Abstract
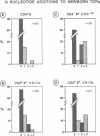
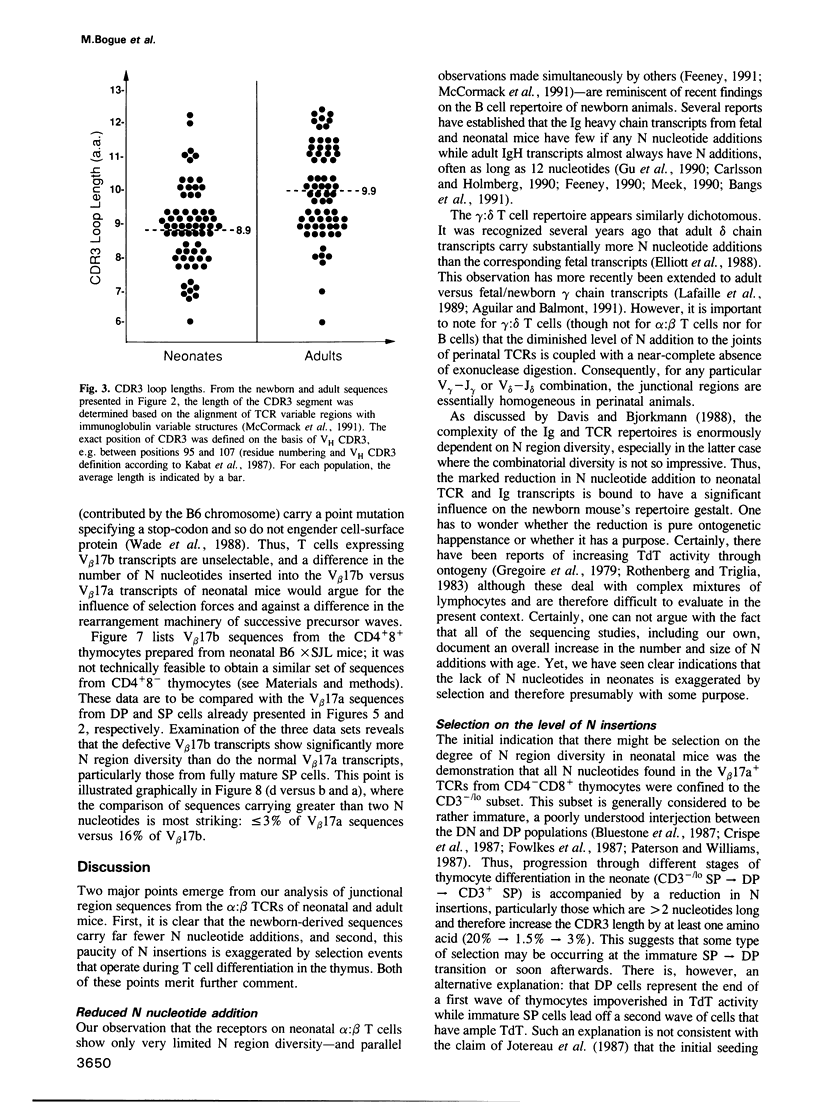
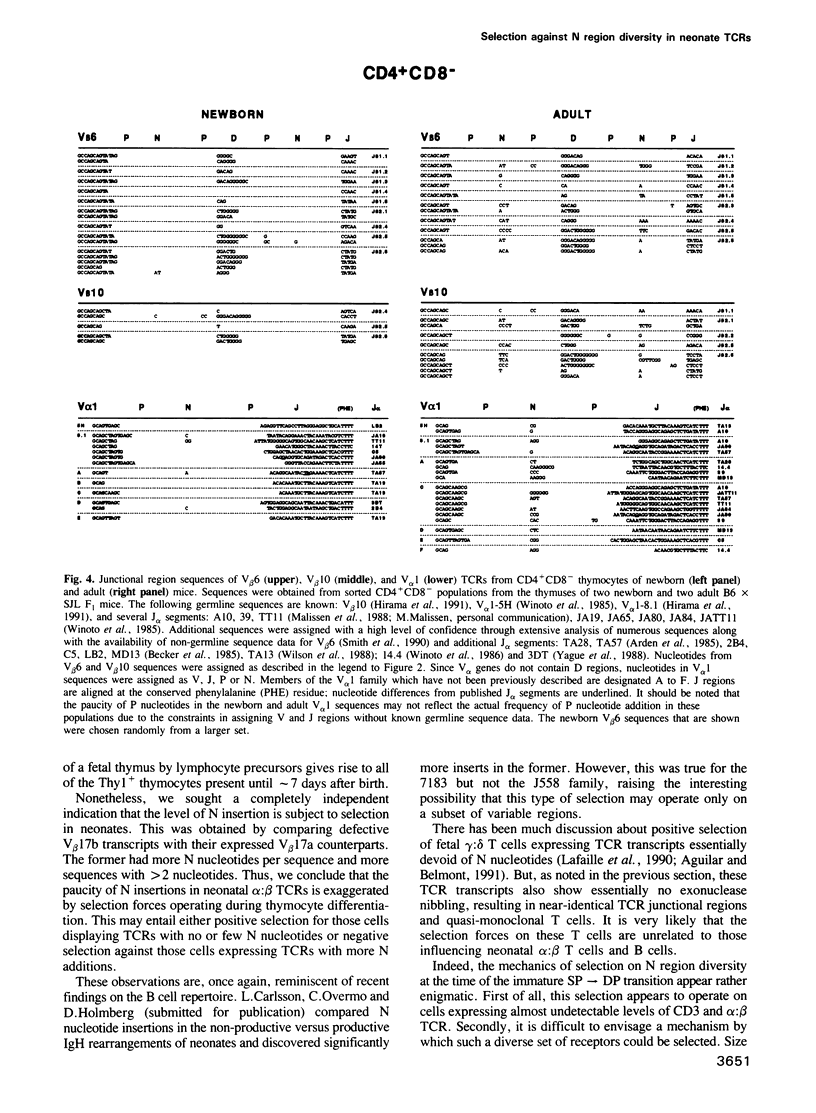
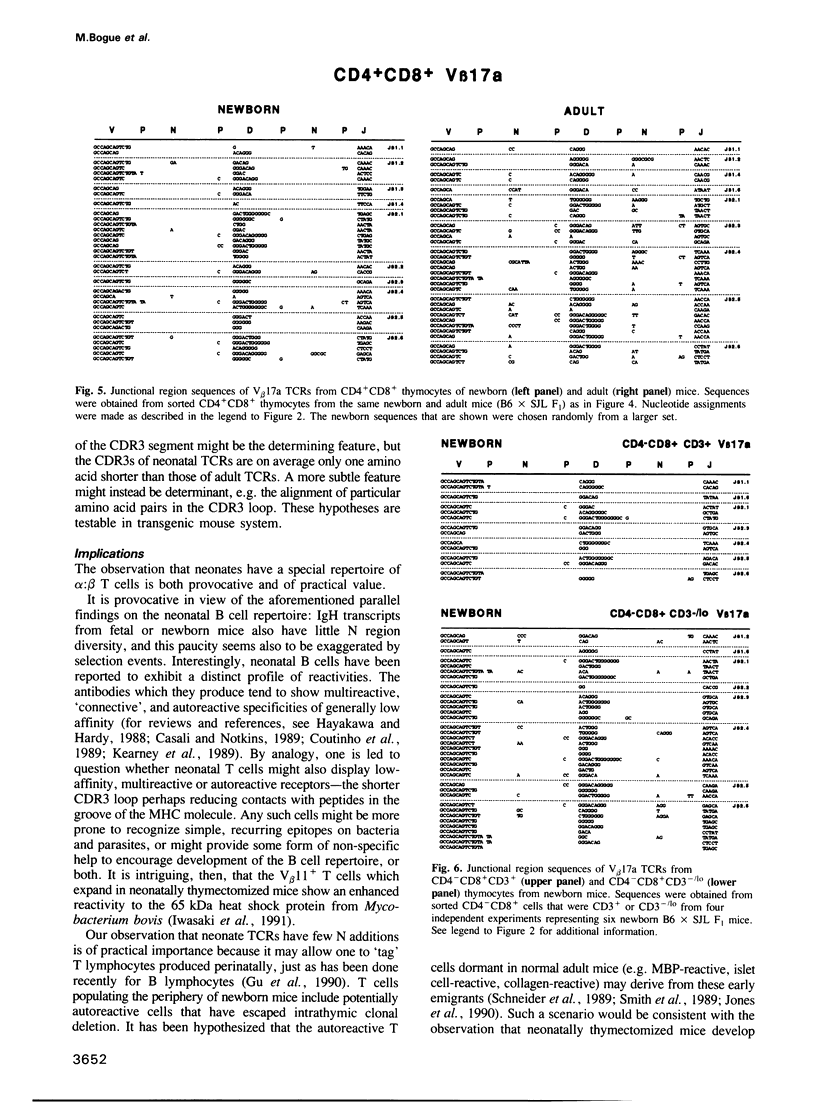
According to several functional criteria, the mature thymocytes of neonatal and adult mice are distinctly different. We wondered whether these differences in function might have a structural correlate: do neonates have a distinct repertoire of alpha:beta T cells? In this study, we have exploited the power of polymerase chain reaction technology to generate large numbers of T cell receptor sequences from sorted thymocyte populations from newborn and adult mice. The newborn-derived sequences show very few N nucleotide additions, usually the major source of diversity in T cell receptors. Most interestingly, the paucity of N insertions appears to be exaggerated by selection events that operate during T cell differentiation in the thymus. The significance of these results is largely: (i) that they parallel recent findings on the B cell repertoire in neonates, raising questions about the reactivities specified by such a special repertoire; and (ii) that they suggest a means to 'tag' T cells exported perinatally, allowing one to test the premise that autoreactive T cells derive preferentially from the newborn repertoire.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar L. K., Belmont J. W. V gamma 3 T cell receptor rearrangement and expression in the adult thymus. J Immunol. 1991 Feb 15;146(4):1348–1352. [PubMed] [Google Scholar]
- Arden B., Klotz J. L., Siu G., Hood L. E. Diversity and structure of genes of the alpha family of mouse T-cell antigen receptor. 1985 Aug 29-Sep 4Nature. 316(6031):783–787. doi: 10.1038/316783a0. [DOI] [PubMed] [Google Scholar]
- Bangs L. A., Sanz I. E., Teale J. M. Comparison of D, JH, and junctional diversity in the fetal, adult, and aged B cell repertoires. J Immunol. 1991 Mar 15;146(6):1996–2004. [PubMed] [Google Scholar]
- Becker D. M., Pattern P., Chien Y., Yokota T., Eshhar Z., Giedlin M., Gascoigne N. R., Goodnow C., Wolf R., Arai K. Variability and repertoire size of T-cell receptor V alpha gene segments. Nature. 1985 Oct 3;317(6036):430–434. doi: 10.1038/317430a0. [DOI] [PubMed] [Google Scholar]
- Benoist C., Mathis D. Positive selection of the T cell repertoire: where and when does it occur? Cell. 1989 Sep 22;58(6):1027–1033. doi: 10.1016/0092-8674(89)90501-1. [DOI] [PubMed] [Google Scholar]
- Berg L. J., Fazekas de St Groth B., Pullen A. M., Davis M. M. Phenotypic differences between alpha beta versus beta T-cell receptor transgenic mice undergoing negative selection. Nature. 1989 Aug 17;340(6234):559–562. doi: 10.1038/340559a0. [DOI] [PubMed] [Google Scholar]
- Bill J., Appel V. B., Palmer E. An analysis of T-cell receptor variable region gene expression in major histocompatibility complex disparate mice. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9184–9188. doi: 10.1073/pnas.85.23.9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone J. A., Pardoll D., Sharrow S. O., Fowlkes B. J. Characterization of murine thymocytes with CD3-associated T-cell receptor structures. Nature. 1987 Mar 5;326(6108):82–84. doi: 10.1038/326082a0. [DOI] [PubMed] [Google Scholar]
- Borgulya P., Kishi H., Müller U., Kirberg J., von Boehmer H. Development of the CD4 and CD8 lineage of T cells: instruction versus selection. EMBO J. 1991 Apr;10(4):913–918. doi: 10.1002/j.1460-2075.1991.tb08024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd R. L., Hugo P. Towards an integrated view of thymopoiesis. Immunol Today. 1991 Feb;12(2):71–79. doi: 10.1016/0167-5699(91)90161-L. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Holmberg D. Genetic basis of the neonatal antibody repertoire: germline V-gene expression and limited N-region diversity. Int Immunol. 1990;2(7):639–643. doi: 10.1093/intimm/2.7.639. [DOI] [PubMed] [Google Scholar]
- Casali P., Notkins A. L. Probing the human B-cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Annu Rev Immunol. 1989;7:513–535. doi: 10.1146/annurev.iy.07.040189.002501. [DOI] [PubMed] [Google Scholar]
- Cazenave P. A., Marche P. N., Jouvin-Marche E., Voegtlé D., Bonhomme F., Bandeira A., Coutinho A. V beta 17 gene polymorphism in wild-derived mouse strains: two amino acid substitutions in the V beta 17 region greatly alter T cell receptor specificity. Cell. 1990 Nov 16;63(4):717–728. doi: 10.1016/0092-8674(90)90138-5. [DOI] [PubMed] [Google Scholar]
- Ceredig R. Intrathymic proliferation of perinatal mouse alpha beta and gamma delta T cell receptor-expressing mature T cells. Int Immunol. 1990;2(9):859–867. doi: 10.1093/intimm/2.9.859. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Waltzinger C. Neonatal mouse CD4+ mature thymocytes show responsiveness to interleukin 2 and interleukin 7: growth in vitro of negatively selected V beta 6- and V beta 11-expressing CD4+ cells from (C57BL/6 x DBA/2)F1 mice. Int Immunol. 1990;2(9):869–877. doi: 10.1093/intimm/2.9.869. [DOI] [PubMed] [Google Scholar]
- Chothia C., Boswell D. R., Lesk A. M. The outline structure of the T-cell alpha beta receptor. EMBO J. 1988 Dec 1;7(12):3745–3755. doi: 10.1002/j.1460-2075.1988.tb03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claverie J. M., Prochnicka-Chalufour A., Bougueleret L. Implications of a Fab-like structure for the T-cell receptor. Immunol Today. 1989 Jan;10(1):10–14. doi: 10.1016/0167-5699(89)90058-3. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Bandeira A., Pereira P., Portnoi D., Holmberg D., Martinez C., Freitas A. A. Selection of lymphocyte repertoires: the limits of clonal versus network organization. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):159–170. doi: 10.1101/sqb.1989.054.01.020. [DOI] [PubMed] [Google Scholar]
- Crispe I. N., Shimonkevitz R. P., Husmann L. A., Kimura J., Allison J. P. Expression of T cell antigen receptor beta-chains on subsets of mouse thymocytes. Analysis by three-color flow cytometry. J Immunol. 1987 Dec 1;139(11):3585–3589. [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Elliott J. F., Rock E. P., Patten P. A., Davis M. M., Chien Y. H. The adult T-cell receptor delta-chain is diverse and distinct from that of fetal thymocytes. Nature. 1988 Feb 18;331(6157):627–631. doi: 10.1038/331627a0. [DOI] [PubMed] [Google Scholar]
- Feeney A. J. Junctional sequences of fetal T cell receptor beta chains have few N regions. J Exp Med. 1991 Jul 1;174(1):115–124. doi: 10.1084/jem.174.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney A. J. Lack of N regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J Exp Med. 1990 Nov 1;172(5):1377–1390. doi: 10.1084/jem.172.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes B. J., Kruisbeek A. M., Ton-That H., Weston M. A., Coligan J. E., Schwartz R. H., Pardoll D. M. A novel population of T-cell receptor alpha beta-bearing thymocytes which predominantly expresses a single V beta gene family. Nature. 1987 Sep 17;329(6136):251–254. doi: 10.1038/329251a0. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Chien Y., Becker D. M., Kavaler J., Davis M. M. Genomic organization and sequence of T-cell receptor beta-chain constant- and joining-region genes. Nature. 1984 Aug 2;310(5976):387–391. doi: 10.1038/310387a0. [DOI] [PubMed] [Google Scholar]
- Gregoire K. E., Goldschneider I., Barton R. W., Bollum F. J. Ontogeny of terminal deoxynucleotidyl transferase-positive cells in lymphohemopoietic tissues of rat and mouse. J Immunol. 1979 Sep;123(3):1347–1352. [PubMed] [Google Scholar]
- Gu H., Förster I., Rajewsky K. Sequence homologies, N sequence insertion and JH gene utilization in VHDJH joining: implications for the joining mechanism and the ontogenetic timing of Ly1 B cell and B-CLL progenitor generation. EMBO J. 1990 Jul;9(7):2133–2140. doi: 10.1002/j.1460-2075.1990.tb07382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidos C. J., Danska J. S., Fathman C. G., Weissman I. L. T cell receptor-mediated negative selection of autoreactive T lymphocyte precursors occurs after commitment to the CD4 or CD8 lineages. J Exp Med. 1990 Sep 1;172(3):835–845. doi: 10.1084/jem.172.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R. Normal, autoimmune, and malignant CD5+ B cells: the Ly-1 B lineage? Annu Rev Immunol. 1988;6:197–218. doi: 10.1146/annurev.iy.06.040188.001213. [DOI] [PubMed] [Google Scholar]
- Hengartner H., Odermatt B., Schneider R., Schreyer M., Wälle G., MacDonald H. R., Zinkernagel R. M. Deletion of self-reactive T cells before entry into the thymus medulla. Nature. 1988 Nov 24;336(6197):388–390. doi: 10.1038/336388a0. [DOI] [PubMed] [Google Scholar]
- Hirama T., Takeshita S., Matsubayashi Y., Iwashiro M., Masuda T., Kuribayashi K., Yoshida Y., Yamagishi H. Conserved V(D)J junctional sequence of cross-reactive cytotoxic T cell receptor idiotype and the effect of a single amino acid substitution. Eur J Immunol. 1991 Feb;21(2):483–488. doi: 10.1002/eji.1830210234. [DOI] [PubMed] [Google Scholar]
- Howe M. L., Goldstein A. L., Battisto J. R. Isogeneic lymphocyte interaction: recognition of self antigens by cells of the neonatal thymus. Proc Natl Acad Sci U S A. 1970 Oct;67(2):613–619. doi: 10.1073/pnas.67.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo P., Boyd R. L., Waanders G. A., Petrie H. T., Scollay R. Timing of deletion of autoreactive V beta 6+ cells and down-modulation of either CD4 or CD8 on phenotypically distinct CD4+8+ subsets of thymocytes expressing intermediate or high levels of T cell receptor. Int Immunol. 1991 Mar;3(3):265–272. doi: 10.1093/intimm/3.3.265. [DOI] [PubMed] [Google Scholar]
- Iwasaki A., Yoshikai Y., Yuuki H., Takimoto H., Nomoto K. Self-reactive T cells are activated by the 65-kDa mycobacterial heat-shock protein in neonatally thymectomized mice. Eur J Immunol. 1991 Mar;21(3):597–603. doi: 10.1002/eji.1830210310. [DOI] [PubMed] [Google Scholar]
- Jones L. A., Chin L. T., Merriam G. R., Nelson L. M., Kruisbeck A. M. Failure of clonal deletion in neonatally thymectomized mice: tolerance is preserved through clonal anergy. J Exp Med. 1990 Nov 1;172(5):1277–1285. doi: 10.1084/jem.172.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jotereau F., Heuze F., Salomon-Vie V., Gascan H. Cell kinetics in the fetal mouse thymus: precursor cell input, proliferation, and emigration. J Immunol. 1987 Feb 15;138(4):1026–1030. [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Wade T., White J., Kushnir E., Blackman M., Bill J., Roehm N., Marrack P. A T cell receptor V beta segment that imparts reactivity to a class II major histocompatibility complex product. Cell. 1987 Apr 24;49(2):263–271. doi: 10.1016/0092-8674(87)90567-8. [DOI] [PubMed] [Google Scholar]
- Kavaler J., Davis M. M., Chien Y. Localization of a T-cell receptor diversity-region element. Nature. 1984 Aug 2;310(5976):421–423. doi: 10.1038/310421a0. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Vakil M., Solvason N. The role of idiotypic interactions and B-cell subsets in development of the B-cell repertoire. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):203–207. doi: 10.1101/sqb.1989.054.01.025. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Lafaille J. J., DeCloux A., Bonneville M., Takagaki Y., Tonegawa S. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989 Dec 1;59(5):859–870. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- Lafaille J. J., Haas W., Coutinho A., Tonegawa S. Positive selection of gamma delta T cells. Immunol Today. 1990 Mar;11(3):75–78. doi: 10.1016/0167-5699(90)90030-d. [DOI] [PubMed] [Google Scholar]
- Lattime E. C., Stutman O. L3T4+, B2A2+ thymocytes from infant mice produce IL 2 after interaction with accessory cells expressing self class II antigens. J Immunol. 1986 Apr 15;136(8):2741–2746. [PubMed] [Google Scholar]
- Le Meur M., Gerlinger P., Benoist C., Mathis D. Correcting an immune-response deficiency by creating E alpha gene transgenic mice. Nature. 1985 Jul 4;316(6023):38–42. doi: 10.1038/316038a0. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Schneider R., Lees R. K., Howe R. C., Acha-Orbea H., Festenstein H., Zinkernagel R. M., Hengartner H. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988 Mar 3;332(6159):40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- Malissen M., Minard K., Mjolsness S., Kronenberg M., Goverman J., Hunkapiller T., Prystowsky M. B., Yoshikai Y., Fitch F., Mak T. W. Mouse T cell antigen receptor: structure and organization of constant and joining gene segments encoding the beta polypeptide. Cell. 1984 Jul;37(3):1101–1110. doi: 10.1016/0092-8674(84)90444-6. [DOI] [PubMed] [Google Scholar]
- Malissen M., Trucy J., Letourneur F., Rebaï N., Dunn D. E., Fitch F. W., Hood L., Malissen B. A T cell clone expresses two T cell receptor alpha genes but uses one alpha beta heterodimer for allorecognition and self MHC-restricted antigen recognition. Cell. 1988 Oct 7;55(1):49–59. doi: 10.1016/0092-8674(88)90008-6. [DOI] [PubMed] [Google Scholar]
- Meek K. Analysis of junctional diversity during B lymphocyte development. Science. 1990 Nov 9;250(4982):820–823. doi: 10.1126/science.2237433. [DOI] [PubMed] [Google Scholar]
- Nikolić-Zugić J. Phenotypic and functional stages in the intrathymic development of alpha beta T cells. Immunol Today. 1991 Feb;12(2):65–70. doi: 10.1016/0167-5699(91)90160-u. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Pircher H., Bürki K., Zinkernagel R. M., Hengartner H. Distinct sequence of negative or positive selection implied by thymocyte T-cell receptor densities. Nature. 1990 Aug 30;346(6287):861–863. doi: 10.1038/346861a0. [DOI] [PubMed] [Google Scholar]
- Paterson D. J., Williams A. F. An intermediate cell in thymocyte differentiation that expresses CD8 but not CD4 antigen. J Exp Med. 1987 Nov 1;166(5):1603–1608. doi: 10.1084/jem.166.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H., Bürki K., Lang R., Hengartner H., Zinkernagel R. M. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989 Nov 30;342(6249):559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- Pullen A. M., Marrack P., Kappler J. W. The T-cell repertoire is heavily influenced by tolerance to polymorphic self-antigens. Nature. 1988 Oct 27;335(6193):796–801. doi: 10.1038/335796a0. [DOI] [PubMed] [Google Scholar]
- Riggs J. E., Stowers R. S., Mosier D. E. Adoptive transfer of neonatal T lymphocytes rescues immunoglobulin production in mice with severe combined immune deficiency. J Exp Med. 1991 Jan 1;173(1):265–268. doi: 10.1084/jem.173.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey E. A., Fowlkes B. J., Gordon J. W., Kioussis D., von Boehmer H., Ramsdell F., Axel R. Thymic selection in CD8 transgenic mice supports an instructive model for commitment to a CD4 or CD8 lineage. Cell. 1991 Jan 11;64(1):99–107. doi: 10.1016/0092-8674(91)90212-h. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Triglia D. Clonal proliferation unlinked to terminal deoxynucleotidyl transferase synthesis in thymocytes of young mice. J Immunol. 1983 Apr;130(4):1627–1633. [PubMed] [Google Scholar]
- Sakaguchi S., Sakaguchi N. Thymus and autoimmunity: capacity of the normal thymus to produce pathogenic self-reactive T cells and conditions required for their induction of autoimmune disease. J Exp Med. 1990 Aug 1;172(2):537–545. doi: 10.1084/jem.172.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R., Lees R. K., Pedrazzini T., Zinkernagel R. M., Hengartner H., MacDonald H. R. Postnatal disappearance of self-reactive (V beta 6+) cells from the thymus of Mlsa mice. Implications for T cell development and autoimmunity. J Exp Med. 1989 Jun 1;169(6):2149–2158. doi: 10.1084/jem.169.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler W., Ruetsch N. R., Amsler M., Bosma M. J. Coding joint formation of endogenous T cell receptor genes in lymphoid cells from scid mice: unusual P-nucleotide additions in VJ-coding joints. Eur J Immunol. 1991 Mar;21(3):589–596. doi: 10.1002/eji.1830210309. [DOI] [PubMed] [Google Scholar]
- Sha W. C., Nelson C. A., Newberry R. D., Kranz D. M., Russell J. H., Loh D. Y. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988 Nov 3;336(6194):73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- Shortman K., Vremec D., Egerton M. The kinetics of T cell antigen receptor expression by subgroups of CD4+8+ thymocytes: delineation of CD4+8+3(2+) thymocytes as post-selection intermediates leading to mature T cells. J Exp Med. 1991 Feb 1;173(2):323–332. doi: 10.1084/jem.173.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H., Chen I. M., Kubo R., Tung K. S. Neonatal thymectomy results in a repertoire enriched in T cells deleted in adult thymus. Science. 1989 Aug 18;245(4919):749–752. doi: 10.1126/science.2788921. [DOI] [PubMed] [Google Scholar]
- Smith L. R., Plaza A., Singer P. A., Theofilopoulos A. N. Coding sequence polymorphisms among V beta T cell receptor genes. J Immunol. 1990 Apr 15;144(8):3234–3237. [PubMed] [Google Scholar]
- Tomonari K. A rat antibody against a structure functionally related to the mouse T-cell receptor/T3 complex. Immunogenetics. 1988;28(6):455–458. doi: 10.1007/BF00355379. [DOI] [PubMed] [Google Scholar]
- Tung K. S., Smith S., Teuscher C., Cook C., Anderson R. E. Murine autoimmune oophoritis, epididymoorchitis, and gastritis induced by day 3 thymectomy. Immunopathology. Am J Pathol. 1987 Feb;126(2):293–302. [PMC free article] [PubMed] [Google Scholar]
- Wade T., Bill J., Marrack P. C., Palmer E., Kappler J. W. Molecular basis for the nonexpression of V beta 17 in some strains of mice. J Immunol. 1988 Sep 15;141(6):2165–2167. [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- Wilson R. K., Lai E., Concannon P., Barth R. K., Hood L. E. Structure, organization and polymorphism of murine and human T-cell receptor alpha and beta chain gene families. Immunol Rev. 1988 Jan;101:149–172. doi: 10.1111/j.1600-065x.1988.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Winoto A., Mjolsness S., Hood L. Genomic organization of the genes encoding mouse T-cell receptor alpha-chain. 1985 Aug 29-Sep 4Nature. 316(6031):832–836. doi: 10.1038/316832a0. [DOI] [PubMed] [Google Scholar]
- Winoto A., Urban J. L., Lan N. C., Goverman J., Hood L., Hansburg D. Predominant use of a V alpha gene segment in mouse T-cell receptors for cytochrome c. Nature. 1986 Dec 18;324(6098):679–682. doi: 10.1038/324679a0. [DOI] [PubMed] [Google Scholar]
- Yague J., Blackman M., Born W., Marrack P., Kappler J., Palmer E. The structure of V alpha and J alpha segments in the mouse. Nucleic Acids Res. 1988 Dec 9;16(23):11355–11364. doi: 10.1093/nar/16.23.11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boehmer H., Byrd W. J. Responsiveness of thymus cells to syngeneic and allogeneic lymphoid cells. Nat New Biol. 1972 Jan 12;235(54):50–52. doi: 10.1038/newbio235050a0. [DOI] [PubMed] [Google Scholar]