Abstract
An approach to improve the diagnosis of Strongyloides stercoralis infection is the use of serologic assays utilising the NIE antigen from S. stercoralis, with good diagnostic sensitivity and excellent specificity reported. Detection of antibody eluted from dried blood spots (DBS) has shown utility in large-scale seroepidemiological studies for a range of conditions and is appealing for use with children where sample collection is difficult. We adapted an existing NIE-enzyme linked immunosorbent assay (ELISA) for the testing of strongyloides antibody response on DBS, and evaluated it in a population screening and mass drug administration programme (MDA) for strongyloidiasis conducted in an Australian indigenous community. Study participants were treated with 200 μg/kg ivermectin (>15 kg) or 3× 400 mg albendazole (<15 kg). The sensitivity of the NIE DBS-ELISA was determined by receiver operator characteristic (ROC) analysis to be 85.7%. A total of 214 DBS were collected from 184 participants across two screening and MDA encounters. A total of 27 of 164 participants (16.5%) tested positive for S. stercoralis NIE-DBS prior to MDA treatment, and 6 of 50 participants (12.0%) tested positive after treatment. These prevalence values are similar to those documented by standard serology in the same community. For 30 participants where a DBS was collected at both MDA 1 and 2, a significant decline in ELISA values was evident post treatment (0.12–0.02, p = 0.0012). These results are in agreement with previous studies documenting the high seroprevalence of S. stercoralis in remote Australian Indigenous communities, and suggest that collection of dried blood spots may be a useful approach for field diagnosis of S. stercoralis seroprevalence.
Keywords: Strongyloides stercoralis, Serological diagnosis, Helminth infection, Indigenous health
1. Introduction
Strongyloides stercoralis is a common soil-transmitted helminth infection in tropical and subtropical regions. While chronic infections can be asymptomatic, hyper-infection is associated with high mortality (Keiser and Nutman, 2004). The sensitivity of stool based parasitological diagnostic methods can be low due to variation in larval output particularly in chronic infections (Krolewiecki et al., 2013), and as such multiple stool collections are recommended to improve sensitivity (Khieu et al., 2013). Molecular methods such as quantitative PCR (Repetto et al., 2013; Schär et al., 2013a; Verweij et al., 2009) are a promising alternative, but still rely on the collection of stool which is impractical for large community screening studies or in public health interventions.
Serologic assays such as those based on crude antigen may offer increased sensitivity (Krolewiecki et al., 2010; Sultana et al., 2012; Yori et al., 2006) but are problematic due to the requirement for large amounts of standardised parasite antigen from patients infected with S. stercoralis (Bon et al., 2010; Boscolo et al., 2007; van Doorn et al., 2007). Potential differences in antigenic profiles between S. stercoralis and antigen collected from experimentally infected animals (such as Strongyloides ratti or Strongyloides venezuelensis) may influence the diagnostic performance of assays using these antigens. Furthermore, cross-reactivity with other helminth infections may reduce specificity (Rodrigues et al., 2007; Sultana et al., 2012). Serologic assays utilising recombinant antigen can potentially overcome these problems, and in this respect a 31-kDA recombinant antigen from S. stercoralis (NIE) has been reported to show good diagnostic sensitivity (75–98%) and excellent specificity (94–100%) (Bisoffi et al., 2014; Krolewiecki et al., 2010; Ramanathan et al., 2008; Ravi et al., 2002).
Enzyme linked immunosorbent assays (ELISAs) utilising finger prick dried blood spots (DBS) on filter paper (DBS-ELISA) offer significant practical benefit for large scale seroepidemiological studies, especially in paediatric patients where venepuncture is problematic (Cook et al., 2010; Hardelid et al., 2008). DBS are easily collected with little technical experience or equipment needed, can be air-dried and have minimal transport and storage requirements (Corran et al., 2008).
In remote indigenous communities in northern Australia, strongyloidiasis is endemic with reported prevalence ranging from 15 to 60%, although these estimates are somewhat confounded by heterogeneity in study design and detection methods (Johnson et al., 2005). A recent mass drug administration (MDA) project primarily utilising 200 μg/kg ivermectin was undertaken in a remote Aboriginal community in East Arnhem land of the Northern Territory, Australia (Kearns et al., 2011a). In this work, we report the adaptation existing NIE-ELISA protocols for use on dried blood spots, and the application of this assay to screen blood spots collected in the East Arnhem MDA study for antibodies to S. stercoralis, including comparison of results before and after treatment.
2. Materials and methods
2.1. Sample collection
Samples were collected in 2010–2011 as part the East Arnhem MDA project, aimed to reduce the prevalence of S. stercoralis and skin infestation with the parasitic mite Sarcoptes scabiei. The study was registered with the Australian New Zealand Clinical Trials Registry (ID 12609000654257). Ethical approval was obtained from the Human Research Ethics Committee of the Northern Territory Department of Health & Menzies School of Health Research (approval 09/34).
Samples were collected during a population census and mass drug administration conducted at month 0 (MDA 1) & 12 (MDA 2). Study enrolment and sample collection was undertaken through house-to-house screening. Participants over 15 kg received a single dose of 200 μg/kg oral ivermectin, children under 15 kg and pregnant women received albendazole at a dose of either 200 mg (6–10 kg) or 400 mg (10–15 kg) for 3 consecutive days, in addition to a single application of topical 5% permethrin.
Written informed consent was obtained from all individuals or guardians before sample collection. In this study, DBS were only collected from a subset of participants not consenting to standard blood collection via venepuncture. Participants also had the option of giving a faecal sample as an alternative to venepuncture or DBS collection. As the consent process was undertaken at both MDA 1 and 2, participants may have elected different sample contribution for the second screening (DBS at MDA 1 and venepuncture at MDA 2 for example). DBS were collected in duplicate onto filter paper cards (903 screening cards, Whatman), stored in ziplock bags containing silica beads as desiccant, and kept at 4–8 °C in the field laboratory. DBS were transported daily to the central laboratory at the Menzies School of Health Research in Darwin where they were held at −20 °C until further analysis. Total storage time of DBS was up to 24 months from collection until assays commenced.
2.2. Antigens and control sera
Recombinant NIE antigen was expressed and purified as described previously (Ravi et al., 2002). An aliquot of the recombinant protein was checked by SDS-PAGE electrophoresis to confirm integrity and concentration prior to use. For assay establishment, a panel of control sera from patients with a confirmed or excluded diagnosis strongyloidiasis by faecal and serology testing (n = 10 positive and 10 negative) (Carroll et al., 1981) were provided by Pathwest Laboratory Medicine, WA, Australia. Positive and negative control blood spots were artificially produced by mixing these Strongyloides negative and positive serum samples 1:1 with blood group O– erythrocytes from a healthy donor from an area non-endemic for Strongyloides, and these ‘spiked’ blood spots spotted onto filter paper. Blood spots collected from three study participants who had a positive faecal diagnosis for S. stercoralis (i.e. inadvertent providers both faecal and DBS samples) were also included as positive controls; additional negative control blood spots were collected from consenting healthy donors from non-endemic areas recruited from within our institution (n = 8). Hence a total of 13 positive controls and 18 negative controls were used for the NIE-DBS-ELISA validation.
2.3. Survey of optimal storage and assay methodology
To elute sera from the DBS, 2.5 mm discs were punched from the spot and placed into low binding 96-well plates (Greiner) containing 150 μL phosphate buffered saline and 0.05% Tween-20 (PBS-T), and plates incubated at room temperature overnight with gentle shaking. To define optimal DBS-ELISA dilutions, pooled positive and negative sera pools (comprising a cocktail of the above 10 positive and negative sera samples) and corresponding spiked blood spot elutions were assayed over a range of dilutions (1:200 to 1:7500, in NaCl PBS-T). The DBS dilution that gave the closest optical density (OD) result to the conventional NIE ELISA dilution (1:200) for the corresponding sera, a high positive to negative DBS ratio, and minimal background absorbance was selected for subsequent assays on test samples. To test the effect of storage conditions on assay performance, positive control DBS were stored at 45 °C, 37 °C, ambient (23 °C), 4 °C, −20 °C and −80 °C for 1, 3 and 7 days prior to analysis by NIE DBS-ELISA.
2.4. NIE DBS-ELISA on dried blood spot elutions
DBS were eluted as described previously, and stored at 4 °C or −20 °C for longer term storage prior to ELISA.
For NIE DBS-ELISA, 96-well plates were coated with 100 μl NIE antigen at 0.125 μg/mL in coating buffer (1 mol/L NaHCO3, 1 mol/L Na2CO3, pH 9.6) and incubated overnight at 4 °C. Plates were washed 5 times with PBS-0.05% Tween 20 (PBS-T) and blocked with 5% skim milk powder in PBS-T at 37 °C for 2 h. After washing in PBS-T, DBS elutions at a 1:500 dilution were added and incubated at 37 °C for 2 h. Each plate included the pooled positive and negative control sera and corresponding spiked DBS elution controls at multiple dilutions, a no-antigen control, and control wells where sera and secondary antibodies were omitted. After washing, goat anti human IgG antibody conjugated to Alkaline Phosphatase (Sigma–Aldrich, Castle Hill, Australia), diluted 1:2500 was added and incubated at 37 °C for 2 h. After washing, phosphatase substrate (PnPP, Sigma–Aldrich) was added, the plate incubated in the dark for 30 min, and the OD read at 405 nm.
2.5. Statistical analysis
To account for inter-assay variation, background corrected optical densities were normalised by calculating the ratio of test sample OD to DBS-positive control OD. To calculate assay sensitivity, specificity, and optimal cut-off, Receiver Operating Characteristic (ROC) analysis (Graphpad Prism v5.0) was done using positive and negative control data. Differences in ELISA values obtained before and after treatment (MDA 1 & 2) in the overall and participant matched data were analysed using non-parametric T-tests (overall-Mann Whitney test, participant matched: Wilcoxon matched pairs signed-rank test).
3. Results
3.1. Optimisation of blood spot elution dilution and effect of storage conditions on blood spot stability
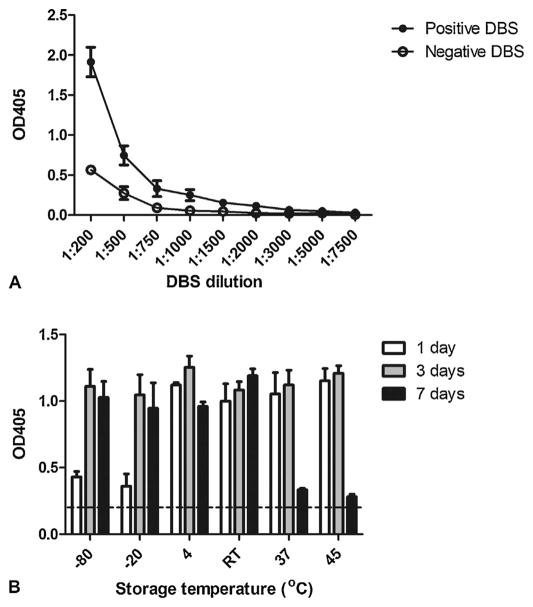
DBS elutions diluted to 1:500 gave good reproducibility and positive:negative ratios, with ODs in a similar range to that obtained for the standard NIE ELISAs which uses sera diluted at 1:200 (Fig. 1a). Lower dilutions of the DBS elutions gave higher ODs for both positive and negative controls indicating non-specific binding, while higher dilutions gave very low ODs for both positive and negative control samples (Fig. 1a).
Fig. 1.
Determination of optimal dried blood spot (DBS) dilutions (A) and storage conditions (B) for NIE DBS-ELISA. (A) Spiked pooled positive and negative control blood spots were prepared and eluted in PBS-T, and different dilutions tested in NIE antigen ELISA. (B) Control blood spots were stored under different conditions for 1, 3 and 7 days. At the conclusion of the storage period all dried blood spots were stored at 4 °C, with elutions and ELISAs of all spots performed in a single assay. Each experiment/storage condition/dilution was repeated twice, and ELISAs were done in triplicate. RT = room temperature. For reference the 0.21 cut-off line is indicated.
A comparison of the effect of different storage temperatures on blood spot stability showed that spots were stable at −80 °C or −20 °C after 3 days storage. Spots were stable at 45 °C, 37 °C or room temperature for up to 3 days, but reactivity decreased thereafter at 37 °C and 45 °C. Spots could also be stored at 4 °C for 7 days with only a slight loss in reactivity (Fig. 1b).
3.2. NIE DBS-ELISA on community samples
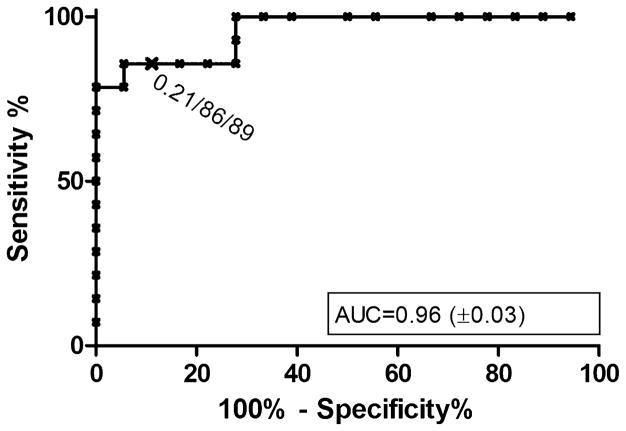
A total of 214 DBS were collected from 184 participants, 124 at MDA 1, and 90 at MDA 2. Of the samples collected at MDA 2, 50 were from participants enrolled and treated at MDA 1, while 40 were from participants new to the study (Table 1). ROC analysis of the positive and negative control spiked blood spots, as well as three blood spots collected from subjects with a positive diagnosis by stool testing, was used to assign ELISA cut-off values. At a cut off of 0.21, the NIE DBS-ELISA had a sensitivity of 85.7% and specificity of 88.9% (ROC Area under curve = 0.96) (Fig. 2). At this designated cut-off among subjects not previously treated, 27/164 (16.5%) tested positive in NIE-DBS ELISA (19/124 [15.3%] at MDA 1, and 8/40 [20.0%] at MDA 2). At MDA 2, the prevalence seropositive in participants screened and treated at MDA 1 was 12.0% (6/50) (Table 1).
Table 1.
Sample details and NIE-DBS ELISA result summary.
| Pre-treatment
|
Post-treatment | |||
|---|---|---|---|---|
| MDA 1 | MDA 2 | Overall pre-treatment | MDA 2 (enrolled/treated at MDA1) | |
| Number of samples | 124 | 40 | 164 | 50 |
| Median age in years (IQR) | 5.3 (3.2–7.8) | 2.3 (1.2–5.1)** | 4.7 (2.3–7) | 4 (2.4–5.5) |
| Median NIE-DBS ELISA result (IQR) | 0.04 (0.01–0.14) | 0.08 (0.08–0.18) | 0.05 (0.02–0.15) | 0.06 (0.01–0.14) |
| NIE-DBS positive (above cut-off 0.21) (%) | 19 (15.3%) | 8 (20.0%) | 27 (16.5%) | 6 (12.0%) |
p = 0.0004.
MDA, mass drug administration; IQR, interquartile range.
Fig. 2.
ROC curve for S. stercoralis NIE-DBS ELISA. The cut-off, sensitivity and specificity indicated were determined using ROC analysis using positive (n = 13) and negative controls (n = 18). AUC = area under curve, ± standard error.
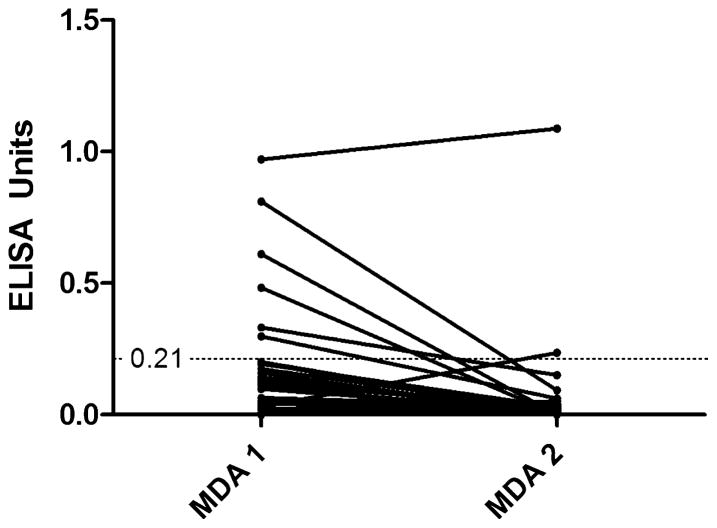
Although no significant difference was observed in median ELISA result between MDA 1 & 2, or before and after treatment (Table 1), a subset of samples from 30 participants where a DBS was collected at both MDA 1 and 2 was available for separate analysis (Fig. 3). Six of these matched samples were NIE-DBS positive at MDA 1, with only one participant remaining positive at MDA 2. One participant with a negative DBS at MDA 1 became positive at MDA 2. Overall comparison of these participant-matched results showed a significant decline in ELISA results at MDA 2 (0.12–0.02, p = 0.0012).
Fig. 3.
Change in ELISA values in participants (n = 30) where a dried blood spot was collected at both MDA 1 & 2. p = 0.0012 (Wilcoxon matched pairs signed rank test).
4. Discussion
Adaptation of the NIE ELISA to the use of DBS for the diagnosis of strongyloidiasis was shown to have good diagnostic performance with 85.7% sensitivity and 88.9% specificity at the designated cutoff. It should be noted however that for this pilot study, there was limited access to DBS from participants or controls subjects who had a parasitologically confirmed diagnosis (only three samples), so most positive controls for this study were indirect, comprising of artificially constructed DBS derived from reference laboratory positive control sera from patients confirmed to be faecal positive for strongyloides, or negative sera and DBS from non-endemic areas with no history of exposure to Strongyloides. We acknowledge these limitations, emphasise the retrospective nature of this study, and note that this study was nested into a much larger community study where DBS collection was not the primary sampling method. This meant that we could not correlate DBS results with corresponding conventional serology or faecal result in the majority of samples. Our estimates of assay sensitivity, cut-off determination and prevalence estimates should therefore be interpreted with caution until further evaluation of a larger, well defined cohort is undertaken. Nevertheless, standard ELISA methods utilising NIE show similar results with sensitivity reported in the range of 84–97%, and specificity of 94–100% (Krolewiecki et al., 2010; Ramanathan et al., 2008; Ravi et al., 2002). It is suggested that compared to ELISA utilising crude antigen derived from S. ratti or S. stercoralis, that the NIE ELISA may have equal or lower sensitivity, although specificity is enhanced with NIE due to the lack of protein homology and consequent low cross-reactivity with other soil transmitted helminths (Ravi et al., 2002). Recent studies report adaptation of the NIE ELISA to a luciferase immunoprecipitation system (NIE-LIPS). This provides enhanced sensitivity, specificity, and wider dynamic range compared to conventional serology (Krolewiecki et al., 2010; Ramanathan et al., 2008). This assay characteristic would be advantageous in screening in non-endemic settings with low prevalence for strongyloidiasis and subsequent requirement for high specificity. Indeed, a recent comparative evaluation of serologic assays for strongyloidiasis indicated a specificity of 100% (Bisoffi et al., 2014), superior to that of conventional NIE serology or our reported NIE-DBS-ELISA. We found a blood spot dilution of 1:500 gave optimal results in terms of reproducible ODs and good signal:background ratio, with higher and lower dilutions giving suboptimal results. It is possible that adapting the NIE-LIPS ELISA to dried blood spots may improve sensitivity over a wider range of dilutions.
Overall, our assay documented a seroprevalence of 16.5% in this small paediatric cohort prior to MDA treatment, decreasing modestly to 12% post treatment at MDA 2. Evaluation of these results was again hindered somewhat by the retrospective design and heterogeneous sampling method for strongyloidiasis in the study and by restrictions on use of ivermectin in paediatric populations. Of the 124 dried blood spots collected at MDA 1, only 30 participants also had a DBS collected at MDA 2, and at MDA 2 some participants were screened and treated at MDA 1 but were sampled by a different method. However, in the 30 matched samples where a blood spot was collected at both MDA 1 and 2, a statistically significant decline in ELISA values at MDA 2 was observed.
In a previously reported study in the same community where testing was undertaken by conventional S. ratti crude antigen ELISA, microscopy and culture of faecal samples, a prevalence of 19% in the 0–10 year old participant group was documented at MDA1, reducing to 14% at MDA 2 (Kearns et al., 2011b). When considering the aforementioned differences in sensitivity and specificity of the two ELISAs, these seroprevalence figures are in reasonable agreement.
Several other studies show a consistent decrease in Strongyloides antibody titre following treatment with follow up periods ranging from 6 to 24 months (Biggs et al., 2009; Karunajeewa et al., 2006; Kobayashi et al., 1994; Loutfy et al., 2002). A serologic follow up period of 6–12 months has been proposed as a reasonable evaluation of treatment efficacy (Loutfy et al., 2002; Requena-Méndez et al., 2013), although serorevision may take longer among patients with very high starting titres (Kobayashi et al., 1994). Of note however, most of these serologic follow up studies have been undertaken in non-endemic settings, where the risk of re-infection is minimal. In a retrospective study evaluation of chronic strongyloides in an endemic Australian indigenous community, Page et al. (2006) documented a reduction in OD from positive to negative or equivocal serostatus in 84.2% of individuals following single dose ivermectin therapy, with a median follow up duration of 367 days. Conversely, a small study in an endemic setting showed that although significant declines in OD were observed, the majority of subjects remained seropositive for up to 542 days following treatment with ivermectin (Lindo et al., 1996). Further follow up would be of interest to investigate if decreases are sustained in subjects beyond 12 months after ivermectin MDA treatment in this endemic setting. With the exception of Costa-Cruz et al. (1998), who detected antibodies to S. ratti using immunofluorescence antibody tests (IFAT), this is the first application of DBS collection for the diagnosis of strongyloidiasis. In contrast, DBS serological testing is commonplace in lymphatic filariasis control programmes and for detection of other parasites such as Toxoplasma gondii, Trypanosoma cruizi and Echinococcus granulosus (Das et al., 2012; Parker and Cubitt, 1999; Terhell et al., 1996; Wattal et al., 2007) (see Smit et al. (2014) for a comprehensive review). Such an approach has recently been applied to malaria transmission studies (Cook et al., 2010). DBS are especially useful for large scale community screening or control programme evaluation, where robust, simple and cost effective collection methods are required in settings with limited laboratory facilities and skilled health care workers. This is of particular relevance to screening for strongyloidiasis in endemic countries, where prevalence may be currently under-estimated and large-scale community screening is advocated (Schär et al., 2013b). In conclusion, the NIE DBS-ELISA showed comparable sensitivity and specificity to routine NIE ELISAs, and this approach could be used more widely to simplify screening for strongyloidiasis, enabling more accurate estimates of prevalence, disease burden, and control programme efficacy of this often neglected but important soil-transmitted helminth.
Acknowledgments
This work was funded by an Australian National Health and Medical Research Council Project Grant (605804), the Cooperative Research Centre for Aboriginal Health, and the Northern Territory Innovation Board. We are grateful to Ian Sampson of Pathwest WA, for provision of control sera and Tegan Harris for database support. We acknowledge the participation of community members and researchers for collection of specimens.
Contributor Information
Kate Mounsey, Email: kmounsey@usc.edu.au, kate.mounsey@qimr.edu.au.
Therese Kearns, Email: therese.kearns@menzies.edu.au.
Melanie Rampton, Email: melanie_rampton@gmail.com.
Stacey Llewellyn, Email: stacey.llewellyn@qimr.edu.au.
Mallory King, Email: mallory.king@qimr.edu.au.
Deborah Holt, Email: deborah.holt@menzies.edu.au.
Bart J. Currie, Email: bart.currie@menzies.edu.au.
Ross Andrews, Email: ross.andrews@menzies.edu.au.
Thomas Nutman, Email: tnutman@niaid.nih.gov.
James McCarthy, Email: j.mccarthy@uq.edu.au.
References
- Biggs BA, Caruana S, Mihrshahi S, Jolley D, Leydon J, Chea L, Nuon S. Management of chronic strongyloidiasis in immigrants and refugees: is serologic testing useful? Am J Trop Med Hyg. 2009;80:788–791. [PubMed] [Google Scholar]
- Bisoffi Z, Buonfrate D, Sequi M, Mejia R, Cimino R, Krolewiecki O, Albonico AJ, Gobbo M, Bonafini M, Angheben S, Requena-Mendez A, Muñoz A, Nutman JTB. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis. 2014;8:e2640. doi: 10.1371/journal.pntd.0002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bon B, Houze S, Talabani H, Magne D, Belkadi G, Develoux M, Senghor Y, Chandenier J, Ancelle T, Hennequin C. Evaluation of a rapid enzyme-linked immunosorbent assay for diagnosis of strongyloidiasis. J Clin Microbiol. 2010;48:1716–1719. doi: 10.1128/JCM.02364-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo M, Gobbo M, Mantovani W, Degani M, Anselmi M, Monteiro GB, Marocco S, Angheben A, Mistretta M, Santacatterina M, Tais S, Bisoffi Z. Evaluation of an indirect immunofluorescence assay for strongyloidiasis as a tool for diagnosis and follow-up. Clin Vaccine Immunol. 2007;14:129–133. doi: 10.1128/CVI.00278-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SM, Karthigasu KT, Grove DI. Serodiagnosis of human strongyloidiasis by an enzyme-linked immunosorbent assay. Trans R Soc Trop Med Hyg. 1981;75:706–709. doi: 10.1016/0035-9203(81)90156-5. [DOI] [PubMed] [Google Scholar]
- Cook J, Reid H, Iavro J, Kuwahata M, Taleo G, Clements A, McCarthy J, Vallely A, Drakeley C. Using serological measures to monitor changes in malaria transmission in Vanuatu. Malar J. 2010;9:169. doi: 10.1186/1475-2875-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corran PH, Cook J, Lynch C, Leenderste H, Manjurano A, Griffin J, Cox J, Abeku T, Bousema T, Ghani AC, Drakeley C, Riley E. Dried blood spots as a source of anti-malarial antibodies for epidemiological studies. Malar J. 2008;7:195. doi: 10.1186/1475-2875-7-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Cruz JM, Machado ER, Campos DM. Seroepidemiological study of human strongyloidiasis with blood samples collected on filter paper, in Abadia dos Dourados (Minas Gerais, Brazil) Rev Inst Med Trop Sao Paulo. 1998;40:329–331. doi: 10.1590/s0036-46651998000500013. [DOI] [PubMed] [Google Scholar]
- Das L, Pani S, Vanamail P, Vijayalakshmi G, Debritto L. Cost-effective antigen testing for delimitation, monitoring and evaluation in bancroftian filariasis. Eur J Clin Microbiol Infect Dis. 2012;31:2069–2075. doi: 10.1007/s10096-011-1542-1. [DOI] [PubMed] [Google Scholar]
- Hardelid P, Williams D, Dezateux C, Tookey PA, Peckham CS, Cubitt WD, Cortina-Borja M. Analysis of rubella antibody distribution from newborn dried blood spots using finite mixture models. Epidemiol Infect. 2008;136:1698–1706. doi: 10.1017/S0950268808000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson FH, Morris PS, Speare R, McCarthy J, Currie B, Ewald D, Page W, Dempsey K. Strongyloidiasis: a review of the evidence for Australian practitioners. Aust J Rural Health. 2005;13:247–254. doi: 10.1111/j.1440-1584.2005.00710.x. [DOI] [PubMed] [Google Scholar]
- Karunajeewa H, Kelly H, Leslie D, Leydon J, Saykao P, Biggs BA. Parasite-specific IgG response and peripheral blood eosinophil count following albendazole treatment for presumed chronic strongyloidiasis. J Travel Med. 2006;13:84–91. doi: 10.1111/j.1708-8305.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- Kearns T, Andrews R, Speare R, Cheng A, McCarthy J, Carapetis J, Holt D, Mulholland E, Currie B, Page WJ, Shield MJ. Ivermectin mass drug administration program to treat endemic scabies and strongyloidiasis in a remote aboriginal community in northern Australia. Trop Med Int Health. 2011a;16:198–199. [Google Scholar]
- Kearns T, Andrews R, Speare R, Cheng A, McCarthy J, Carapetis J, Holt D, Mulholland E, Currie B, Page W, McDonnell J, Shield J. Epidemiology of strongyloidiasis and an ivermectin MDA in a remote Aboriginal community in the Northern Territory. Ann Aust Coll Trop Med. 2011b;12:46. [Google Scholar]
- Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17:208–217. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khieu V, Schär F, Marti H, Sayasone S, Duong S, Muth S, Odermatt P. Diagnosis, treatment and risk factors of Strongyloides stercoralis in schoolchildren in Cambodia. PLoS Negl Trop Dis. 2013;7:e2035. doi: 10.1371/journal.pntd.0002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi J, Sato Y, Toma H, Takara M, Shiroma Y. Application of enzyme immunoassay for postchemotherapy evaluation of human strongyloidiasis. Diagn Microbiol Infect Dis. 1994;18:19–23. doi: 10.1016/0732-8893(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Krolewiecki AJ, Lammie P, Jacobson J, Gabrielli AF, Levecke B, Socias E, Arias LM, Sosa N, Abraham D, Cimino R, Echazu A, Crudo F, Vercruysse J, Albonico M. A public health response against Strongyloides stercoralis: time to look at soil-transmitted helminthiasis in full. PLoS Negl Trop Dis. 2013;7:e2165. doi: 10.1371/journal.pntd.0002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolewiecki AJ, Ramanathan R, Fink V, McAuliffe I, Cajal SP, Won K, Juarez M, Di Paolo A, Tapia L, Acosta N, Lee R, Lammie P, Abraham D, Nutman TB. Improved diagnosis of Strongyloides stercoralis using recombinant antigen-based serologies in a community-wide study in northern Argentina. Clin Vaccine Immunol. 2010;17:1624–1630. doi: 10.1128/CVI.00259-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindo JF, Atkins NS, Lee MG, Robinson RD, Bundy DA. Parasite-specific serum IgG following successful treatment of endemic strongyloidiasis using ivermectin. Trans R Soc Trop Med Hyg. 1996;90:702–703. doi: 10.1016/s0035-9203(96)90444-7. [DOI] [PubMed] [Google Scholar]
- Loutfy MR, Wilson M, Keystone JS, Kain KC. Serology and eosinophil count in the diagnosis and management of strongyloidiasis in a non-endemic area. Am J Trop Med Hyg. 2002;66:749–752. doi: 10.4269/ajtmh.2002.66.749. [DOI] [PubMed] [Google Scholar]
- Page WA, Dempsey K, McCarthy JS. Utility of serological follow-up of chronic strongyloidiasis after anthelminthic chemotherapy. Trans R Soc Trop Med Hyg. 2006;100:1056–1062. doi: 10.1016/j.trstmh.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Parker SP, Cubitt WD. The use of the dried blood sport in epidemiological studies. J Clin Pathol. 1999;52:633–639. doi: 10.1136/jcp.52.9.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan R, Burbelo PD, Groot S, Iadarola MJ, Neva FA, Nutman TB. A luciferase immunoprecipitation systems assay enhances the sensitivity and specificity of diagnosis of Strongyloides stercoralis infection. J Infect Dis. 2008;198:444–451. doi: 10.1086/589718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi V, Ramachandran S, Thompson RW, Andersen JF, Neva FA. Characterization of a recombinant immunodiagnostic antigen (NIE) from Strongyloides stercoralis L3-stage larvae. Mol Biochem Parasitol. 2002;125:73–81. doi: 10.1016/s0166-6851(02)00214-1. [DOI] [PubMed] [Google Scholar]
- Repetto SA, Alba Soto CD, Cazorla SI, Tayeldin ML, Cuello S, Lasala MB, Tekiel VS, González Cappa SM. An improved DNA isolation technique for PCR detection of Strongyloides stercoralis in stool samples. Acta Trop. 2013;126:110–114. doi: 10.1016/j.actatropica.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Requena-Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Muñoz J. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis. 2013;7:e2002. doi: 10.1371/journal.pntd.0002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues RM, de Oliveira MC, Sopelete MC, Silva DA, Campos DM, Taketomi EA, Costa-Cruz JM. IgG1, IgG4, and IgE antibody responses in human strongyloidiasis by ELISA using Strongyloides ratti saline extract as heterologous antigen. Parasitol Res. 2007;101:1209–1214. doi: 10.1007/s00436-007-0602-z. [DOI] [PubMed] [Google Scholar]
- Schär F, Odermatt P, Khieu V, Panning M, Duong S, Muth S, Marti H, Kramme S. Evaluation of real-time PCR for Strongyloides stercoralis and hookworm as diagnostic tool in asymptomatic schoolchildren in Cambodia. Acta Trop. 2013a;126:89–92. doi: 10.1016/j.actatropica.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, Vounatsou P, Odermatt P. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013b;7:e2288. doi: 10.1371/journal.pntd.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit PW, Elliott I, Peeling RW, Mabey D, Newton PN. An overview of the clinical use of filter paper in the diagnosis of tropical diseases. Am J Trop Med Hyg. 2014;90:195–210. doi: 10.4269/ajtmh.13-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana Y, Gilbert GL, Ahmed B, Lee R. Strongyloidiasis in a high risk community of Dhaka, Bangladesh. Trans R Soc Trop Med Hyg. 2012;106:756–762. doi: 10.1016/j.trstmh.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Terhell A, Haarbrink M, Abadi K, Bronneberg D, Tieleman M, Asri M, Yazdanbakhsh M. A filter paper technique for the detection of anti-filarial IgG4 in lymphatic filariasis. Trans R Soc Trop Med Hyg. 1996;90:196–198. doi: 10.1016/s0035-9203(96)90140-6. [DOI] [PubMed] [Google Scholar]
- van Doorn HR, Koelewijn R, Hofwegen H, Gilis H, Wetsteyn JC, Wismans PJ, Sarfati C, Vervoort T, van Gool T. Use of enzyme-linked immunosorbent assay and dipstick assay for detection of Strongyloides stercoralis infection in humans. J Clin Microbiol. 2007;45:438–442. doi: 10.1128/JCM.01735-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij JJ, Canales M, Polman K, Ziem J, Brienen EA, Polderman AM, van Lieshout L. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans R Soc Trop Med Hyg. 2009;103:342–346. doi: 10.1016/j.trstmh.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Wattal S, Dhariwal A, Ralhan P, Tripathi V, Regu KS, Lal KS. Evaluation of Og4C3 antigen ELISA as a tool for detection of bancroftian filariasis under lymphatic filariasis elimination program. J Commun Dis. 2007;39:75–84. [PubMed] [Google Scholar]
- Yori PP, Kosek M, Gilman RH, Cordova J, Bern C, Chavez CB, Olortegui MP, Montalvan C, Sanchez GM, Worthen B, Worthen J, Leung F, Oré CV. Seroepidemiology of strongyloidiasis in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74:97–102. [PMC free article] [PubMed] [Google Scholar]