL, changes in 52 were consistent with these criteria (Table 4). Among the 9 differentially expressed miRNAs, changes in 5 were consistent with corresponding changes in their mRNA targets. These miRNAs had 3 possible categorizations based on relevancy to luteal physiology and adiposity: (1) known links to both CL and adiposity; known links to (2) CL only or (3) adiposity only.
Table 4. Integrated Analysis of miRNAs and their mRNA Targets after High Fat High Fructose Diet.
| MicroRNA (Fold Change) | Target mRNA Gene Symbol (Name) | Fold Change |
|---|---|---|
| hsa-miR-186-5p (298) | NAA38 (LSM8 homolog, U6 small nuclear RNA associated) | -29.0 |
| UBE2B (ubiquitin-conjugating enzyme E2B) | -22.0 | |
| FAM204A (family with sequence similarity 204, member A) | -7.8 | |
| EIF2S2 (eukaryotic translation initiation factor 2, subunit 2 beta) | -4.4 | |
| MPC2 (mitochondrial pyruvate carrier 2) | -3.3 | |
| ACTR10 (actin-related protein 10 homolog) | -2.8 | |
| PDCD10 (programmed cell death 10) | -2.8 | |
| hsa-miR-193b-3p (-15,448) | SRSF6 (serine/arginine-rich splicing factor 6) | 41.0 |
| TRIB2 (tribbles pseudokinase 2) | 12.0 | |
| UBP1 (upstream binding protein 1 (LBP-1a) | 12.0 | |
| TAOK2 (TAO kinase 2) | 7.0 | |
| SCARF1 (scavenger receptor class F, member 1) | 6.0 | |
| GABPA (GA binding protein transcription factor, α subunit) 60kDa) | 5.0 | |
| GPANK1(G patch domain and ankyrin repeats 1) | 5.0 | |
| PPM1F (protein phosphatase, Mg2+/Mn2+ dependent, 1F) | 5.0 | |
| STX16 (syntaxin 16) | 5.0 | |
| ABI2 (abl-interactor 2) | 4.0 | |
| CCNG2 (cyclin G2) | 4.0 | |
| FGF12 (fibroblast growth factor 12) | 4.0 | |
| CREBRF (CREB3 regulatory factor) | 3.0 | |
| FAM53C (family with sequence similarity 53, member C) | 3.0 | |
| RAPGEF5 (Rap guanine nucleotide exchange factor (GEF) 5) | 3.0 | |
| RGL1 (ral guanine nucleotide dissociation stimulator-like 1) | 3.0 | |
| ZBTB40 (zinc finger and BTB domain containing 40) | 3.0 | |
| DLG1 (discs, large homolog 1 (Drosophila) | 2.0 | |
| DYRK1A (dual-specificity TYR-(Y)-phos. regulated kinase 1A) | 2.0 | |
| ERAP2 (endoplasmic reticulum aminopeptidase 2) | 2.0 | |
| JAK2 (Janus kinase 2) | 2.0 | |
| SLC23A2 (solute carrier family 23,ascorbiate transporter, member 2 | 2.0 | |
| ZNF562 (zinc finger protein 562) | 2.0 | |
| hsa-miR-26a-5p (61,129) | MSMO1 (methylsterol monooxygenase 1) | -15.0 |
| VMA21 (VMA21 vacuolar H+-ATPase homolog) | -9.9 | |
| NT5DC1 (5'-nucleotidase domain containing 1) | -9.0 | |
| MAT2A (methionine adenosyltransferase II, alpha) | -6.0 | |
| BCCIP (BRCA2 and CDKN1A interacting protein) | -4.9 | |
| MTFMT (mitochondrial methionyl-tRNA. Fformyltransferase) | -4.6 | |
| B4GALT4 (UDP-Gal:betaGlcNAc beta 1,4- GST, polypeptide 4 | -3.4 | |
| MCUR1 (mitochondrial calcium uniporter regulator 1) | -3.0 | |
| PDCD10 (programmed cell death 10) | -2.8 | |
| SRP19 (signal recognition particle 19kDa) | -2.6 | |
| hsa-miR-28-3p (4,831) | BCCIP (BRCA2 and CDKN1A interacting prtein) protein | -4.9 |
| PARL (presenilin associated, rhomboid-like) | -4.8 | |
| hsa-miR-486-5p (-25,555) | PTEN (phosphatase and tensin homolog) | 6.0 |
| FLRT2 (fibronectin leucine rich transmembrane protein 2) | 5.0 | |
| ARHGAP5 (Rho GTPase activating protein 5) | 4.0 | |
| ZNF701 (zinc finger protein 701) | 4.0 | |
| GRAP (GRB2-related adaptor protein) | 3.0 | |
| TEK (TEK tyrosine kinase, endothelial) | 3.0 | |
| TTC31 (tetratricopeptide repeat domain 31) | 3.0 | |
| VPS37B (vacuolar protein sorting 37 homolog B) | 3.0 | |
| DYRK1A (dual-specificity TYR-(Y)-phos. regulated kinase 1A) | 2.0 | |
| PPP1R16A (protein phosphatase 1, regulatory subunit 16A) | 2.0 |
Among the miRNAs that have been individually shown to be important for both CL function and adiposity, changes in miR-26 and miR-28 were notable. In addition to inhibition of sex steroid production in vitro by both of these miRNA [45], miR-28 is over expressed during the E2 drop following dominant follicle selection [53]. In our setting, both were up-regulated in response to HFHF diet, which is consistent with dysregulation of CL function. A notable down-regulated mRNA target of miR-28 was PARL, a critical regulator of mitochondrial morphology and function. Reduced PARL levels correlate with mitochondrial abnormalities in obesity and are linked with insulin resistance [54–56]. Maternal obesity and a high fat diet resulted in decreased expression of PARL in rats and it may play a role in metabolic programming [54]. Another down-regulated mRNA target of miR-28 was BCCIP, a cofactor for BRCA2, which functions as a progesterone-responsive gene involved in DNA repair and cell cycle control [57]. Among miR-26 targets, genes with the highest level of down-regulation were MSMO1 and VMA21. MSMO1 mediates LH stimulation of cholesterol biosynthesis [58, 59]. Deficiency in VMA21 results in impaired autophagy and endoplasmic reticulum stress [60, 61] and is associated with development of metabolic syndrome [62]. Taken together, our observed down-regulated mRNA targets of up-regulated miRNAs provide new mechanistic links between weight gain and CL function.
Among the miRNAs with known impact on CL function, only miR-186 was up-regulated. In follicular fluid, miR-186 increases in response to exogenous progesterone after ovulation [63]. EIF2S2, an mRNA target of miR-186 was down-regulated in this study. It has been implicated in differentiation of granulosa cells [64] and has been causally linked to a genetic variant of diminished ovarian reserve [65]. EIF2S2 is a translation initiation factor that functions in the early steps of protein synthesis. It regulates angiogenesis via VEGF signaling due to accumulation of denatured proteins in stress and its dysfunction induces apoptosis of follicles [66]. Thus, down-regulation of EIF2S2 implies decreased CL formation due to decreased angiogenesis.
Among the miRNA affected only in adiposity, miR-486 was down-regulated. MiR-486 has been shown to inhibit adipogenesis in human in vitro and animal in vivo obesity models [67, 68]. Thus, down-regulation of miR-486 may promote adipogenesis. In our setting, several of its up-regulated mRNA targets with known impact on CL function were detected. The target mRNA with the highest up-regulation was PTEN, a tumor suppressor and cell cycle regulator that inhibits CL granulosa cell differentiation and survival [69, 70]. Similarly, TEK/Tie2, an angiopoietin receptor, is implicated in CL angiogenesis and may mediate follicular atresia [71].
After HGHF diet, miR-193 was significantly down-regulated. It is down-regulated in adipose tissue from obese patients and is negatively correlated with BMI [72, 73]. Among its up-regulated mRNA targets, several were notable for known links with either CL function or diet-induced adiposity. Increased CREBRF (Luman recruiting factor) promotes apoptosis of granulosa cells [74]. TRIB2 is known to promote visceral and ectopic fat accumulation [75, 76] and is implicated in dominant follicle selection [77]. Other up-regulated genes that are also targets of miR-193 included mediators of obesity, related inflammatory cytokine and leptin signaling (JAK2) [78, 79] as well as atherosclerosis (SCARF2/SREC-I) [80].
Discussion
In this 10 month-long dietary intervention study, we have established a nonhuman primate (NHP) model to examine de novo weight gain in relation to luteal physiology. Due to their close phylogenetic relationship to humans, NHP are of special interest in modeling human disease. In studies of female reproduction, it is advantageous to use NHP models as they exhibit close similarities in the endocrine control of the menstrual cycle with regulatory mechanisms that are distinct from estrous species [81, 82]. Vervets are a small NHP species with a well-characterized menstrual cycle that mimics women in length and hormonal regulation [25–28]. Development of obesity in long-term vervet studies demonstrated an associated metabolic profile analogous to humans [30]. Our model allowed for invasive interventions, removal of the meticulously timed CL to collect tissue for analysis and paired design to maximize power in this costly setting. As ovulation was confirmed in all luteectomy cycles, this is the first study to uncover the response of the ovulating ovary to weight gain. Importantly, obesity is common in ovulatory women [8], leads to profound consequences on offspring health [10] and has not been adequately examined with respect to CL function. Thus, our work bridges a knowledge gap by creating a model of diet-induced weight gain and its effects on ovarian function. Use of a menstrual NHP species allowed us to apply invasive investigative tools that cannot be practically used in humans.
Monkeys consuming HFHF diet more than doubled their total body fat mass (106%). The average weight gain (20%) and the average percentage of body fat gain (66%) in the intervention group were comparable to other NHP high-fat feeding experiments [39, 83, 84]. While multiple NHP studies have examined the impact of adiposity on various organ systems, this is the first report of the ovary as the principal target organ, and represents the first comprehensive analysis of changes in expression of mRNAs and non-coding RNAs in the vervet CL in response to diet-induced weight gain.
Based upon homology to the human genome, we identified and jointly analyzed both mRNAs and 50 unique miRNAs that were expressed at detectable levels in the vervet CL. Specific changes in miRNA and mRNA expression profiles were consistent with impaired folliculogenesis and sex steroid function. Overall, HFHF diet-induced DEGs were consistent with a dysfunctional CL. We identified a multitude of genes highly relevant for luteal physiology, thus substantiating the choice of the model and approach used as profiling of vervet CL has not been published before. The DEGs included genes linked to premature ovarian failure (NTRK2)[85, 86], polycystic ovary syndrome (MEF2A)[87], signaling pathways regulating follicular development and angiogenesis, growth and survival of granulosa cells, and responses to gonadotropins (BAK1[88], BMP1[89], BMP4[89, 90], GADD45A[91], IFNGR1[92], IGFBP1[93], JAK2[94], MAPILC3A[95, 96], NOS3[97, 98], NOTCH2[99], PLK2[100, 101], PTEN[70], SFRP4[102], VCAN[103], YY1[104]). Further, transcripts implicated in steroidogenesis, cholesterol biosynthesis, transport and metabolism (AUP1[105], FDFT1[106], PAWR[107], SLCO2B1[108], VAMP4[109]), progesterone synthesis (BLVRB[110], BMP4[90]) and linked to obesity-related reduced fertility (FANCC[111], PPT1[112], SFRP4[102]) exhibited significant changes in expression (p<0.05, FDR<0.15). This underscores physiological relevance and utility of this model for further exploration in targeted and longer-term studies of high-fat feeding and its effects of hypothalamic-pituitary-ovarian axis, gestation and neonatal health.
Our CL miRNA data are novel with respect to the response to the intervention and joint analysis of target- miRNA interactions. While some of observed DEGs were previously reported in studies of either obesity or reproduction separately, we expand these data by the newly identified co-occurring links between the metabolic and reproductive axes.
Published reports suggest that let7b, miR-26a and miR-28 inhibit sex steroid secretion from GC, while let7b, miR-26a, miR-28 and miR-143 decrease GC proliferation and let7b and miR-26a promote GC apoptosis [46]. Providing mechanistic insight into impaired CL function with weight gain, several down regulated target genes were observed coincident with up-regulation of their predicted miRNA regulators. Several such genes represent our DEGs with the highest level of expression in response to HFHF and are known to regulate CL angiogenesis and differentiation, as well as cholesterol biosynthesis in response to LH, CL angiogenesis and differentiation. Specifically, down-regulated DEGs likely to regulate CL dysfunction include MSMO1 (methylsterol monooxygenase located in endoplasmic reticulum membrane, involved in cholesterol biosynthesis and induced by LH in GCs [58, 59]), VMA21 (vacuolar ATPase deficiency linked to autophagy and ER stress and metabolic syndrome [60–62]), and PARL (an integral mitochondrial protein that decreases mitochondrial abundance and integrity in response to insulin resistance [54–56]).
Conversely, CREBRF and PTEN were up-regulated and observed jointly with down-regulation of their predicted miRNA regulators, miR-193 and miR-486, respectively. CREBRF regulates apoptosis of GC cells [74], while ovary-specific deletion of PTEN causes premature activation of the primordial ovarian follicles [113], a process that is linked with diminished ovarian reserve and function and important for follicular atresia [114]. Other observed changes in DEGs were also consistent with systemic changes in metabolic regulation, mitochondrial dysfunction and ER stress, inflammation and lipotoxicity, which may underlie the reported decrease in ovarian function, steroid hormone secretion and concomitant reduction in fertility in obese women [115–118].
Limitations of our study included the fact that sequencing coverage was comprehensive but restricted by incomplete annotation of the vervet genome. Approximately 60% of potential mRNA sequences present in the draft vervet genome remain unannotated after human genome lift-over. Thus, we relied on genes with the highest homology to the human reference (i.e. the human lift-over) for both mRNA and miRNA analyses. While Next Generation sequencing of RNAs has been shown to be highly reproducible with little technical variation [119, 120], the limited amount of CL tissue obtained from the surgical procedures required the entire specimen to be used to provide sufficient materials for library preparation and sequencing analysis. Animal welfare and economic considerations did not allow procurement of additional independent samples for secondary analyses of protein or transcript levels. We initially hypothesized that weight gain due to HFHF diet would lead to decreased ovarian sex steroid secretion, as observed in obese ovulatory women [12, 16, 121]. However, our assessment of reproductive hormones did not reveal any significant changes after intervention, possibly due to inter-individual variation in hormone levels within each group and insufficient time of dietary intervention to develop overt reproductive endocrine dysfunction. Nonetheless, our observed phenotypic variation in response to dietary intervention is similar to that observed in humans and was unequivocally accompanied by significant weight, body fat and metabolic changes. Thus, we have established that our model represents an early, pre-clinical response of the ovarian transcriptome to an acute gain in adiposity.
In summary, we have demonstrated that a 10 month administration of an adipogenic diet results in doubling of fat mass, significant weight gain and substantial gene expression changes in the vervet CL. We report hitherto unreported links for genes and miRNAs regulating both luteal physiology and response to adiposity. The current report bridges a knowledge gap by creating a model of diet-induced weight gain and its effects on ovarian function in a menstrual NHP species with confirmed ovulation in each analyzed cycle. A critical shortcoming of using in vivo animal work to model women’s reproductive health is the long duration required for oocyte recruitment, CL timespan and gestation in women. Unlike rodents, vervets exhibit a well-characterized menstrual cycle with secretion of ovarian steroids and patterns of pituitary gonadotropins that mimic those of women. Vervets also develop obesity and associated metabolic profile similar to humans, while allowing invasive tissue acquisition for analysis. Using this model, we have documented for the first time changes in the CL transcriptome in response to diet-induced weight gain, which provide insight into the potential mechanisms mediating the CL dysfunction characteristic of obesity in humans. Alterations in miRNA expression that are implicated in regulation of folliculogenesis, sex steroid synthesis, and their corresponding target mRNA levels may underlie reduced fertility and adverse pregnancy outcomes linked to obesity. Future studies are well positioned to expand this model with full annotation of the whole vervet genome sequencing and mapping of more genes. The attractiveness of using this NHP model for reproductive studies is underscored by the phylogenetic link to people, close similarity of reproductive endocrine dynamics and the ability to apply powerful investigative tools that cannot practically be used in humans.
Supporting Information
(TIFF)
(DOCX)
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors gratefully acknowledge Drs. Richard Stouffer, Nanette Santoro and Jay Kaplan for sharing their expertise in reproductive physiology due the overall planning of this project. We thank Dr. Kylie Kavanagh for her expertise regarding obesity and metabolic perturbations in vervet monkeys and Dewayne Cairnes, Edison Floyd, Debbie Golden and Maryanne Post for technical contributions. We thank Matt Jorgensen for facilitating the screening and acquisition of subjects from the Wake Forest Vervet Research Colony. We give special thanks to Drs. Alberto Carrillo and Tamer Yalcinkaya as well as Julie Miller (Wake Forest Reproductive Medicine Laboratory) for assistance with serum estradiol assays. We thank Drs. Anthony Comuzzie and Dr. Maureen Charron for lending their expertise in planning of the dietary intervention and gene expression experiments. The authors also gratefully acknowledge the assistance of Drs. Bill Lasley, Nancy Gee and the Endocrine Core at the California National Primate Research Center, who supplied conjugate and antibody for the urinary steroid assays. Finally, we thank Drs. Nelson B Freimer and Anna Jasinska for assistance with vervet genome database.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by: NIH R21HD060944 (AJP and SEA), and the VRC grant RR019963 (currently OD010965). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. World Health Organisation. World Health Statistics 2012: WHO Press. [Google Scholar]
- 2. Lake JK, Power C, Cole TJ. Women's reproductive health: the role of body mass index in early and adult life. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1997;21(6):432–8. . [DOI] [PubMed] [Google Scholar]
- 3. Stothard KJ, Tennant PWG, Bell R, Rankin J. Maternal Overweight and Obesity and the Risk of Congenital Anomalies. JAMA: The Journal of the American Medical Association. 2009;301(6):636–50. 10.1001/jama.2009.113 [DOI] [PubMed] [Google Scholar]
- 4. Smith GC, Shah I, Pell JP, Crossley JA, Dobbie R. Maternal obesity in early pregnancy and risk of spontaneous and elective preterm deliveries: a retrospective cohort study. Am J Public Health. 2007;97(1):157–62. Epub 2006/12/02. 10.2105/ajph.2005.074294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cnattingius S, Villamor E, Johansson S, Edstedt Bonamy AK, Persson M, Wikstrom AK, et al. Maternal obesity and risk of preterm delivery. JAMA. 2013;309(22):2362–70. Epub 2013/06/13. 10.1001/jama.2013.6295 . [DOI] [PubMed] [Google Scholar]
- 6. Metwally M, Saravelos SH, Ledger WL, Li TC. Body mass index and risk of miscarriage in women with recurrent miscarriage. Fertility and sterility. 2010;94(1):290–5. 10.1016/j.fertnstert.2009.03.021 [DOI] [PubMed] [Google Scholar]
- 7. Metwally M, Ong KJ, Ledger WL, Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertility and sterility. 2008;90(3):714–26. . [DOI] [PubMed] [Google Scholar]
- 8. Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Human Reproduction. 2007;22(2):414–20. 10.1093/humrep/del400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Polotsky AJ, Hailpern SM, Skurnick JH, Lo JC, Sternfeld B, Santoro N. Association of adolescent obesity and lifetime nulliparity—The Study of Women's Health Across the Nation (SWAN). Fertility and sterility. 2010;93(6):2004–11. 10.1016/j.fertnstert.2008.12.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heerwagen MJR, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology. 2010;299(3):R711–R22. 10.1152/ajpregu.00310.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tworoger SS, Eliassen AH, Missmer SA, Baer H, Rich-Edwards J, Michels KB, et al. Birthweight and body size throughout life in relation to sex hormones and prolactin concentrations in premenopausal women. Cancer Epidemiology Biomarkers & Prevention. 2006;15(12):2494–501. [DOI] [PubMed] [Google Scholar]
- 12. Yeung E, Zhang C, Albert P, Mumford S, Ye A, Perkins N, et al. Adiposity and sex hormones across the menstrual cycle: the BioCycle Study. International Journal of Obesity. 2012;37(2):237–43. 10.1038/ijo.2012.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Santoro N, Lasley B, McConnell D, Allsworth J, Crawford S, Gold EB, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women's Health across the Nation (SWAN) Daily Hormone Study. Journal of Clinical Endocrinology & Metabolism. 2004;89(6):2622–31. . [DOI] [PubMed] [Google Scholar]
- 14. Bordini B, Littlejohn E, Rosenfield RL. Blunted sleep-related luteinizing hormone rise in healthy premenarcheal pubertal girls with elevated body mass index. Journal of Clinical Endocrinology & Metabolism. 2009;94(4):1168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taylor AE, McCourt B, Martin KA, Anderson EJ, Adams JM, Schoenfeld D, et al. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism. 1997;82(7):2248–56. [DOI] [PubMed] [Google Scholar]
- 16. Jain A, Polotsky AJ, Rochester D, Berga SL, Loucks T, Zeitlian G, et al. Pulsatile Luteinizing Hormone Amplitude and Progesterone Metabolite Excretion Are Reduced in Obese Women. Journal of Clinical Endocrinology & Metabolism. 2007;92(7):2468–73. 10.1210/jc.2006-2274 [DOI] [PubMed] [Google Scholar]
- 17. Iwanaga R, Wang CA, Micalizzi DS, Harrell JC, Jedlicka P, Sartorius CA, et al. Expression of Six1 in luminal breast cancers predicts poor prognosis and promotes increases in tumor initiating cells by activation of extracellular signal-regulated kinase and transforming growth factor-beta signaling pathways. Breast Cancer Res. 2012;14(4):R100 Epub 2012/07/07. 10.1186/bcr3219 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lewis BP, Shih I-h, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115(7):787–98. [DOI] [PubMed] [Google Scholar]
- 19. Otsuka M, Zheng M, Hayashi M, Lee JD, Yoshino O, Lin S, et al. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. The Journal of clinical investigation. 2008;118(5):1944–54. 10.1172/JCI33680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Korch C, Spillman MA, Jackson TA, Jacobsen BM, Murphy SK, Lessey BA, et al. DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification, redundancy and contamination. Gynecologic Oncology. 2012;127(1):241–8. 10.1016/j.ygyno.2012.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lei L, Jin S, Gonzalez G, Behringer RR, Woodruff TK. The regulatory role of Dicer in folliculogenesis in mice. Molecular and cellular endocrinology. 2010;315(1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hennebold JD. Preventing Granulosa Cell Apoptosis Through the Action of a Single MicroRNA. Biology of reproduction. 2010;83(2):165–7. 10.1095/biolreprod.110.086173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rajewsky N. microRNA target predictions in animals. Nature genetics. 2006;38:S8–S13. [DOI] [PubMed] [Google Scholar]
- 24. Sirotkin AV, Ovcharenko D, Grossmann R, Laukova¡ M, Mlynanek M. Identification of MicroRNAs controlling human ovarian cell steroidogenesis via a genome scale screen. Journal of cellular physiology. 2009;219(2):415–20. 10.1002/jcp.21689 [DOI] [PubMed] [Google Scholar]
- 25. Kundu MC, May MC, Chosich J, Bradford AP, Lasley B, Gee N, et al. Assessment of luteal function in the vervet monkey as a means to develop a model for obesity-related reproductive phenotype. Systems biology in reproductive medicine. 59(2):74–81. 10.3109/19396368.2012.752547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carroll RL, Mah K, Fanton JW, Maginnis GN, Brenner RM, Slayden OD. Assessment of menstruation in the vervet (Cercopithecus aethiops). American Journal of Primatology. 2007;69(8):901–16. . [DOI] [PubMed] [Google Scholar]
- 27. Stephens SM, Pau FKY, Yalcinkaya TM, May MC, Berga SL, Post MD, et al. Assessing the Pulsatility of Luteinizing Hormone in Female Vervet Monkeys (Chlorocebus aethiops sabaeus). Comparative medicine. 2013;63(5):432–8. [PMC free article] [PubMed] [Google Scholar]
- 28. Molskness TA, Hess DL, Maginnis GM, Wright JW, Fanton JW, Stouffer RL. Characteristics and regulation of the ovarian cycle in vervet monkeys. Am J Primatol. 2007;69:890–900. [DOI] [PubMed] [Google Scholar]
- 29. Newman TK, Fairbanks LA, Pollack D, Rogers J. Effectiveness of human microsatellite loci for assessing paternity in a captive colony of vervets (Chlorocebus aethiops sabaeus). American Journal of Primatology. 2002;56(4):237–43. [DOI] [PubMed] [Google Scholar]
- 30. Kavanagh K, Fairbanks LA, Bailey JN, Jorgensen MJ, Wilson M, Zhang L, et al. Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obesity. 2007;15(7):1666–74. [DOI] [PubMed] [Google Scholar]
- 31. Munro C, Stabenfeldt G, Cragun J, Addiego L, Overstreet J, Lasley B. Relationship of serum estradiol and progesterone concentrations to the excretion profiles of their major urinary metabolites as measured by enzyme immunoassay and radioimmunoassay. Clinical Chemistry. 1991;37(6):838–44. [PubMed] [Google Scholar]
- 32. Kundu MC, May MC, Chosich J, Bradford AP, Lasley B, Gee N, et al. Assessment of luteal function in the vervet monkey as a means to develop a model for obesity-related reproductive phenotype. Systems biology in reproductive medicine. 2013;59(2):74–81. 10.3109/19396368.2012.752547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bogan RL, Murphy MJ, Hennebold JD. Dynamic changes in gene expression that occur during the period of spontaneous functional regression in the rhesus macaque corpus luteum. Endocrinology. 2009;150(3):1521–9. 10.1210/en.2008-1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Higgins PB, Bastarrachea RA, Lopez-Alvarenga JC, Garcia-Forey M, Proffitt JM, Voruganti VS, et al. Eight week exposure to a high sugar high fat diet results in adiposity gain and alterations in metabolic biomarkers in baboons (Papio hamadryas sp.). Cardiovasc Diabetol. 2010;9:71 Epub 2010/11/03. 10.1186/1475-2840-9-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mubiru JN, Garcia-Forey M, Higgins PB, Hemmat P, Cavazos NE, Dick EJ, et al. A preliminary report on the feeding of cynomolgus monkeys (Macaca fascicularis) with a high-sugar high-fat diet for 33 weeks. Journal of Medical Primatology. 2011;40(5):335–41. 10.1111/j.1600-0684.2011.00495.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ewing B, Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome research. 1998;8(3):186–94. [PubMed] [Google Scholar]
- 37. Anders S, Huber W. Differential expression analysis for sequence count data. Genome biol. 2010;11(10):R106 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jasinska AJ, Lin MK, Choi O-W, DeYoung J, Grujic O, Kong S-Y, et al. A non-human primate system for large-scale genetic studies of complex traits. Human molecular genetics. 2012;21(15):3307–16. 10.1093/hmg/dds160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Duffy DM, Stewart DR, Stouffer RL. Titrating Luteinizing Hormone Replacement to Sustain the Structure and Function of the Corpus Luteum after Gonadotropin-Releasing Hormone Antagonist Treatment in Rhesus Monkeys 1. The Journal of Clinical Endocrinology & Metabolism. 1999;84(1):342–9. [DOI] [PubMed] [Google Scholar]
- 40. Ahn HW, Morin RD, Zhao H, Harris RA, Coarfa C, Chen ZJ, et al. MicroRNA transcriptome in the newborn mouse ovaries determined by massive parallel sequencing. Molecular human reproduction. 2010;16(7):463–71. 10.1093/molehr/gaq017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McBride D, Carre W, Sontakke SD, Hogg CO, Law A, Donadeu FX, et al. Identification of miRNAs associated with the follicular-luteal transition in the ruminant ovary. Reproduction. 2012;144(2):221–33. 10.1530/REP-12-0025 . [DOI] [PubMed] [Google Scholar]
- 42. Hossain MM, Ghanem N, Hoelker M, Rings F, Phatsara C, Tholen E, et al. Identification and characterization of miRNAs expressed in the bovine ovary. BMC genomics. 2009;10:443 10.1186/1471-2164-10-443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Christenson LK. MicroRNA control of ovarian function. Animal reproduction / Colegio Brasileiro de Reproducao Animal. 2010;7(3):129–33. [PMC free article] [PubMed] [Google Scholar]
- 44. Baley J, Li J. MicroRNAs and ovarian function. J Ovarian Res. 2012;5(8):80724–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sirotkin AV, Ovcharenko D, Grossmann R, Laukova M, Mlynček M. Identification of MicroRNAs controlling human ovarian cell steroidogenesis via a genome‐scale screen. Journal of cellular physiology. 2009;219(2):415–20. 10.1002/jcp.21689 [DOI] [PubMed] [Google Scholar]
- 46. Nothnick WB. The role of micro-RNAs in the female reproductive tract. Reproduction. 2012;143(5):559–76. 10.1530/REP-11-0240 [DOI] [PubMed] [Google Scholar]
- 47. Zhang J, Ji X, Zhou D, Li Y, Lin J, Liu J, et al. miR-143 is critical for the formation of primordial follicles in mice. Frontiers in bioscience (Landmark edition). 2012;18:588–97. [DOI] [PubMed] [Google Scholar]
- 48. Jasinska AJ, Schmitt CA, Cantor RM, Dewar K, Jentsch JD, Kaplan JR, et al. Systems Biology of the Vervet Monkey. Institute of Laboratory Animal Resources 2013;54(2):122–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee YS, Shibata Y, Malhotra A, Dutta A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs). Genes & development. 2009;23(22):2639–49. 10.1101/gad.1837609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dey BK, Mueller AC, Dutta A. Non-micro-short RNAs: the new kids on the block. Molecular biology of the cell. 2012;23(24):4664–7. 10.1091/mbc.E12-10-0716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Garcia-Silva MR, Cabrera-Cabrera F, Guida MC, Cayota A. Hints of tRNA-Derived Small RNAs Role in RNA Silencing Mechanisms. Genes. 2012;3(4):603–14. 10.3390/genes3040603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Haussecker D, Huang Y, Lau A, Parameswaran P, Fire AZ, Kay MA. Human tRNA-derived small RNAs in the global regulation of RNA silencing. Rna. 2010;16(4):673–95. 10.1261/rna.2000810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Salilew-Wondim D, Ahmad I, Gebremedhn S, Sahadevan S, Hossain MM, Rings F, et al. The Expression Pattern of microRNAs in Granulosa Cells of Subordinate and Dominant Follicles during the Early Luteal Phase of the Bovine Estrous Cycle. PLoS One. 2014;9(9):e106795 10.1371/journal.pone.0106795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Borengasser SJ, Faske J, Kang P, Blackburn ML, Badger TM, Shankar K. In Utero Exposure to Pre-Pregnancy Maternal Obesity and Post-weaning High Fat Diet Impairs Regulators of Mitochondrial Dynamics in Rat Placenta and Offspring. Physiological genomics. 2014:physiolgenomics 00059 2014. 10.1152/physiolgenomics.00059.2014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Civitarese AE, MacLean PS, Carling S, Kerr-Bayles L, McMillan RP, Pierce A, et al. Regulation of skeletal muscle oxidative capacity and insulin signaling by the mitochondrial rhomboid protease PARL. Cell metabolism. 2010;11(5):412–26. 10.1016/j.cmet.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Civitarese AE, Ravussin E. Mitochondrial energetics and insulin resistance. Endocrinology. 2008;149(3):950–4. 10.1210/en.2007-1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Woźny M, Brzuzan P, Wolińska L, Góra M, Łuczyński MK. Differential gene expression in rainbow trout (Oncorhynchus mykiss) liver and ovary after exposure to zearalenone. Comparative Biochemistry and Physiology 2012;156(3):221–8. [DOI] [PubMed] [Google Scholar]
- 58. Vickers KC, Landstreet SR, Levin MG, Shoucri BM, Toth CL, Taylor RC, et al. MicroRNA-223 coordinates cholesterol homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(40):14518–23. 10.1073/pnas.1215767111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang F, Yang J, Wang H, Xia G. Gonadotropin-regulated expressions of lanosterol 14alpha-demethylase, sterol Delta14-reductase and C-4 sterol methyl oxidase contribute to the accumulation of meiosis-activating sterol in rabbit gonads. Prostaglandins & other lipid mediators. 2010;92(1–4):25–32. 10.1016/j.prostaglandins.2010.02.002 . [DOI] [PubMed] [Google Scholar]
- 60. Nogalska A, D'Agostino C, Terracciano C, Engel WK, Askanas V. Impaired autophagy in sporadic inclusion-body myositis and in endoplasmic reticulum stress-provoked cultured human muscle fibers. The American journal of pathology. 2010;177(3):1377–87. 10.2353/ajpath.2010.100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ramachandran N, Munteanu I, Wang P, Ruggieri A, Rilstone JJ, Israelian N, et al. VMA21 deficiency prevents vacuolar ATPase assembly and causes autophagic vacuolar myopathy. Acta neuropathologica. 2013;125(3):439–57. 10.1007/s00401-012-1073-6 . [DOI] [PubMed] [Google Scholar]
- 62. Ryter SW, Koo JK, Choi AM. Molecular regulation of autophagy and its implications for metabolic diseases. Current opinion in clinical nutrition and metabolic care. 2014;17(4):329–37. 10.1097/MCO.0000000000000068 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhao Y, Zacur H, Cheadle C, Ning N, Fan J, Vlahos NF. Effect of luteal-phase support on endometrial microRNA expression following controlled ovarian stimulation. Reprod Biol Endocrinol. 2012;10:72 10.1186/1477-7827-10-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang J, Li H, Wu Z, Tan X, Liu F, Huang X, et al. Differentiation of rat iPS cells and ES cells into granulosa cell-like cells in vitro. Acta biochimica et biophysica Sinica. 2013;45(4):289–95. 10.1093/abbs/gmt008 . [DOI] [PubMed] [Google Scholar]
- 65. Fogli A, Rodriguez D, Eymard-Pierre E, Bouhour F, Labauge P, Meaney BF, et al. Ovarian failure related to eukaryotic initiation factor 2B mutations. American journal of human genetics. 2003;72(6):1544–50. 10.1086/375404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fogli A, Gauthier-Barichard F, Schiffmann R, Vanderhoof VH, Bakalov VK, Nelson LM, et al. Screening for known mutations in EIF2B genes in a large panel of patients with premature ovarian failure. BMC women's health. 2004;4(1):8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim YJ, Hwang SH, Lee SY, Shin KK, Cho HH, Bae YC, et al. miR-486-5p induces replicative senescence of human adipose tissue-derived mesenchymal stem cells and its expression is controlled by high glucose. Stem cells and development. 2011;21(10):1749–60. 10.1089/scd.2011.0429 [DOI] [PubMed] [Google Scholar]
- 68. Civelek M, Hagopian R, Pan C, Che N, Yang W-p, Kayne PS, et al. Genetic regulation of human adipose microRNA expression and its consequences for metabolic traits. Human Molecular Genetics. 2013;22(15):3023–37. 10.1093/hmg/ddt159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fan HY, Liu Z, Cahill N, Richards JS. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Molecular endocrinology. 2008;22(9):2128–40. 10.1210/me.2008-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McLaughlin M, Kinnell HL, Anderson RA, Telfer EE. Inhibition of phosphatase and tensin homologue (PTEN) in human ovary in vitro results in increased activation of primordial follicles but compromises development of growing follicles. Molecular human reproduction. 2014;20(8):736–44. 10.1093/molehr/gau037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277(5322):55–60. . [DOI] [PubMed] [Google Scholar]
- 72. Arner E, Mejhert N, Kulyté A, Balwierz PJ, Pachkov M, Cormont M, et al. Adipose tissue microRNAs as regulators of CCL2 production in human obesity. Diabetes. 2012;61(8):1986–93. 10.2337/db11-1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Meerson A, Traurig M, Ossowski V, Fleming J, Mullins M, Baier L. Human adipose microRNA-221 is upregulated in obesity and affects fat metabolism downstream of leptin and TNF-α. Diabetologia. 2013;56(9):1971–9. 10.1007/s00125-013-2950-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yang Y, Lin P, Chen F, Wang A, Lan X, Song Y, et al. Luman recruiting factor regulates endoplasmic reticulum stress in mouse ovarian granulosa cell apoptosis. Theriogenology. 2013;79(4):633–9 e1-3. 10.1016/j.theriogenology.2012.11.017 . [DOI] [PubMed] [Google Scholar]
- 75. Fox CS, White CC, Lohman K, Heard-Costa N, Cohen P, Zhang Y, et al. Genome-wide association of pericardial fat identifies a unique locus for ectopic fat. PLoS genetics. 2012;8(5):e1002705 10.1371/journal.pgen.1002705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nakayama K, Ogawa A, Miyashita H, Tabara Y, Igase M, Kohara K, et al. Positive natural selection of TRIB2, a novel gene that influences visceral fat accumulation, in East Asia. Human genetics. 2013;132(2):201–17. 10.1007/s00439-012-1240-9 . [DOI] [PubMed] [Google Scholar]
- 77. Ndiaye K, Fayad T, Silversides DW, Sirois J, Lussier JG. Identification of downregulated messenger RNAs in bovine granulosa cells of dominant follicles following stimulation with human chorionic gonadotropin. Biol Reprod. 2005;73(2):324–33. 10.1095/biolreprod.104.038026 . [DOI] [PubMed] [Google Scholar]
- 78. Khan S, Shukla S, Sinha S, Meeran SM. Role of adipokines and cytokines in obesity-associated breast cancer: therapeutic targets. Cytokine & growth factor reviews. 2013;24(6):503–13. 10.1016/j.cytogfr.2013.10.001 . [DOI] [PubMed] [Google Scholar]
- 79. Park HK, Ahima RS. Leptin signaling. F1000prime reports. 2014;6:73 10.12703/P6-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sano M, Korekane H, Ohtsubo K, Yamaguchi Y, Kato M, Shibukawa Y, et al. N-glycans of SREC-I (scavenger receptor expressed by endothelial cells): essential role for ligand binding, trafficking and stability. Glycobiology. 2012;22(5):714–24. 10.1093/glycob/cws010 . [DOI] [PubMed] [Google Scholar]
- 81. Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96(1):219–26. . [DOI] [PubMed] [Google Scholar]
- 82. Gallo RV. Pulsatile LH release during periods of low level LH secretion in the rat estrous cycle. Biology of Reproduction. 1981;24(4):771–7. . [DOI] [PubMed] [Google Scholar]
- 83. Stephens SM, Pau FK, Yalcinkaya TM, May MC, Berga SL, Post MD, et al. Assessing the pulsatility of luteinizing hormone in female vervet monkeys (Chlorocebus aethiops sabaeus). Comparative medicine. 2013;63(5):432 [PMC free article] [PubMed] [Google Scholar]
- 84. Lewis D, Bertrand H, McMahan C, McGill H Jr, Carey K, Masoro E. Influence of preweaning food intake on body composition of young adult baboons. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1989;257(5):R1128–R35. [DOI] [PubMed] [Google Scholar]
- 85. Dorfman MD, Garcia-Rudaz C, Alderman Z, Kerr B, Lomniczi A, Dissen GA, et al. Loss of Ntrk2/Kiss1r signaling in oocytes causes premature ovarian failure. Endocrinology. 2014;155(8):3098–111. 10.1210/en.2014-1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kerr B, Garcia-Rudaz C, Dorfman M, Paredes A, Ojeda SR. NTRK1 and NTRK2 receptors facilitate follicle assembly and early follicular development in the mouse ovary. Reproduction. 2009;138(1):131–40. 10.1530/REP-08-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Carvajal R, Rosas C, Kohan K, Gabler F, Vantman D, Romero C, et al. Metformin augments the levels of molecules that regulate the expression of the insulin-dependent glucose transporter GLUT4 in the endometria of hyperinsulinemic PCOS patients. Hum Reprod. 2013;28(8):2235–44. 10.1093/humrep/det116 . [DOI] [PubMed] [Google Scholar]
- 88. Li H, Zeng WS, Luo S, Xing FQ. [Inhibitory effect of RNA interference targeting BaxBak on apoptosis of human granulosa cells]. Nan fang yi ke da xue xue bao = Journal of Southern Medical University. 2009;29(12):2367–70. . [PubMed] [Google Scholar]
- 89. Canty-Laird E, Carre GA, Mandon-Pepin B, Kadler KE, Fabre S. First evidence of bone morphogenetic protein 1 expression and activity in sheep ovarian follicles. Biol Reprod. 2010;83(1):138–46. 10.1095/biolreprod.109.082115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Khalaf M, Morera J, Bourret A, Reznik Y, Denoual C, Herlicoviez M, et al. BMP system expression in GCs from polycystic ovary syndrome women and the in vitro effects of BMP4, BMP6, and BMP7 on GC steroidogenesis. European journal of endocrinology / European Federation of Endocrine Societies. 2013;168(3):437–44. 10.1530/EJE-12-0891 . [DOI] [PubMed] [Google Scholar]
- 91. Hayashi KG, Ushizawa K, Hosoe M, Takahashi T. Differential genome-wide gene expression profiling of bovine largest and second-largest follicles: identification of genes associated with growth of dominant follicles. Reproductive biology and endocrinology: RB&E. 2010;8:11 10.1186/1477-7827-8-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ma T, Jiang H, Gao Y, Zhao Y, Dai L, Xiong Q, et al. Microarray analysis of differentially expressed microRNAs in non-regressed and regressed bovine corpus luteum tissue; microRNA-378 may suppress luteal cell apoptosis by targeting the interferon gamma receptor 1 gene. Journal of applied genetics. 2011;52(4):481–6. 10.1007/s13353-011-0055-z . [DOI] [PubMed] [Google Scholar]
- 93. Brogan RS, Mix S, Puttabyatappa M, VandeVoort CA, Chaffin CL. Expression of the insulin-like growth factor and insulin systems in the luteinizing macaque ovarian follicle. Fertility and sterility. 2010;93(5):1421–9. 10.1016/j.fertnstert.2008.12.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wu S, Divall S, Hoffman GE, Le WW, Wagner KU, Wolfe A. Jak2 is necessary for neuroendocrine control of female reproduction. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(1):184–92. 10.1523/JNEUROSCI.2974-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kim SH, Hwang SY, Min KS, Yoon JT. Molecular cloning and expression analyses of porcine MAP1LC3A in the granulosa cells of normal and miniature pig. Reproductive biology and endocrinology: RB&E. 2013;11:8 10.1186/1477-7827-11-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kim SH, Min KS, Kim NH, Yoon JT. Differential expression of programmed cell death on the follicular development in normal and miniature pig ovary. PloS one. 2012;7(10):e46194 10.1371/journal.pone.0046194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mitchell LM, Kennedy CR, Hartshorne GM. Expression of nitric oxide synthase and effect of substrate manipulation of the nitric oxide pathway in mouse ovarian follicles. Hum Reprod. 2004;19(1):30–40. . [DOI] [PubMed] [Google Scholar]
- 98. Zamberlam G, Sahmi F, Price CA. Nitric oxide synthase activity is critical for the preovulatory epidermal growth factor-like cascade induced by luteinizing hormone in bovine granulosa cells. Free radical biology & medicine. 2014;74:237–44. 10.1016/j.freeradbiomed.2014.06.018 . [DOI] [PubMed] [Google Scholar]
- 99. Vanorny DA, Prasasya RD, Chalpe AJ, Kilen SM, Mayo KE. Notch signaling regulates ovarian follicle formation and coordinates follicular growth. Molecular endocrinology. 2014;28(4):499–511. 10.1210/me.2013-1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li F, Jo M, Curry TE Jr., Liu J. Hormonal induction of polo-like kinases (Plks) and impact of Plk2 on cell cycle progression in the rat ovary. PloS one. 2012;7(8):e41844 10.1371/journal.pone.0041844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Weiss J, Hurley LA, Harris RM, Finlayson C, Tong M, Fisher LA, et al. ENU mutagenesis in mice identifies candidate genes for hypogonadism. Mammalian genome: official journal of the International Mammalian Genome Society. 2012;23(5–6):346–55. 10.1007/s00335-011-9388-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hsieh M, Boerboom D, Shimada M, Lo Y, Parlow AF, Luhmann UF, et al. Mice null for Frizzled4 (Fzd4-/-) are infertile and exhibit impaired corpora lutea formation and function. Biol Reprod. 2005;73(6):1135–46. 10.1095/biolreprod.105.042739 . [DOI] [PubMed] [Google Scholar]
- 103. Russell DL, Ochsner SA, Hsieh M, Mulders S, Richards JS. Hormone-regulated expression and localization of versican in the rodent ovary. Endocrinology. 2003;144(3):1020–31. 10.1210/en.2002-220434 . [DOI] [PubMed] [Google Scholar]
- 104. Griffith GJ, Trask MC, Hiller J, Walentuk M, Pawlak JB, Tremblay KD, et al. Yin-yang1 is required in the mammalian oocyte for follicle expansion. Biol Reprod. 2011;84(4):654–63. 10.1095/biolreprod.110.087213 . [DOI] [PubMed] [Google Scholar]
- 105. Jo Y, Hartman IZ, DeBose-Boyd RA. Ancient ubiquitous protein-1 mediates sterol-induced ubiquitination of 3-hydroxy-3-methylglutaryl CoA reductase in lipid droplet-associated endoplasmic reticulum membranes. Molecular biology of the cell. 2013;24(3):169–83. 10.1091/mbc.E12-07-0564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. McReynolds S, Dzieciatkowska M, McCallie BR, Mitchell SD, Stevens J, Hansen K, et al. Impact of maternal aging on the molecular signature of human cumulus cells. Fertility and sterility. 2012;98(6):1574–80 e5. 10.1016/j.fertnstert.2012.08.012 . [DOI] [PubMed] [Google Scholar]
- 107. Rao JU, Shah KB, Puttaiah J, Rudraiah M. Gene expression profiling of preovulatory follicle in the buffalo cow: effects of increased IGF-I concentration on periovulatory events. PloS one. 2011;6(6):e20754 10.1371/journal.pone.0020754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Brown KA, Bouchard N, Lussier JG, Sirois J. Down-regulation of messenger ribonucleic acid encoding an importer of sulfoconjugated steroids during human chorionic gonadotropin-induced follicular luteinization in vivo. The Journal of steroid biochemistry and molecular biology. 2007;103(1):10–9. 10.1016/j.jsbmb.2006.07.005 . [DOI] [PubMed] [Google Scholar]
- 109. Urano Y, Watanabe H, Murphy SR, Shibuya Y, Geng Y, Peden AA, et al. Transport of LDL-derived cholesterol from the NPC1 compartment to the ER involves the trans-Golgi network and the SNARE protein complex. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(43):16513–8. 10.1073/pnas.0807450105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Arianmanesh M, McIntosh RH, Lea RG, Fowler PA, Al-Gubory KH. Ovine corpus luteum proteins, with functions including oxidative stress and lipid metabolism, show complex alterations during implantation. The Journal of endocrinology. 2011;210(1):47–58. 10.1530/JOE-10-0336 . [DOI] [PubMed] [Google Scholar]
- 111. Koomen M, Cheng NC, van de Vrugt HJ, Godthelp BC, van der Valk MA, Oostra AB, et al. Reduced fertility and hypersensitivity to mitomycin C characterize Fancg/Xrcc9 null mice. Hum Mol Genet. 2002;11(3):273–81. . [DOI] [PubMed] [Google Scholar]
- 112. Liu Y, Zhao W, Gu G, Lu L, Feng J, Guo Q, et al. Palmitoyl-protein thioesterase 1 (PPT1): an obesity-induced rat testicular marker of reduced fertility. Molecular reproduction and development. 2014;81(1):55–65. 10.1002/mrd.22281 . [DOI] [PubMed] [Google Scholar]
- 113. Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319(5863):611–3. 10.1126/science.1152257 [DOI] [PubMed] [Google Scholar]
- 114. Kaipia A, Hsueh AJ. Regulation of ovarian follicle atresia. Annual review of physiology. 1997;59(1):349–63. [DOI] [PubMed] [Google Scholar]
- 115. Wu LL-Y, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, et al. High-fat diet causes lipotoxicity responses in cumulus–oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151(11):5438–45. 10.1210/en.2010-0551 [DOI] [PubMed] [Google Scholar]
- 116. Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, et al. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PloS one. 2010;5(4):e10074 10.1371/journal.pone.0010074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151(8):4039–46. 10.1210/en.2010-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wu LL, Russell DL, Wong SL, Chen M, Tsai TS, St John JC, et al. Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development. 2015;142(4):681–91. 10.1242/dev.114850 . [DOI] [PubMed] [Google Scholar]
- 119. Fang Z, Cui X. Design and validation issues in RNA-seq experiments. Briefings in bioinformatics. 2011;12(3):280–7. 10.1093/bib/bbr004 . [DOI] [PubMed] [Google Scholar]
- 120. Marioni JC, Mason CE, Mane SM, Stephens M, Gilad Y. RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome research. 2008;18(9):1509–17. 10.1101/gr.079558.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sherman BM, Korenman SG. Measurement of serum LH, FSH, estradiol and progesterone in disorders of the human menstrual cycle: the inadequate luteal phase. The Journal of clinical endocrinology and metabolism. 1974;39(1):145–9. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIFF)
(DOCX)
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.