Abstract
BADH1 and BADH2 are two homologous genes, encoding betaine aldehyde dehydrogenase in rice. In the present study, we scanned BADHs sequences of 295 rice cultivars, and 10 wild rice accessions to determine the polymorphisms, gene functions and domestication of these two genes. A total of 16 alleles for BADH1 and 10 alleles for BADH2 were detected in transcribed region of cultivars and wild species. Association study showed that BADH1 has significant correlation with salt tolerance in rice during germination stage, the SNP (T/A) in exon 4 is highly correlated with salt tolerance index (STI) (P<10−4). While, BADH2 was only responsible for rice fragrance, of which two BADH2 alleles (8 bp deletion in exon 7 and C/T SNP in exon 13) explain 97% of aroma variation in our germplasm. Theses indicate that there are no overlapping functions between the two homologous genes. In addition, a large LD block was detected in BADH2 region, however, there was no large LD blocks in a 4-Mb region of BADH1. We found that BADH2 region only showed significant bias in Tajima’s D value from the balance. Extended haplotype homozygosity study revealed fragrant accessions had a large LD block that extended around the mutation site (8 bp deletion in exon 7) of BADH2, while both of the BADH1 alleles (T/A in exon 4) did not show large extended LD block. All these results suggested that BADH2 was domesticated during rice evolution, while BADH1 was not selected by human beings.
Introduction
Betaine aldehyde dehydrogenase (BADH) is an enzyme found in a large number of plant species, and its catalyst glycine betaine (GB) is a powerful osmoprotectant associated with salt and drought stress tolerance [1]. In many plant species, such as mangrove, spinach (Spinacia oleracea L.), amaranth (Aramanthus spp.), barley (Hordeum vulgare L.) and sorghum (Sorghum bicolor L.), BADH plays a role in abiotic stress tolerance through the accumulation of GB from betaine aldehyde (BA). Conversely, plant species such as tobacco (Nicotiana tabacum L.), tomato (Solanum lycopersicum L.) and rice (Oryza sativa L.) reportedly do not accumulate GB due to insufficient BA [1–3].
Rice is regarded as a typical non-glycine betaine-accumulating species with two functional genes coding BADH (EC 1.2.1.8). BADH1 (Os04g0464200) is located on chromosome 4 and BADH2 (Os08g0424500) is found on chromosome 8 [1,4]. Both genes have 15 exons and show 75% sequence homology at the amino acid level [5]. In vitro studies showed that rice BADH1 has very low activity on BA but the function and mechanism of BADH1 are uncertain [6]. The rice BADH1 has been reported close correlation with salt tolerance [7], while as Singh et al. (2010) argued BADH1 is associated with rice fragrance [1]. In contrast, BADH2 has been identified as a recessive gene conferring fragrance in rice, rather than abiotic stress tolerance[8]. The loss of function of BADH2 is the reason for aromatic rice, accumulating the main flavoring components 2-AP (2-acetyl-1-pyrroline).
Fitzgerald et al. (2008) reported that as the salt concentration increased, the transcript level of BADH1 increased significantly in leaf tissues of both non-fragrant and fragrant rice, while no consistent relationship was observed between BADH2 transcript levels and salt concentration during the seedling stage [5]. However, Fitzgerald et al. (2010) found a highly significant difference in the ability to produce mature seed between the fragrant and non-fragrant rice in the presence of salt [2]. Thus, BADH1 and BADH2 may be responsible for salt stress tolerance, but at different growth stages.
BADH2 has been extensively studied at the genetic and molecular levels for rice fragrance. Many studies on the BADH2 have been reported since Bradbury et al. (2005) found a strong allele with eight base-pairs deletion in exon 7, causing 2AP-dependent strong fragrance in rice [8]. Numerous BADH2 alleles, at least 15 alleles in transcribed region, have been detected in diverse rice germplasms [8–16]. However, BADH1 has not been studied as extensively as BADH2. Only three single-nucleotide polymorphisms (SNPs) have been reported so far [1].
Investigating the domestication of the agriculturally important gene is one of the methods to enlighten the rice evolutionary history, which are receiving increasing research attention in recent years [11,17–19]. In rice, a number of genes have been proved as the domesticated genes, such as sh4 [20], rc [21], prog1 [22], GS3 [23] including badh2 [11].The badh2.1 allele has been significantly selected during the aromatic rice evolution [11]. The evolutionary path of BADH1, however, has not been fully explored. It is of great interest to explore whether rice BADH1 gene was also involved in the domestication.
The goal of this study were to: (1) identify sequence polymorphism in the BADH1 and BADH2 gene from 295 cultivated rice and 10 wild rice by re-sequencing and (2) investigate the function of BADHs on the salt tolerance at germination stage and rice fragrance, and their overlapping functions. Lastly, we (3) investigated evolutionary path of BADHs in Asian rice.
Materials and Methods
Plant materials
Two hundred and ninety-five rice varieties used for the present study were collected from RDA gene bank of Korea. Among the 295 accessions, 137 accessions were selected from worldwide 4,046 accessions, based on 15 SSRs by a heuristic approach using PowerCore software [24,25], the rest 158 accessions were selected from Korean breeding varieties. All rice accessions were planted in a paddy field at Kongju National University, Korea, in 2012 and 2013.
Salt stress evaluation
Seeds of each accession were sterilized in sodium hypochlorite (1%) solution for 10 min and then washed twice with deionized distilled water. Then, the seeds were placed in petri dishes with two-layer filter paper and soaked in 200 mM NaCl solution, which was previously determined as an the optimal concentration (data not shown) to distinguish salt stress tolerance and sensitivity. The salt tolerance index (STI) was used to evaluate salt stress tolerance, where STI (%) = (total dry weight of the samples/total dry weight of the control) × 100.
Assay of rice fragrance
The aroma of rice grains and leaves were measured by a sensory evaluation according to the method of Amarawathi et al. (2008) with minor modifications. Ten milled rice grains were placed in a 15 ml tube containing 8 ml of 1.7% KOH and incubated at 30°C water bath for 10 min with lids on. The lids were then opened and samples were smelled. The aroma of young leaves was checked by the same method to confirm the results for rice grain.
Resequencing of 295 rice accessions and allele detection for BADH1 and BADH2
HiSeq 2000 and HiSeq 2500 were employed for whole-genome resequencing of the 295 rice accessions. Raw sequences were first processed to obtain an average of quality score (QS) per read ≤ 20 by trimming 3'-end of reads using SICKLE (https://github.com/najoshi/sickle). High-quality reads were aligned to the rice reference genome IRGSP-1.0 (http://rapdb.dna.affrc.go.jp/download/irgsp1.html) using the Burrows-Wheeler Aligner (BWA) (version 0.7.5a) with default parameters [26]. The reads that does not meet BWA quality criteria or not match the reference genome were removed. PCR duplicate reads were removed by using PICARD (version 1.88) (http://broadinstitute.github.io/picard/). By using Genome Analysis Toolkit (GATK) (version 2.3.9 Lite) [27], regional realignment and quality score recalibration were carried out. SNPs and InDels were, then, identified with ≥ 3X of read depth coverage. Overall, the mapping depth about 9X in average. Sequence data for 10 WT rice accessions (5 Oryza rufipogon and 5 Oryza nivara) were downloaded from the National Centre for Biotechnology Information Sequence Read Archive 023116 [19]. The polymorphisms of BADH1 and BADH2 were purified from whole-genome resequencing data.
Association study
Genotypic and phenotypic data files were prepared and imported to TASSEL4.0 [28] for association test. The general linear model (GLM), containing the SNP tested as a fixed effect, was applied to test the association between phenotypic variation (aroma and STI) and 19 alleles, which located in transcribed region of BADHs without any missing data. To get reliable results, P-value ≤ 0.001 was considered to have a significant effect on each trait.
Linkage disequibrium (LD) block detection, nucleotide diversity, Tajima’s D analysis and extended haplotype homozygosity (EHH) analysis
To identify LD blocks in both wild and cultivated rice populations, we used Haploview software with “-dprime–minMAF 0.05 –hwcutoff 0.001 –blockoutput GAB –pairwiseTagging” parameters [29]. Average pairwise divergence within the population was estimated in a 4-Mb interval of BADH genes. Sliding windows of 1 kb and 3k were used to estimate nucleotide diversity for the 4-Mb region of each gene. In each window, the π and Tajima’s D value was calculated using VCFtools [30]. The pattern of the results were plotted using R scripts. Extended haplotype homozygosity (EHH) was calculated using the R package rehh [31].
Results
Salt stress tolerance and rice fragrance
A total of 205 rice accessions, without any missing sequence information of BADH1 and BADH2, were evaluated for salt stress index and rice fragrance. The average STI is 1.14. Among these, RWG-140 had the highest stress tolerance (STI, 1.50). However, RWG-222 had the lowest STI (0.26), which is salt stress sensitive. In the rice aroma test, total 12 accessions were evaluated to be fragrant rice (S3 Table).
Polymorphisms of BADH1 and BADH2
In this study, we used the whole genome resequencing data to study BADH1 and BADH2. From our resequencing data set (cultivated rice), seven SNPs were found in the exon region, three SNPs and one insertion were in the untranslated region (UTR) in BADH1 (S1 Table). For BADH2, three SNPs, two deletions and one insertion were located in exon part, while four SNPs and three insertions were detected in untranslated region (S2 Table). When we checked those regions in the wild rice, we found, to our surprise, very high sequence diversity in BADH1 gene region, including four SNPs in UTR and twelve SNPs in exon region. However, only four heterozygous SNPs were found in BADH2 exon part and five heterozygous SNPs and one insertion in UTR (S1 and S2 Tables).
Among the 16 exonic SNPs of BADH1, only seven were non-synonymous mutations, others were synonymous mutations. In our rice germplasm, five non-synonymous mutations were detected. Here we are using “P1” and “P2” to represent the gene positions in BADH1 and BADH2, respectively. The five mutations are described as follows: 1) one G/A SNP (P1141) in exon 1 leading to substitution from arginine to histidine; 2) one T/A SNP (P11483) in exon 4 leading to asparagine to lysine substitution; 3) one C/A SNP (P13605) in exon 11 leading to substitution from glutamine to lysine; 4) one T/C SNP (P13612) in exon 12 leading to substitution from isoleucine to threonine; 5) one G/T SNP (P13883) in exon 12 leading to substitution from glutamic acid to aspartic acid. Two non-synonymous mutations were represented in wild type as heterozygous SNPs. One is T/A SNP (P11232) in exon 3 leading to a substitution from aspartic acid to glutamic acid, the other was a G/T SNP (P13672) leading to a substitution from arginine to leucine in exon 11 (S1 Table).
Eighteen alleles were detected in BADH2 transcribed region. Ten of 18 were located in coding region, and eight were located in untranslated region. In our materials, 1) 8-bp deletion in exon 7 (P23036) leading to premature transcription termination; 2) one C/A SNP (P24488) in exon 10 leading to amino acid substitution from alanine to glutamic acid; 3) three base-pair deletion (P25240) in exon 12 leading to loss of one amino acid; 4) one C/T SNP (P25390) in exon 13 leading to substitution from alanine to valine; and 5) one base pair insertion in exon 14 resulting in frameshift and premature transcription termination have been detected. One synonymous SNP (P24528) in exon 10 has been found in two varieties. Two non-synonymous SNP were detected in wild rice, 1) C/G SNP (P2205) in exon 1 resulting to alanine to glycine substitution; and 2) C/T SNP (P22685) in exon 5 resulting to alanine to valine substitution. Those two alleles were heterozygous and initially detected in BADH2. In the UTR, three InDels, P214 (1 bp insertion), P236 (1 bp deletion) and P241 (insertion in the 5’UTR) and two SNPs (P224 C/T in the 3’UTR and P26038 in the 3’UTR) were detected.
Association analysis of salt tolerance, aroma and BADHs alleles
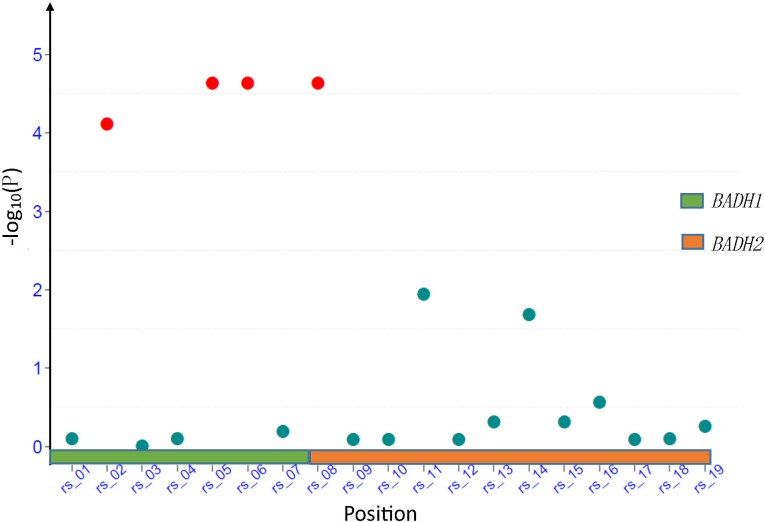
Due to the limitations of whole-genome resequencing, missing sequence data were inevitable. In this study, we sequenced total 295 rice samples, most had high sequencing coverage both in BADH1 and BADH2 region, but some accessions had low coverage or missing sequence in these gene regions. After removing accessions with missing sequence data, a total of 205 accessions remained. Among these 205 accessions, totally 19 SNPs and InDels were detected in BADH1 and BADH2 transcribed regions (S3 Table), and subsequently used for the association studies (Table 1).
Table 1. The association study of salt tolerance index and candidate regions.
| Marker | Locus | Locus_pos | Marker_F | Marker_P | Marker_R2 |
|---|---|---|---|---|---|
| rs-01 | BADH1 | 46 | 0.24703 | 0.7814 | 0.00295 |
| rs-02 | BADH1 | 87 | 16.44522 | 7.65E-05 | 0.08916 |
| rs-03 | BADH1 | 101 | 7.53E-04 | 0.97814 | 4.48E-06 |
| rs-04 | BADH1 | 181 | 0.24703 | 0.7814 | 0.00295 |
| rs-05 | BADH1 | 1483 | 11.39543 | 2.30E-05 | 0.12008 |
| rs-06 | BADH1 | 3605 | 11.39543 | 2.30E-05 | 0.12008 |
| rs-07 | BADH1 | 3883 | 0.22252 | 0.63774 | 0.00132 |
| rs-08 | BADH1 | 4811 | 11.39543 | 2.30E-05 | 0.12008 |
| rs-09 | BADH2 | 14 | 0.05781 | 0.81028 | 3.44E-04 |
| rs-10 | BADH2 | 24 | 0.05781 | 0.81028 | 3.44E-04 |
| rs-11 | BADH2 | 36 | 6.5564 | 0.01133 | 0.03756 |
| rs-12 | BADH2 | 41 | 0.05781 | 0.81028 | 3.44E-04 |
| rs-13 | BADH2 | 42 | 0.49606 | 0.48221 | 0.00294 |
| rs-14 | BADH2 | 49 | 3.97914 | 0.0205 | 0.04549 |
| rs-15 | BADH2 | 115 | 0.49606 | 0.48221 | 0.00294 |
| rs-16 | BADH2 | 3036 | 1.22893 | 0.2692 | 0.00726 |
| rs-17 | BADH2 | 4488 | 0.05781 | 0.81028 | 3.44E-04 |
| rs-18 | BADH2 | 4528 | 0.06933 | 0.79264 | 4.13E-04 |
| rs-19 | BADH2 | 5390 | 0.37313 | 0.54213 | 0.00222 |
Locus_pos: Physical distance from the transcription initiation site of the gene.
The association study demonstrated a relatively high correlation (R 2) of 0.12 between P11483/P13605/P14811 and STI (P<10−4) in BADH1 (Table 1 and Fig 1), while no correlation was observed in BADH2 in this regard. On the other hand, it was found that two BADH2 alleles (P23036, P25390) explain 97% of aroma variation in our germplasm. However, no association existed between aroma and BADH1 (S4 Table). It is suggested that BADH1 and BADH2 have different functions in rice.
Fig 1. Associations across transcribed regions of BADH1 and BADH2.
Extended haplotype homozygosity study
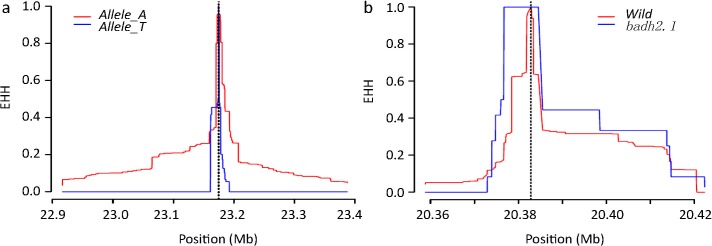
According to the association study, P23036 (8-bp deletion in exon 7) is the major allele which is associated with rice fragrance (S4 Table). P23036 also called badh2.1 by Kovach et al. (2009). Here we follow the method which was used by Kovach et al. (2009) for domestication study. Firstly, we divided the whole 205 accessions into two subgroups: the one that have 8-bp deletion in exon 7 (badh2.1), and without 8-bp deletion (Wild). Then we investigated the extended haplotype homozygosity of BADH1 and BADH2 regions to find the evidence for artificial selections. We calculated the EHH for BAHD2 gene by comparing the haplotypes of fragrant (badh2.1) and wild type (with functional BADH2 gene) group. The badh2.1 had a large LD block that extended around the mutation site, while LD decreased rapidly around the wild type alleles (Fig 2B).
Fig 2. EHH plot across the BADH1 and BADH2 genomic region.
(a) EHH of BADH1 genomic region. Wild type (T) and P11483 (A) did not show large LD block. (b) EHH of BADH2 genomic region. The fragrant accessions carrying the P23036 (badh2.1 which has 8 bp deletion in exon 7) allele exhibited a large block of extended LD around the FNP, while wild type allele declined rapidly.
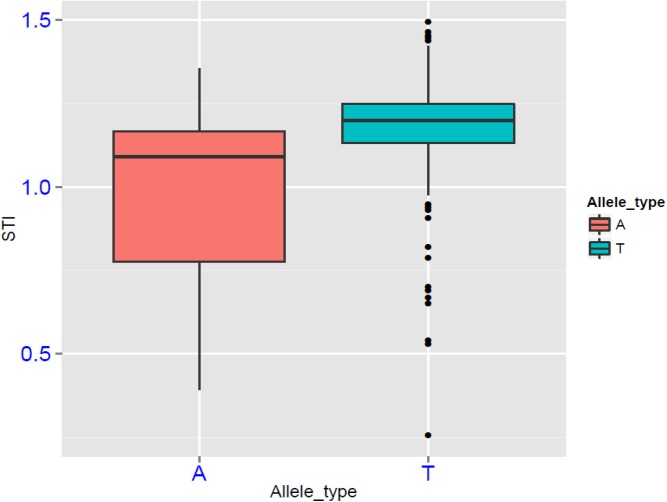
Since the P11483 (T/A) allele was significantly correlated with salt tolerance, we separated the 205 individuals into two subgroups, one included “T” allele (Allele_T group), another one included “A” allele (Allele_A group). STI values of the “T” allele subgroup were significantly higher than the “A” allele subgroup (P<0.05; Fig 3). The EHH study for the BADH1 gene was compared the extended haplotypes of (P11483 A) and wide type (P11483 T). However, these two groups carrying A or T alleles did not exhibit large difference, both of them did not show large extended LD block, reducing rapidly around P11483 (Fig 2A).
Fig 3. Boxplot of STI (salt tolerance index) for different allele type at P11483 (T/A).
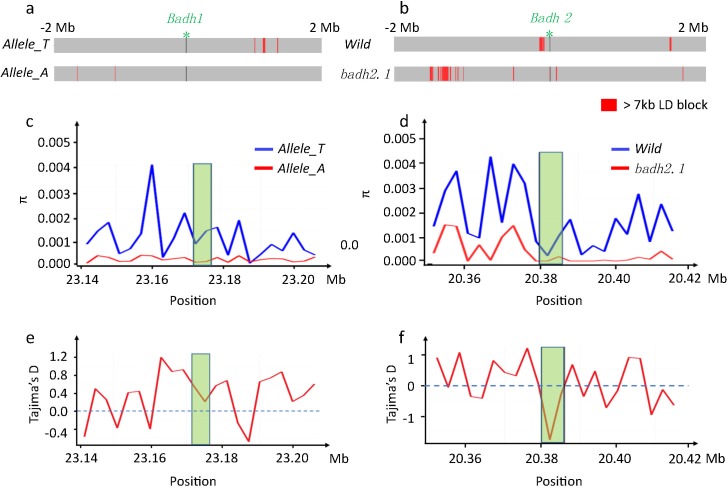
LD block, nucleotide diversity and Tajima’s D analysis in different subpopulations
In order to find more evidence whether the domestication rate for BADH1 and BADH2 are different, we analyzed the LD block, nucleotide diversity and Tajima’s D for each subgroup. For BADH2, it clearly showed that wild group has no block in badh2 gene region, while badh2.1 group had the block which over 7 kb in the gene region (Fig 4B). The nucleotide diversity study showed that wild group had higher diversity than badh2.1 in both the flanking 2 Mb of BADH2 and BADH2 gene region. Interestingly, the nucleotide diversity in BADH2 gene region was reduced in two subgroups. The wild type was still higher than badh2.1 and the diversity of badh2.1 was almost as low as 0 (Fig 4D). Using 205 individual’s sequence, we calculated the Tajima’s D value in BADHs regions. It is clearly showed that in BADH2 region the Tajima’s D value (less than -1) was significantly biased from the balance (Fig 4F). These three results strongly suggested that badh2.1 had been artificially selected during the rice domestication.
Fig 4. LD block, nucleotide diversity and Tajima’s D in BADHs regions (Green part are the gene regions).
(a) LD block of BADH1 at different groups. (b) LD block of BADH2 at different groups (badh2.1 has 8bp deletion in exon 7). (c) Nucleotide diversity of BADH1 region with 3kb slide window. (d) Nucleotide diversity of BADH2 region with 3kb slide window. (e) Tajima’s D of BADH1 region with 3kb slide window. (f) Tajima’s D of BADH2 region with 3kb slide window.
On the other hand, we failed to find the large LD block in BADH1 region in both Allele_T group and Allele_A group (Fig 4A). The nucleotide diversity study showed that the Allele_T group had higher diversity than Allele_A in both flanking region and gene region of BADH1 (Fig 4C), but Allele_A group had low diversity level in this region. The Tajima’s D value also kept a stationary value in the BADH1 gene region (Fig 4E). From these three analyses, we are failed to get any clear evidence that BADH1 was also selected by human beings, suggesting that BADH1 is not a domesticated gene in rice.
Discussion
Polymorphism and association study
BADH2 was widely known as the gene associated with fragrance in rice [11]. As the homologous gene of BADH2, BADH1 is considered to be the candidate gene controlling fragrance or salt tolerance in rice. Recently, Fitzgerald et al. (2010) suggested that BADH2 is also responsible for salt stress tolerance. The relationship between these two BADH genes and salt stress tolerance or fragrance in rice is unclear. Various studies have reported conflicting results [1,2,5]. These inconsistencies could be attributable to the differences either in rice germplasm materials or growth stages that they investigated for their studies.
Here, we used whole genome resequencing data to study BADH1 and BADH2 genes. For BADH1, we found a total of 10 genetic variants, seven SNPs were in the exon region and two SNPs and one insertion were in the untranslated region (S1 Table). Among the seven SNPs in exon region, four of them were novel (S1 Fig). It was observed that four SNPs and three insertions were detected in the UTR of BADH2. Three SNPs, two deletions and one insertion were located in the exon region of BADH2, extending the number of BADH2 exonic alleles from 15 to 18 (S2 Table and S2 Fig) [8–11,13].
After removing the accessions with missing sequence information, we finally discovered eight alleles in BADH1, and eleven alleles in BADH2 from the 205 accessions (S3 Table). Eight alleles in BADH1 generated 7 haplotypes and eleven alleles result in 9 haplotypes in BADH2 region (S3 Table). We performed the GLM based association study for STI using19 alleles. As a result, we discovered that P11483 (T/A), P13605 (C/A) in translated region and P14811 in the 3’UTR were highly correlated with STI with, P < 10−4 and R 2 around 0.12 (Table 1 and Fig 1). However, similar correlations were not found for BADH2 (Table 1), indicating BADH2 may not be associated with salt tolerance.
Fitzgerald et al. (2008) reported that the expression level of BADH1 in leaf tissue is highly associated in response to salt treatment at the seedling stage. In contrast, such relationship between BADH2 transcript levels and salt treatment has not been found yet. More recently, however, Fitzgerald et al. (2010) did show a difference between fragrant (badh2) and non-fragrant (BADH2) rice for the ability to produce mature seed in the presence of salt. Tang et al. (2014) proved that BADH1 was associated with rice salt (NaCl) tolerance at seedling stage by using RNAi-directed knock down of BADH1 [32]. Thus, BADH1 and BADH2 are both suggested to be responsible for salt stress tolerance in rice, but, possibly, at different growth stages. Since our STI was tested at germination stage, we extended the conclusion that BADH1 is response for rice salt stress at germination and seedling stage, while BADH2 may be accountable at the reproductive stage.
P23036 (8 bp deletions) and P25390 (C/T) were the main alleles that explained the aroma variation with 74% and 23% respectively (S4 Table), which are consistent with previous studies in that the lack of function of BADH2 has a significant effect on rice aroma [8,11]. However, we found no association between BADH1 and aroma (S4 Table).
Singh et al. (2010) reported that there’s no association between salt tolerance and common BADH1 but few exceptions. None the less, our results are rather consistent with the result from Fitzgerald et al. (2008), demonstrating that BADH1 (and not BADH2) contributes to salt stress tolerance. However, we cannot exclude the possibility that these contradictions may be resulted from the different conditions undertaken.
Domestication of BADH1 and BADH2
Domestication of crops has historically been critical for human reproduction and development. The domestication of rice were explained by several models [11]. The recent increase in molecular marker density through next generation sequencing (NGS) technology has facilitated to build more solid hypotheses on the domestication process of rice [11,33]. Investigating the domestication history of a specific gene is one way to decipher the history of rice and its impact on humans [11]. An EHH study of BADH2 led by Kovach et al. (2009) suggested that BADH2 is one of the domesticated genes responsible for rice fragrance. In this study, we attempted to determine if BADH1 is also a domesticated gene.
The association mapping study identified three alleles (P11483, P13605 and P14811) in BADH1 highly correlated with STI. These three alleles were consistently present together in the rice genome. We selected P11483 (T/A) as the key allele for an evolution study of BADH1. To validate our method, we tested the domestication signal of BADH2 using the same method (EHH) as Kovach et al. (2009). Our EHH test result was similar to the results of Kovach et al. (2009). The fragrant accessions which carrying 8 bp deletions in exon 7 showed a large LD block extended around the mutation site, while the wild type alleles relatively reduced rapidly (Fig 2B). To obtain more clear evidence, we further investigated LD block, nucleotide diversity, and Tajima’s D for the 4-Mb flanking regions of BADHs, which had always been tested for mutual verification for a domestication process [11,18,19,34]. For BADH2, LD block study showed that there were a lot of LD blocks in the fragrant rice group (badh2.1) over 7 kb, while fewer LD blocks were found in wild type group (Fig 4B). The diversity study suggested that all groups show reduced diversity in BADH2 gene region, but the badh2.1 approaches zero for this region (Fig 4D). Furthermore, the Tajima’s D test showed a significantly deviated signal in BADH2 region (Fig 4F). All of those results support the conclusions that badh2.1 was domesticated, which is totally consistent with previous study [11].
Employing the same tests, finding clear domesticated signals for BADH1 was difficult. We failed in finding LD blocks in BADH1 for the “Allele_A” and “Allele_T” groups (Fig 4A). The diversity of Allele_A group was consistently low without any violent swings in BADH1 gene region (Fig 4C). Moreover, Tajima’s D value was not significant for BADH1 (Fig 4E). Lastly, EHH was significantly diminished in the two BADH1 groups (Fig 2A). Thus, we concluded that BADH1 had not been domesticated in Asian rice.
Interestingly, Wang et al. (2014) found that the genetic diversity is strongly reduced in the BADH1 region of O. glaberrima, indicating BADH1 was possibly domesticated in O. glaberrima [35]. O. glaberrima is widely known to be better at tolerating biotic or abiotic stress than O. sativa [36]. As a gene associated with abiotic stress tolerance, BADH1 was likely domesticated in O. glaberrima, which is not the case for O. sativa [36]. Our results supports that the BADH1 is responsible for salt stress in rice germination stage, explaining 12% variation of phenotype for salt stress in O. sativa (Table 1). However, this effect might not be strong enough to be selected during the evolution of Asian cultivated rice.
Supporting Information
badh1.3, badh1.5 and badh1.6 were detected by previous study [1]. badh1.1, badh1.2, badh1.4 and badh1.7 were detected in this study.
(DOCX)
badh2.1~badh2.2 [11]; badh2.11~badh2.12, badh2.14 [10]; badh2.13 [16]; badh2.15 [12]. badh2.16~badh2.18 were detected in this study.
(DOCX)
(DOCX)
(DOCX)
(Excel)
(XLSX)
(DOCX)
Acknowledgments
We thank Dr. Kyu-Won Kim for his technical supports on data analysis.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No.PJ01116101), Rural Development Administration, Republic of Korea. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Seedpia Inc provided support in the form of salaries for authors [YHC], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1. Singh A, Singh PK, Singh R, Pandit A, Mahato AK, Gupta DK, et al. (2010) SNP haplotypes of the BADH1 gene and their association with aroma in rice (Oryza sativa L.). Mol Breed 26: 325–338. [Google Scholar]
- 2. Fitzgerald TL, Waters DLE, Brooks LO, Henry RJ (2010) Fragrance in rice (Oryza sativa) is associated with reduced yield under salt treatment. Environ Exp Bot 68: 292–300. [Google Scholar]
- 3. Sakamoto A, Murata AN (1998) Metabolic engineering of rice leading to biosynthesis of glycinebetaine and tolerance to salt and cold. Plant Mol Biol 38: 1011–1019. [DOI] [PubMed] [Google Scholar]
- 4. Nakamura T, Yokota S, Muramoto Y, Tsutsui K, Oguri Y, Fukui K, et al. (1997) Expression of a betaine aldehyde dehydrogenase gene in rice, a glycinebetaine nonaccumulator, and possible localization of its protein in peroxisomes. The Plant Journal 11: 1115–1120. [DOI] [PubMed] [Google Scholar]
- 5. Fitzgerald TL, Waters DLE, Henry RJ (2008) The effect of salt on betaine aldehyde dehydrogenase transcript levels and 2-acetyl-1-pyrroline concentration in fragrant and non-fragrant rice (Oryza sativa). Plant Sci 175: 539–546. [Google Scholar]
- 6. Bradbury LM, Gillies SA, Brushett DJ, Waters DL, Henry RJ (2008) Inactivation of an aminoaldehyde dehydrogenase is responsible for fragrance in rice. Plant Mol Biol 68: 439–449. 10.1007/s11103-008-9381-x [DOI] [PubMed] [Google Scholar]
- 7. Hasthanasombut S, Ntui V, Supaibulwatana K, Mii M, Nakamura I (2010) Expression of Indica rice OsBADH1 gene under salinity stress in transgenic tobacco. Plant Biotechnology Reports 4: 75–83. [Google Scholar]
- 8. Bradbury LM, Fitzgerald TL, Henry RJ, Jin Q, Waters DL (2005) The gene for fragrance in rice. Plant Biotechnol J 3: 363–370. [DOI] [PubMed] [Google Scholar]
- 9. Shi W, Yang Y, Chen S, Xu M (2008) Discovery of a new fragrance allele and the development of functional markers for the breeding of fragrant rice varieties. Mol Breed 22: 185–192. [Google Scholar]
- 10. Shao G, Tang S, Chen M, Wei X, He J, Luo J, et al. (2013) Haplotype variation at Badh2, the gene determining fragrance in rice. Genomics 101: 157–162. 10.1016/j.ygeno.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 11. Kovach MJ, Calingacion MN, Fitzgerald MA, McCouch SR (2009) The origin and evolution of fragrance in rice (Oryza sativa L.). Proceedings of the National Academy of Sciences 106: 14444–14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi Y, Zhao G, Xu X, Li J (2014) Discovery of a new fragrance allele and development of functional markers for identifying diverse fragrant genotypes in rice. Mol Breed 33: 701–708. [Google Scholar]
- 13. Bourgis F, Guyot R, Gherbi H, Tailliez E, Amabile I, Salse J, et al. (2008) Characterization of the major fragance gene from an aromatic japonica rice and analysis of its diversity in Asian cultivated rice. Theor Appl Genet 117: 353–368. 10.1007/s00122-008-0780-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Myint KM, Arikit S, Wanchana S, Yoshihashi T, Choowongkomon K, Vanavichit A (2012) A PCR-based marker for a locus conferring the aroma in Myanmar rice (Oryza sativa L.). Theor Appl Genet 125: 887–896. 10.1007/s00122-012-1880-0 [DOI] [PubMed] [Google Scholar]
- 15. Ootsuka K, Takahashi I, Tanaka K, Itani T, Tabuchi H, Yoshihashi T, et al. (2014) Genetic polymorphisms in Japanese fragrant landraces and novel fragrant allele domesticated in northern Japan. Breeding science 64: 115–124. 10.1270/jsbbs.64.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amarawathi Y, Singh R, Singh AK, Singh VP, Mohapatra T, Sharma TR, et al. (2008) Mapping of quantitative trait loci for basmati quality traits in rice (Oryza sativa L.). Mol Breed 21: 49–65. [Google Scholar]
- 17. Huang X, Kurata N, Wei X, Wang Z-X, Wang A, Zhao Q, et al. (2012) A map of rice genome variation reveals the origin of cultivated rice. Nature 490: 497–501. 10.1038/nature11532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lam H-M, Xu X, Liu X, Chen W, Yang G, Wong F-L, et al. (2010) Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nat Genet 42: 1053–1059. 10.1038/ng.715 [DOI] [PubMed] [Google Scholar]
- 19. Xu X, Liu X, Ge S, Jensen JD, Hu F, Li X, et al. (2012) Resequencing 50 accessions of cultivated and wild rice yields markers for identifying agronomically important genes. Nat Biotechnol 30: 105–111. [DOI] [PubMed] [Google Scholar]
- 20. Li C, Zhou A, Sang T (2006) Rice domestication by reducing shattering. science 311: 1936–1939. [DOI] [PubMed] [Google Scholar]
- 21. Sweeney MT, Thomson MJ, Cho YG, Park YJ, Williamson SH, Bustamante CD, et al. (2007) Global dissemination of a single mutation conferring white pericarp in rice. PLoS Genet 3: e133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin J, Huang W, Gao J-P, Yang J, Shi M, Zhu M-Z, et al. (2008) Genetic control of rice plant architecture under domestication. Nat Genet 40: 1365–1369. 10.1038/ng.247 [DOI] [PubMed] [Google Scholar]
- 23. Takano-Kai N, Jiang H, Kubo T, Sweeney M, Matsumoto T, Kanamori H, et al. (2009) Evolutionary history of GS3, a gene conferring grain length in rice. Genetics 182: 1323–1334. 10.1534/genetics.109.103002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim K-W, Chung H-K, Cho G-T, Ma K-H, Chandrabalan D, Gwag J-G, et al. (2007) PowerCore: a program applying the advanced M strategy with a heuristic search for establishing core sets. Bioinformatics 23: 2155–2162. [DOI] [PubMed] [Google Scholar]
- 25. Zhao W, Cho G-T, Ma K-H, Chung J-W, Gwag J-G, Park Y-J (2010) Development of an allele-mining set in rice using a heuristic algorithm and SSR genotype data with least redundancy for the post-genomic era. Mol Breed 26: 639–651. [Google Scholar]
- 26. Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25: 1754–1760. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20: 1297–1303. 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23: 2633–2635. [DOI] [PubMed] [Google Scholar]
- 29. Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21: 263–265. [DOI] [PubMed] [Google Scholar]
- 30. Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, et al. (2011) The variant call format and VCFtools. Bioinformatics 27: 2156–2158. 10.1093/bioinformatics/btr330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gautier M, Vitalis R (2012) rehh: an R package to detect footprints of selection in genome-wide SNP data from haplotype structure. Bioinformatics 28: 1176–1177. 10.1093/bioinformatics/bts115 [DOI] [PubMed] [Google Scholar]
- 32. Tang W, Sun J, Liu J, Liu F, Yan J, Gou X, et al. (2014) RNAi-directed downregulation of betaine aldehyde dehydrogenase 1 (OsBADH1) results in decreased stress tolerance and increased oxidative markers without affecting glycine betaine biosynthesis in rice (Oryza sativa). Plant Mol Biol 86: 443–454. 10.1007/s11103-014-0239-0 [DOI] [PubMed] [Google Scholar]
- 33. Huang X, Wei X, Sang T, Zhao Q, Feng Q, Zhao Y, et al. (2010) Genome-wide association studies of 14 agronomic traits in rice landraces. Nat Genet 42: 961–967. 10.1038/ng.695 [DOI] [PubMed] [Google Scholar]
- 34. Jin L, Lu Y, Xiao P, Sun M, Corke H, Bao J (2010) Genetic diversity and population structure of a diverse set of rice germplasm for association mapping. Theor Appl Genet 121: 475–487. 10.1007/s00122-010-1324-7 [DOI] [PubMed] [Google Scholar]
- 35. Wang M, Yu Y, Haberer G, Marri PR, Fan C, Goicoechea JL, et al. (2014) The genome sequence of African rice (Oryza glaberrima) and evidence for independent domestication. Nat Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Linares OF (2002) African rice (Oryza glaberrima): history and future potential. Proceedings of the National Academy of Sciences 99: 16360–16365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
badh1.3, badh1.5 and badh1.6 were detected by previous study [1]. badh1.1, badh1.2, badh1.4 and badh1.7 were detected in this study.
(DOCX)
badh2.1~badh2.2 [11]; badh2.11~badh2.12, badh2.14 [10]; badh2.13 [16]; badh2.15 [12]. badh2.16~badh2.18 were detected in this study.
(DOCX)
(DOCX)
(DOCX)
(Excel)
(XLSX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.