Abstract
BACKGROUND
Epigenetic differences exist between trauma-exposed individuals with and without posttraumatic stress disorder (PTSD). It is unclear whether these epigenetic differences preexist, or arise following, trauma and PTSD onset.
METHODS
In pre- and post-trauma samples from a subset of Detroit Neighborhood Health Study participants, DNA methylation (DNAm) was measured at DNMT1, DNMT3A, DNMT3B, and DNMT3L. Pre-trauma DNAm differences and changes in DNAm from pre- to post-trauma were assessed between and within PTSD cases (n=30) and age-, gender-, and trauma exposure-matched controls (n=30). Pre-trauma DNAm was tested for association with post-trauma symptom severity (PTSS) change. Potential functional consequences of DNAm differences were explored via bioinformatic search for putative transcription factor binding sites (TFBS).
RESULTS
DNMT1 DNAm increased following trauma in PTSD cases (p=0.001), but not controls (p=0.067). DNMT3A and DNMT3B DNAm increased following trauma in both cases (DNMT3A: p=0.009; DNMT3B: p<0.001) and controls (DNMT3A: p=0.002; DNMT3B: p<0.001). In cases only, pre-trauma DNAm was lower at a DNMT3B CpG site that overlaps with a TFBS involved in epigenetic regulation (p=0.001); lower pre-trauma DNMT3B DNAm at this site was predictive of worsening of PTSS post-trauma (p=0.034). Some effects were attenuated following correction for multiple hypothesis testing.
CONCLUSIONS
DNAm among trauma-exposed individuals shows both longitudinal changes and preexisting epigenetic states that differentiate individuals who are resilient vs. susceptible to PTSD. These distinctive DNAm differences within DNMT loci may contribute to genome-wide epigenetic profiles of PTSD.
Keywords: posttraumatic stress disorder, epigenetics, DNA methylation, susceptibility, longitudinal, epidemiology
Introduction
PTSD is a prevalent and debilitating mental health disorder that may arise following exposure to a potentially traumatic event (Association, 2013). While the lifetime prevalence of traumatic exposure is 50–90%(Kessler et al., 1995), PTSD in the general U.S. population is estimated to be only 6.8%(Kessler and Wang, 2008). Although the majority of persons exposed to trauma display resiliency (Kessler et al., 1995; Breslau et al., 1998; Acierno et al., 2007; Kessler and Wang, 2008), the molecular underpinnings of risk remain poorly characterized. The identification of risk markers, and particularly biomarkers, that distinguish between persons at high and low risk of developing PTSD following trauma exposure has been identified as a priority research goal by the Institute of Medicine (Medicine, 2012), Department of Defense(Congressionally Directed Medical Research Programs, 2011), and the National Institute of Mental Health (NIMH, 2008). Ideally, the ability to identify persons at high risk of developing PTSD would enable providers to target evidence-based interventions to high-risk groups(Andrews and Neises, 2012). The identification of robust predictive biomarkers may also improve our understanding of the pathophysiology of PTSD and lead to more effective pharmacological interventions.
Although much work has been done to identify social and environmental factors that contribute to PTSD risk [e.g. (Kulka et al., 1990; Breslau et al., 1991; Brewin et al., 2000; Koenen et al., 2003; Breslau et al., 2004; DiGrande et al., 2008; Galea et al., 2008; Kun et al., 2009)], the biological undergirding of differential PTSD risk and resiliency remains to be more fully elucidated. Twin studies have demonstrated heritability and genetic contribution to PTSD risk(True et al., 1993; Koenen et al., 2002; Stein et al., 2002) and targeted gene and GWAS approaches have identified both genetic risk loci(Lu et al., 2008; Ressler et al., 2011; Chang et al., 2012; Logue et al., 2013) and important gene-by-environment interactions (Binder et al., 2008; Xie et al., 2010; Uddin et al., 2013) that contribute to risk for the disorder; nevertheless, a substantial proportion of biologically mediated variance in PTSD risk has yet to be explained.
Epigenetic variability is considered a plausible and increasingly empirically supported contributor to the etiology of phenotypes with marked genetic and environmental influences(Meaney, 2010), including certain psychopathologies(Toyokawa et al., 2012). Indeed, recent advances have revealed that PTSD risk and resiliency is associated with differential epigenetic variation(El-Sayed et al., 2012). Epigenetic mechanisms – including histone modifications, non-protein coding RNAs, and, most notably, DNA methylation (DNAm) – affect gene expression and cellular phenotype without altering the underlying DNA sequence(Feinberg, 2008; Meaney, 2010). DNAm is stably heritable across mitotic replications, but is modifiable throughout the life course in response to lived experiences and environmental exposures(Bird, 2002). In primordial mammalian germ cells, global DNAm is removed (with the exception of imprinted loci)(Reik et al., 2001), with new patterns established by de novo methyltransferases DNMT3A, DNMT3B, and DNMT3L following fertilization(Bourc’his et al., 2001; Bourc’his and Bestor, 2004; Kaneda et al., 2004; Kato et al., 2007; Ooi et al., 2007). These reprogrammed DNAm patterns are largely maintained throughout mitotic DNA replication by the action of the maintenance methyltransferase, DNMT1(Li et al., 1992; Seisenberger et al., 2013).
Although influenced by other variables, global DNAm patterns are largely established and maintained by the activity of the DNA methyltransferases, DNMT1, DNMT3A, DNMT3B, and DNMT3L(Feng and Fan, 2009). Gene expression evidence suggests that these DNMTs may be active throughout the life course(Robertson et al., 1999; Feng et al., 2005; Siegmund et al., 2007), including in brain tissue(Goto et al., 1994; Veldic et al., 2004; Feng et al., 2005) and in association with mental disorders(Veldic et al., 2004; Veldic et al., 2005). In addition, protein-level expression of DNMT1(Inano et al., 2000; Veldic et al., 2005) and DNMT3A(Feng et al., 2005) has been demonstrated in the mouse and human brain. With respect to PTSD, recent work confirms that DNMT activity plays a role in mediating risk for PTSD-related phenotypes, including fear conditioning and memory consolidation (Miller and Sweatt, 2007; Feng et al., 2010). Together, these findings suggest that DNAm and DNA methyltransferases represent promising targets for the identification of epigenetic underpinnings of differential PTSD risk and resiliency.
Studies of epigenetic variation have provided important insights into PTSD risk, but have been largely limited by cross-sectional analyses of post-trauma samples. Most notably, epidemiological cohorts from Detroit(Uddin et al., 2010) and Atlanta(Smith et al., 2011) have been the basis of research that has demonstrated cross-sectional differential DNAm that distinguishes between trauma-exposed individuals with vs. without PTSD. DNMT3B and DNMT3L were among the differentially methylated loci identified in the Detroit study(Uddin et al., 2010). More recently, longitudinal DNAm data among PTSD cases and controls have been reported, including studies using samples from a cohort of U.S. military personnel deployed to Iraq and Afghanistan(Rusiecki et al., 2012; Rusiecki et al., 2013). To further elucidate whether differential DNAm between trauma exposed controls and PTSD cases represent pre-existing susceptibility/resiliency factors or downstream biomarkers of PTSD, additional longitudinal analyses are required. Finally, while the identification of epigenetic variation associated with mental health outcomes is important, work must begin to test the putative functionality of mental health-associated differential DNAm. For example, the identification of transcription factor binding sites (TFBS) that overlap with differentially methylated CpG sites and to which transcription factor binding may be disrupted offer one possibility of supporting DNAm functionality(Weaver et al., 2004).
Here, we analyze DNAm from individuals pre- and post-trauma to identify differences that characterize individuals who are susceptible vs. resilient to PTSD following trauma. To assess potential functional consequences of examined DNAm differences, we then perform a bioinformatic search for the presence of putative transcription factor binding sites(Weaver et al., 2004). Results from this work suggest that PTSD-relevant DNAm differences in DNMT loci may exist both prior to and following trauma, with implications for future targeted interventions.
Methods and Materials
Subjects
Samples are from a subset of participants from the Detroit Neighborhood Health Study (DNHS), a longitudinal, community-representative cohort of adult residents in Detroit, MI. The current study draws on peripheral blood samples and survey data obtained at two time points were from 60 DNHS participants. Forty-six were female and fourteen male; forty-six were African-American and 12 were Caucasian, and 2 were Hispanic. The average age was 55.1 years. PTSD diagnosis was assessed via structured interview administered via telephone(Breslau et al., 1998). PTSD symptoms were assessed in reference to both the traumatic event the participant regarded as their worst and one randomly selected traumatic event from the remaining traumas the participant experienced. Lifetime PTSD cases met all six DSM-IV criteria in reference to either the worst or random traumatic event. The diagnostic interview showed good validity against the Clinician Administered PTSD Scale (Blake et al., 1995) as described elsewhere(Uddin et al., 2010). The Institutional Review Board of the University of Michigan reviewed and approved the study protocol. Incident cases (n=30) of PTSD were identified in either waves 2, 3, or 4 of DNHS data collection among individuals for whom blood samples were available at both the wave of first PTSD diagnosis and the immediately previous, pre-incident trauma wave. Non-PTSD controls (n=30) were matched to cases on the basis of age, sex, and number of traumatic event types. DNA samples were isolated from both pre- and post-trauma time points for both cases and controls. The time between pre- and post-trauma time points was approximately 1 year. Cases and controls had no history of PTSD prior to the post-trauma wave.
Methylation quantification by targeted bisulfite pyrosequencing
DNA Isolation
DNA was isolated from whole blood acquired via venipuncture when available from DNHS participants selected for inclusion in this study. Blood spots were used as an alternate source of whole blood-derived DNA when venipuncture samples were unavailable. The exact tissue type was shared between matched case-control pairs in all instances. Venipuncture- and bloodspot-derived whole blood represent the same tissue and therefore should not differ with respect to DNAm, as confirmed by numerous studies to date (Wong et al., 2008; Aberg et al., 2013; Hollegaard et al., 2013).
Whole blood
DNA was isolated from whole blood using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA) and the QuickGene DNA Whole blood Kit S (Lifesciences, FujiFilm, Tokyo, Japan) using manufacturers’ recommended protocols.
Blood spots
DNA was isolated using the QIAamp DNA Micro Kit (Qiagen) using the manufacturer’s recommended protocol. For each sample, one 6mm punch was taken from dried blood spots using a disposable, sterile biopsy punch (Miltex, York, PA) within a sterile field and placed immediately into a sterile 1.7ml microcentrifuge tube. New gloves, biopsy punches, and sterile fields were utilized for each sample. Negative controls in the form of blank extractions were included with all DNA isolations.
Bisulfite conversion
For each sample, ~750ng of DNA was bisulfite converted using the EpiTect Bisulfite Kit (Qiagen) using the manufacturer’s recommended protocol. Negative controls in the form of bisulfite conversion of water were included with each bisulfite conversion.
Pyrosequencing
Assays to assess the methylation levels of CpG sites found in the DNMT1, DNMT3A, and DNMT3L and DNMT3B (see below for assay-specific details) were custom designed using the Pyromark Q24 Assay Design Software 2.0 (Qiagen). Targeted CpG sites were selected based on prior evidence(Uddin et al., 2010) of involvement in epigenetic regulation of PTSD risk (DNMT3B, DNMT3L) and to investigate whether longitudinal, PTSD-associated DNAm differences exist across DNA methyltransferase genes more broadly (DNMT1, DNMT3A, DNMT3B, and DNMT3L). Because the DNMT3B target CpG is located in a CpG island, our designed assay captures DNAm at 12 CpG sites in an approximately 70 base pair region of exon 1 (see DNMT3B assay section below for details). Single CpG sites were assessed at DNMT1, DNMT3A, and DNMT3L loci (see individual assay section below for details); these CpG sites did not fall into CpG islands. DNMT1, DNMT3A, and DNMT3L CpG sites and 2 DNMT3B CpG sites assessed are also found on the HM27 and HM450K methylation bead chips from Illumina (see below for actual HG19 nucleotide location). The capacity for each assay to capture DNAm levels ranging from 0–100% was validated using commercially available demethylated and highly methylated DNA at dilutions of 1:0 (unmethylated), 3:1, 1:1, 1:3, and 0:1 (highly methylated). PCR amplification of target sequences was performed on 20ng of bisulfite-converted DNA samples using the PyroMark PCR kit (Qiagen). Bisulfite-converted, PCR-amplified DNA was pyrosequenced on the Pyromark Q24 Pyrosequencer (Qiagen) using the manufacturer’s recommended protocol and default settings. All methylation analyses were conducted in triplicate with appropriate negative controls included at each of the following steps: DNA isolation, bisulfite conversion, PCR amplification, and pyrosequencing reaction.
Details of each custom assay are listed below.
DNMT1
PCR forward primer: TTTTTTTAGGTGTGATGGGGATAAAG
PCR reverse primer (biotinylated): CAAAAACTCTCACAAACCCTTAAA
PCR program (50 cycles):
| Initial | 15 minutes at 95°C |
| Denaturation | 30 seconds at 94°C |
| Annealing | 30 seconds at 58°C |
| Extension | 30 seconds at 72°C |
| Final | 10 minutes at 72°C |
| Hold | 4°C |
Sequencing primer: GTGATGGGGATAAAGT
Target sequence: AGCGAGAAGCCCCCAAGGGTTTGTGAGA (CpG target in bold; hg19: chr19:10,305,909–10,305,936)
DNMT3A
PCR forward primer: GGTGGGAGGTTGAATGAAATGA
PCR reverse primer (biotinylated): AATACCCAACCCCAAATCCTAC
PCR program (50 cycles):
| Initial | 15 minutes at 95°C |
| Denaturation | 30 seconds at 94°C |
| Annealing | 30 seconds at 58°C |
| Extension | 30 seconds at 72°C |
| Final | 10 minutes at 72°C |
| Hold | 4°C |
Sequencing primer: AGTTGGAAGATTTTGTG
Target sequence:
TGTGCCTACACACCGCCCTCACCCCTTCACYGTGGGGGCTGTTCTCCTTCCCCATGGAGYGCTCAGGGCTCTAGGTTCCTGACTTGGGGCACCTCTGTCTAATTCCACCAGCACAGCCACTCACTATGTGCTCATCTCACTCCTCCAGCAGCYGCTGTAGGACTTG GGGCTGGGCACC (CpG target in bold; hg19: chr2:25,565,782–25,565,959)
DNMT3B
PCR forward primer: GGGGTTAAGTGGTTTAAGTAAAT
PCR reverse primer (biotinylated): CCTCCAAAAATCCCTAAAAAAAATCTCTCC
PCR program (45 cycles):
| Initial | 15 minutes at 95°C |
| Denaturation | 30 seconds at 94°C |
| Annealing | 30 seconds at 52°C |
| Extension | 30 seconds at 72°C |
| Final | 10 minutes at 72°C |
| Hold | 4°C |
Sequencing primer: GTTAAGTGGTTTAAGTAAATTTAG
Target sequence:
CTCGGCGATCGGCGCCGGAGATTCGCGAGCCCAGCGCCCTGCACGGCCGCCAGCCGGCCTCCCGCCAGCCAGCCCCGACCCGCGGCTCCGCCGCCCAGCCGCGCCCCAGCCAGCCCTGCGGCAGGTGAGCGCCCCGGGGCCC (CpG targets in bold; hg19: chr20:31,350,382–31,350,523)
DNMT3L
PCR forward primer: AGTTTTTTTTATTGGGGTAGTTAGG
PCR reverse primer (biotinylated): CTTAAAACCAAAAAACCACATTTTATTCA
PCR program (45 cycles):
| Initial | 15 minutes at 95°C |
| Denaturation | 30 seconds at 94°C |
| Annealing | 30 seconds at 50°C |
| Extension | 30 seconds at 72°C |
| Final | 10 minutes at 72°C |
| Hold | 4°C |
Sequencing primer: GATTTAGGGATAGAGAGGG
Target sequence: GCGGTAGGGAGTGGGAAATCTGAATAA (CpG target in bold; hg19: chr21:45,683,527–45,683,553)
To demonstrate the ability of our assays to resolve DNAm differences as small as reported, we computed intraclass correlation coefficients (ICC) between triplicate replicates for each assay. Average within-sample coefficient of variation was computed using a two-way mixed model, using an absolute agreement definition (Shrout and Fleiss, 1979), as implemented in SPSS. ICCs for the 15 total CpG sites assayed ranged from 0.703 to 0.937, with a mean ICC of 0.855 (standard deviation: 0.066). This strongly supports the conclusion that these assays are capable of consistently resolving small DNAm differences.
Transcription factor binding site prediction
Putative TFBS were identified that overlap target CpG sites using the MatInspector(Cartharius et al., 2005) tool from Genomatix, with default parameters. Input sequence included 200bp up and downstream of the CpG site. Only putative TFBS that directly overlapped CpG sites of interest were retained.
Statistical analyses
Statistical testing was performed using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY). DNAm at DNMT3B CpG sites was treated on a regional and an individual CpG site basis, similar to previous work (Rusiecki et al., 2013). Regional values were calculated as the mean of 12 CpG sites. Paired-sample t-tests were used to test for differences in pre-trauma DNAm between cases and controls and to test for differences between pre- and post-trauma time points within cases and controls. Linear regression was used to test whether pre-trauma DNAm levels are predictive of post-trauma symptom severity (PTSS) changes. PTSS change was calculated as the difference between post-trauma PTSS and pre-trauma PTSS. Analyses included severity scores of individual symptom criteria (hyperarousal, avoidance, or intrusion symptoms) as well as a total severity score that is inclusive of each symptom subdomain. Regression models were adjusted for age, gender, and pre-trauma symptom severity. The contribution of pre-trauma DNAm to post-trauma PTSS change was tested via the change in R square values comparing full to reduced models. We present primary results uncorrected for multiple testing as is consistent with the current state of the science of DNAm variation in association with psychiatric endpoints(Perroud et al., 2011; Unternaehrer et al., 2012; Perroud et al., 2013; Rusiecki et al., 2013). In addition, to assess the extent to which our results may be attenuated by multiple hypothesis testing correction, we calculated stringent Bonferroni-corrected significance values(Dunn, 1961) as well as false discovery rate (FDR) Q values(Benjamini, 1995). FDR has recently been utilized to correct multiple hypothesis testing in studies utilizing DNAm data, with user-defined Q-values ranging from 0.05 to 0.2(Provencal et al., 2013; Zhao et al., 2013).
Results
PTSD cases and controls do not differ in age, gender, ethnicity, or pre-trauma symptom severity, including individual symptoms of intrusion, avoidance, and hyperarousal (Table 1).
Table 1.
Demographic and pre-trauma characteristics of 30 posttraumatic stress disorder (PTSD) case-control pairs.
| Controls (N = 30)
|
PTSD (N = 30)
|
t (d.f.) | p-value | |||
|---|---|---|---|---|---|---|
| Mean/# | s.d./% | Mean/# | s.d./% | |||
| Age (years) | 55.37 | 12.97 | 53.71 | 12.94 | −0.47 (29) | 0.638 |
| Female | 23 | 76.7% | 23 | 76.7% | NA (1) | 1.000 |
| African-American | 22 | 73.3% | 24 | 80% | 0.01 (1) | 0.938 |
| Lifetime traumas | 3.80 | 3.83 | 4.43 | 3.70 | −0.84 (29) | 0.407 |
| Assaultive Violence | 0.87 | 1.33 | 1.07 | 1.55 | −0.55 (29) | 0.589 |
| Other Injury or Shccking Experience | 0.83 | 1.32 | 1.27 | 1.48 | −1.51 (29) | 0.141 |
| Learning about traumas to others | 1.20 | 1.50 | 1.17 | 1.37 | 0.12 (29) | 0.909 |
| Sudden Death | 0.77 | 0.43 | 0.70 | 0.47 | 0.63 (29) | 0.536 |
| Other Event | 0.13 | 0.35 | 0.23 | 0.43 | −1.36 (29) | 0.184 |
| Pre-trauma PTS | 26.90 | 10.96 | 39.33 | 16.20 | −1.12 (29) | 0.275 |
| Intrusion | 9.58 | 7.90 | 11.65 | 6.08 | −0.99 (29) | 0.333 |
| Avoidance | 11.65 | 11.04 | 14.69 | 6.97 | −1.14 (29) | 0.267 |
| Hyperarousal | 8.60 | 7.98 | 11.24 | 5.11 | −1.24 (29) | 0.226 |
| Post-trauma PTS | 25.41 | 7.01 | 54.20 | 11.59 | 11.4 (29) | <0.001 |
Pre-trauma DNAm variation is associated with PTSD
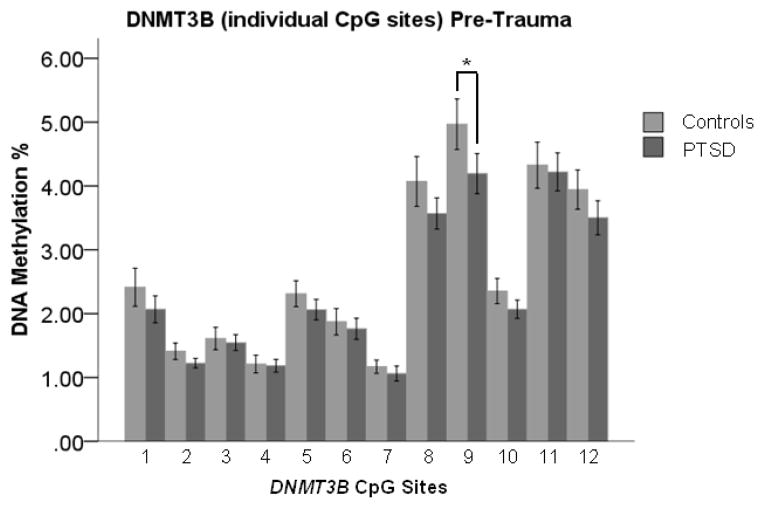
PTSD-associated DNAm variation may both pre-exist trauma and be associated with post-trauma PTSD outcome. To test for pre-existing protective/risk factors, pre-trauma DNAm at DNMT1, DNMT3A, DNMT3B, and DNMT3L loci was compared between trauma exposed individuals with vs. without PTSD. Pre-trauma DNAm was higher in cases compared with controls at a single DNMT3B CpG site (CpG 9) (Figure 1; t=2.250, 29 df, p=0.032); no difference in pre-trauma DNMT3B regional DNAm mean was observed (t=1.538, 29 df, p=0.135). We observed no pre-trauma differences between cases and controls at DNMT1 (t=0.582, 29 df, p=0.565), DNMT3A (t=0.579, 29 df, p=0.567), and DNMT3L (t=1.386, 29df, p=0.176) loci.
Figure 1.
Pre-trauma “DNA methyltransferase 3B” (DNMT3B) DNA methylation (DNAm) is significantly higher in trauma-exposed controls compared to posttraumatic stress disorder (PTSD) cases at CpG 9. Pre-trauma DNAm did not differ between cases and controls at the other 11 DNMT3B CpG sites assessed. Light gray bars indicate mean DNAm of controls. Dark gray bars indicate mean DNAm of PTSD cases. Error bars represent standard error of the mean. Difference between controls and cases was tested by paired-sample t-tests (N = 60; 30 cases and 30 matched controls). *: p<0.05.
Pre-trauma DNAm variation predicts post-trauma changes in trauma symptom severity
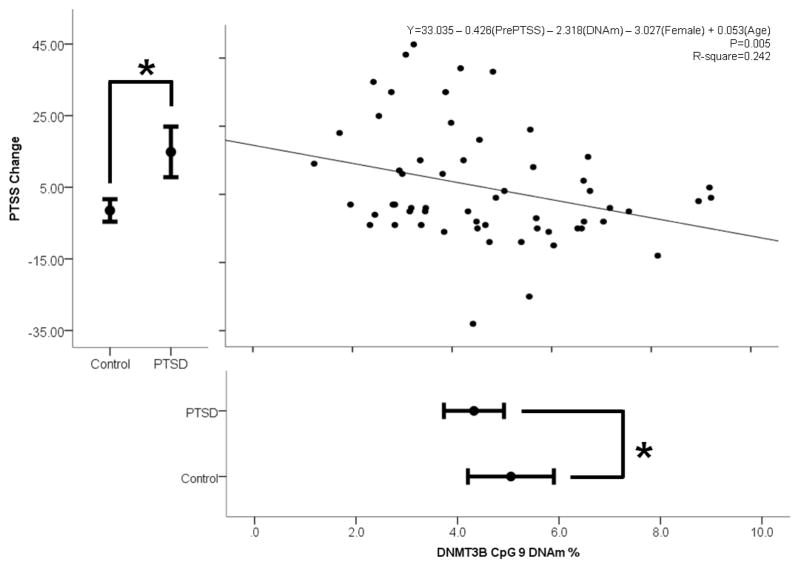
To explore whether this PTSD-associated pre-trauma DNAm is predictive of trauma response, we performed linear regression analyses with pre-trauma DNAm of DNMT3B at CpG 9 and PTSS change as predictor and outcome variables, respectively. Controlling for age, gender, and pre-trauma symptom severity, pre-trauma DNAm of CpG 9 (Figure 2; unstandardized B=−2.318, SE=1.25 p=0.034) predicted post-trauma symptom severity change. In this model, only pre-trauma symptom severity and pre- trauma DNAm were significant predictor variables. DNMT3B CpG 9 DNAm explained approximately 6.8% of the variance in PTS severity change, as revealed by a comparison of the full and reduced models. The full model that included DNMT3B CpG 9 DNAm, age, gender, and pre-trauma symptom severity explained approximately 24% of the variance in post-trauma PTSS change (Adjusted R Square=0.242, p=0.005).
Figure 2.
Linear regression model of symptom severity (PTSS) change post-trauma and pre-trauma “DNA methyltransferase 3B” (DNMT3B) CpG 9 DNA methylation (DNAm), adjusting for age, gender, and pre-trauma symptom severity (N=60). Only pre-trauma PTSS and DNAm were significant variables in this model. Error bar plots represent the mean plus/minus the 95% confidence intervals. Differences between posttraumatic stress disorder cases and trauma-exposed controls were tested by paired-sample t-tests (N = 60; 30 PTSD cases and 30 matched controls). *: p<0.05.
Because the relationship between pre-trauma DNAm and post-trauma changes in PTS symptom severity may be driven by distinct symptom subdomains (hyperarousal, avoidance, and intrusion), we regressed separately each subdomain symptom severity change onto pre-trauma DNAm, controlling for age, gender, and pre-trauma symptom severity of the relevant subdomain. Pre-trauma DNAm of DNMT3B CpG 9 (hyperarousal: p=0.249; avoidance: p=0.137; intrusion: p=0.071) did not predict change in subdomain symptom severity.
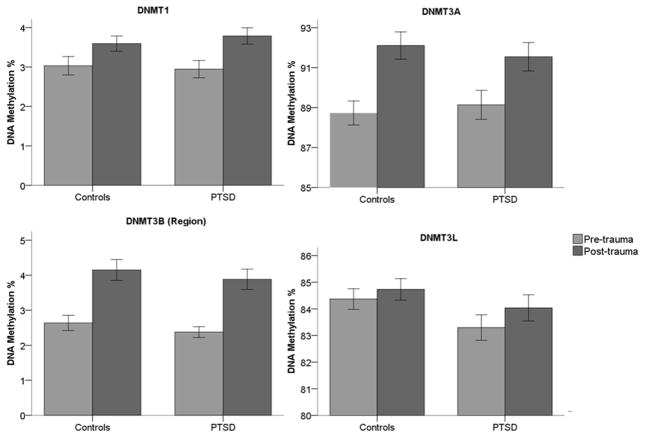
Trauma induces PTSD-associated DNAm modifications
DNAm differences may arise following trauma and be associated with PTSD development. To test this, we compared pre-trauma DNAm with post-trauma DNAm within PTSD cases and within trauma-exposed, healthy controls. Both PTSD-associated and PTSD-independent changes in DNAm following trauma were observed at DNMT loci (Figure 3). DNMT1 DNAm increased (Figure 3A; t=3.887, 29 df, p=0.001) following trauma in the PTSD group, but not the control group (t=1.903, 29 df, p=0.067). At DNMT3A (Figure 3B) and DNMT3B (Figure 3C) loci, DNAm increased following trauma in both PTSD case (DNMT3A: t=2.806, 29 df, p=0.009; DNMT3B: t=4.286, 29 df, p<0.001) and control (DNMT3A: t=3.421, 29 df, p=0.002; DNMT3B: t=3.938, 29 df, p<0.001) groups. No change was observed in DNMT3L (Figure 3D) DNAm in either cases (t=1.551, 29 df, p=0.132) or controls (t=1.146, 29 df, p=0.261). Table 3 presents a summary including uncorrected p values, Bonferroni-corrected p values, and FDR values, as well as accompanying effect sizes, of our results described above.
Figure 3.
Longitudinal DNA methylation (DNAm) modifications of DNA methyltransferase (DNMT) loci in response to trauma in posttraumatic stress disorder (PTSD) cases and trauma-exposed controls. DNMT3B (region) represents the mean of 12 CpG sites. Differences between PTSD cases and trauma-exposed controls were tested by paired-sample t-tests (N = 60; 30 PTSD cases and 30 matched controls). Error bars represent standard error of the mean. *: p<0.05; **:p<0.01; ***:p<0.001.
Table 3.
Observed and corrected significance values of tests. The list of observed p-values is sorted from smallest to largest (indicated by the Rank column). All tests above and including those bolded meet significance at p<0.05 for various corrections for multiple hypothesis testing. Corrected significance thresholds at p<0.05 are listed using two controlling procedures: Bonferroni and False Discovery Rate (FDR) using the procedure of Benjamini and Hochberg (BH, (Benjamini, 1995)). Bolded values meet significance at p<0.05 for the various correction procedures.
| Test | Mean Difference | SE | Observed P-value | Rank (i) | Bonferroni threshold | FDR (BH) thresholds |
|---|---|---|---|---|---|---|
| Pre- vs. post-trauma DNMT3B in Cases |
1.51 | 0.35 | 0.000 | 1 | 0.002 | 0.002 |
| Pre- vs. post-trauma DNMT3B in Controls |
1.51 | 0.38 | 0.000 | 2 | 0.002 | 0.003 |
| Pre- vs. post-trauma DNMT1 in Cases |
0.84 | 0.22 | 0.001 | 3 | 0.002 | 0.005 |
| Pre- vs. post-trauma DNMT3A in Controls |
3.38 | 0.99 | 0.002 | 4 | 0.002 | 0.006 |
| Pre- vs. post-trauma DNMT3A in Cases |
2.41 | 0.86 | 0.009 | 5 | 0.002 | 0.008 |
| Cases vs. Controls DNMT3B (CpG 9) pre-trauma |
0.77 | 0.34 | 0.032 | 6 | 0.002 | 0.009 |
| CpG 9 Regression analysis (all symptoms) | B: −2.318 | 1.251 | 0.034 | 7 | 0.002 | 0.011 |
| Cases vs. Controls DNMT3B (CpG 2) pre-trauma |
0.19 | 0.11 | 0.057 | 8 | 0.002 | 0.013 |
| Pre- vs. post-trauma DNMT1 in Controls |
0.56 | 0.30 | 0.067 | 9 | 0.002 | 0.014 |
| CpG 9 Regression analysis (Intrusion symptoms) | B: −0.851 | 0.458 | 0.071 | 10 | 0.002 | 0.016 |
| Cases vs. Controls DNMT3B (CpG 12) pre-trauma |
0.44 | 0.24 | 0.122 | 11 | 0.002 | 0.017 |
| Cases vs. Controls DNMT3B (CpG 10) pre-trauma |
0.28 | 0.19 | 0.127 | 12 | 0.002 | 0.019 |
| Pre- vs. post-trauma DNMT3L in Cases |
0.74 | 0.47 | 0.132 | 13 | 0.002 | 0.020 |
| Cases vs. Controls DNMT3B pre-trauma |
0.27 | 0.17 | 0.135 | 14 | 0.002 | 0.022 |
| CpG 9 Regression analysis (Avoidance symptoms) | B: −0.821 | 0.541 | 0.137 | 15 | 0.002 | 0.023 |
| Cases vs. Controls DNMT3B (CpG 1) pre-trauma |
0.35 | 0.23 | 0.151 | 16 | 0.002 | 0.025 |
| Cases vs. Controls DNMT3B (CpG 8) pre-trauma |
0.50 | 0.33 | 0.158 | 17 | 0.002 | 0.027 |
| Cases vs. Controls DNMT3L pre-trauma |
0.91 | 0.65 | 0.176 | 18 | 0.002 | 0.028 |
| Cases vs. Controls DNMT3B (CpG 5) pre-trauma |
0.25 | 0.19 | 0.221 | 19 | 0.002 | 0.030 |
| CpG 9 Regression analysis (Hyperarousal symptoms) | B: −0.466 | 0.399 | 0.249 | 20 | 0.002 | 0.031 |
| Pre- vs. post-trauma DNMT3L in Controls |
0.36 | 0.32 | 0.261 | 21 | 0.002 | 0.033 |
| Cases vs. Controls DNMT3L post-trauma |
0.58 | 0.56 | 0.304 | 22 | 0.002 | 0.034 |
| Cases vs. Controls DNMT1 post-trauma |
−0..19 | 0.19 | 0.323 | 23 | 0.002 | 0.036 |
| Cases vs. Controls DNMT3B post-trauma |
0.18 | 0.22 | 0.351 | 24 | 0.002 | 0.038 |
| Cases vs. Controls DNMT3A post-trauma |
0.56 | 0.66 | 0.356 | 25 | 0.002 | 0.039 |
| Cases vs. Controls DNMT3B (CpG 7) pre-trauma |
0.11 | 0.12 | 0.487 | 26 | 0.002 | 0.041 |
| Cases vs. Controls DNMT1 pre-trauma |
0.09 | 0.20 | 0.565 | 27 | 0.002 | 0.042 |
| Cases vs. Controls DNMT3A pre-trauma |
−0.41 | 0.73 | 0.567 | 28 | 0.002 | 0.044 |
| Cases vs. Controls DNMT3B (CpG 6) pre-trauma |
0.11 | 0.17 | 0.603 | 29 | 0.002 | 0.045 |
| Cases vs. Controls DNMT3B (CpG 3) pre-trauma |
0.06 | 0.15 | 0.711 | 30 | 0.002 | 0.047 |
| Cases vs. Controls DNMT3B (CpG 11) pre- trauma |
0.11 | 0.39 | 0.773 | 31 | 0.002 | 0.048 |
| Cases vs. Controls DNMT3B (CpG 4) pre-trauma |
0.03 | 0.14 | 0.881 | 32 | 0.002 | 0.050 |
Note: For regression analyses, “B” represents unstandardized Beta values.
Transcription factor binding site prediction
DNAm is associated with gene expression. One mechanism by which increased DNAm can lead to decreased gene expression is by affecting the binding of trans-activating factors to cis-regulatory elements. To contextualize our DNAm findings, we used bioinformatic methods to identify putative TFBS that overlap CpG sites showing PTSD-associated DNAm differences. In total, we identified 24 putative TFBS, including 2, 3, 14, and 5 that overlap DNMT1, DNMT3A, DNMT3B, and DNMT3L CpG target sites, respectively (Table 2). Notable among these 24 TFBS are those that overlap with CpG sites at which we identified PTSD-associated differential methylation (2 overlap the DNMT1 CpG; 3 overlap DNMT3B CpG 9). Binding sites for heat shock factor 1 and E2F-4/DP-2 heterodimeric complex were identified to overlap with the DNMT1 CpG site at which an increase in DNAm was observed in PTSD cases, but not controls. Overlapping with DNMT3B CpG site 9, at which increased pre-trauma DNAm was associated with PTSD development and predictive of worsening of PTSS, we identified binding sites for Human motif ten element, ZF5 POZ domain zinc finger, and the insulator protein CTCF.
Table 2.
Putative transcription factor binding sites overlap DNA methyltransferase (DNMT) CpG sites of interest. V$ matrix families indicate Genomatix-annotated transcription factor binding site matrix families. DNMT3B CpG sites are described in the Methods.
| Gene | Matrix Family | Matrix Information | Core similarity | DNMT3B CpG overlap |
|---|---|---|---|---|
| DNMT1 | V$HEAT | Heat shock factor 1 | 1.000 | - |
| V$E2FF | E2F-4/DP-2 heterodimeric complex | 0.847 | - | |
|
| ||||
| DNMT3A | V$SP1F | TGFbeta-inducible early gene (TIEG)/Early growth response gene alpha (EGRalpha) | 0.750 | - |
| V$SP1F | Stimulating protein 1, ubiquitous zinc finger transcription factor | 1.000 | - | |
| V$EGRF | EGR1, early growth response 1 | 0.802 | - | |
| V$GCMF | Glial cells missing homolog 1, chorion-specific transcription factor GCMa | 1.000 | - | |
| V$KLFS | Kidney-enriched kruppel-like factor, KLF15 | 1.000 | - | |
|
| ||||
| DNMT3B | O$MTEN | Human motif ten element | 0.839 | 1, 2, 3, 4, 5 |
| V$PAX5 | PAX5 paired domain protein | 0.789 | 1, 2, 3, 4, 5, 6, 7 | |
| V$E2FF | E2F transcription factor 3 (secondary DNA binding preference) | 1.000 | 2, 3, 4, 5 | |
| V$E2FF | E2F transcription factor 3 (secondary DNA binding preference) | 1.000 | 2, 3, 4, 5, 6, 7 | |
| V$ETSF | Ets variant 4 | 1.000 | 3, 4, 5, 6, 7 | |
| O$MTEN | Human motif ten element | 0.961 | 7, 8, 9 | |
| V$ZF5F | ZF5 POZ domain zinc finger, zinc finger protein 161 (secondary DNA binding preference) | 0.775 | 8, 9, 10 | |
| V$CTCF | Insulator protein CTCF (CCCTC-binding factor) | 0.818 | 9, 10, 11, 12 | |
| V$HDBP | Huntington’s disease gene regulatory region-binding protein 1 and 2 (SLC2A4 regulator and papillomavirus binding factor) | 1.000 | 10, 11, 12 | |
| V$EGRF | Collagen krox protein (zinc finger protein 67-zfp67) | 1.000 | 11, 12 | |
| V$PLAG | Pleomorphic adenoma gene (PLAG) 1, a developmentally regulated C2H2 zinc finger protein | 0.958 | 12 | |
| V$ZF02 | Transcriptional repressor, binds to elements found predominantly in genes that participate in lipid metabolism | 0.776 | 12 | |
|
| ||||
| DNMT3L | V$KLFS | Basic transcription element (BTE) binding protein, BTEB3, FKLF-2 | 1.000 | - |
| V$SP1F | Stimulating protein 1, ubiquitous zinc finger transcription factor | 1.000 | - | |
| V$MYBL | C-Myb, important in hematopoesis, cellular equivalent to avian myoblastosis virus oncogene v-myb | 0.797 | - | |
| V$GLIF | Zinc finger transcription factor, Zic family member 2 (odd-paired homolog, Drosophila) | 1.000 | - | |
| V$CP2F | LBP-1c (leader-binding protein-1c), LSF (late SV40 factor, CP2, SEF (SAA3 enhancer factor) | 0.875 | - | |
Discussion
Our data represent preliminary findings suggesting that pre-trauma DNAm states and post-trauma DNAm modifications differ between those who develop PTSD following trauma and those who display resiliency. While baseline PTS symptoms did not differ between cases and controls, baseline DNAm at a DNMT3B CpG site was higher in resilient individuals compared to those who eventually developed PTSD. Additionally, longitudinal change in DNAm at a DNMT1 CpG site was associated with PTSD, with an increase in DNAm being observed in those with PTSD but not controls. Finally, increases in DNAm were observed following trauma at DNMT3A and DNMT3B loci that were independent of PTSD outcome, being observed in both PTSD cases and trauma-exposed controls. Although some of these results were attenuated following correction for multiple hypothesis testing, our findings suggest that epigenetic variation plays a complex regulatory role in PTSD risk and etiology.
One way in which DNAm may regulate gene transcription is by altering the strength and occupancy of transcription factor binding (Weaver et al., 2004). To provide insight into potential functional consequences of the observed PTSD-associated differences, we conducted a secondary analysis of TFBS overlapping the distinguishing CpG sites. Among the sites identified was a binding site for CTCF, a transcription factor known to be involved in chromatin remodeling(Barkess and West, 2012). We identified this binding site overlapping with DNMT3B CpG site 9, at which higher DNAm was identified as a protective/risk factor for PTSD and symptom severity change following trauma exposure. Differential methylation at this site is particularly compelling as a determinant of PTSD risk, given that DNAm at CTCF binding sites has been shown to significantly affect CTCF occupancy(Wang et al., 2012) and downstream levels of gene transcription(Renaud et al., 2007). Due to the nature of our samples, we are unable to test directly whether DNAm at these identified TFBS influences gene expression. Where available, we have utilized ENCODE data(Consortium, 2011) to provide evidence for or against transcription factor binding at the PTSD-associated sites in blood-derived cell types. Among the TFBS identified that overlap PTSD-associated CpG sites (DNMT1 and DNMT3B CpG 9), ENCODE data includes binding of CTCF and E2F4. ENCODE data supports the binding of CTCF to DNMT3B in blood tissue (specifically b-lymphocyte cell lines: GM12864 and GM12874), but does not support the binding of E2F4 to DNMT1. This supports the potential functionality of observed DNAm differences at DNMT3B CpG 9 in pre-trauma samples in cases vs. controls.
DNMTs have been previously implicated in PTSD, anxiety, and fear conditioning. In suicide completers relative to controls, DNMT3B was upregulated in the frontopolar cortex, hypothalamus, and dorsal vagal complex and down regulated, along with DNMT1, in the hippocampus(Poulter et al., 2008). Additionally, de novo methyltransferases have been shown to be upregulated during contextual fear conditioning, also in the hippocampus (Miller and Sweatt, 2007); DNMTs are required for fear conditioning and memory consolidation as demonstrated, respectively, by administration of DNMT inhibitors (Miller and Sweatt, 2007) and the creation of mice with the combined knockout of DNMT1 and DNMT3A(Feng et al., 2010). Our results thus add to the growing evidence implicating DNMTs in phenotypes of relevance to PTSD, and of psychiatric phenotypes more broadly.
The expression of DNMTs at the mRNA(Goto et al., 1994; Veldic et al., 2004; Kang et al., 2011; Sterner et al., 2012) and protein(Inano et al., 2000; Feng et al., 2005; Veldic et al., 2005) levels in post-mitotic neurons of the central nervous system suggests that they are involved in methyltransferase activity that persists into adulthood and that is unrelated to DNA replication(Goto et al., 1994). Indeed, previous work has identified DNMT1 protein expression in multiple brain regions in rodents (e.g. cortex, cerebellum(Inano et al., 2000)), as well as in specific cortical regions in adult humans (e.g. Broadmann’s Area 9(Veldic et al., 2005)). Furthermore, recent work suggests that our epigenetic findings in peripheral blood may be relevant to brain tissue: environmental exposures such as trauma have been shown to induce parallel epigenetic modifications in peripheral blood and brain (McGowan et al., 2011; Klengel et al., 2013). Although the current study, based on living participants drawn from a population-based cohort, precludes such work, future research is needed to address whether the epigenetic determinants of risk observed here in peripheral blood-derived DNA is also found in brain-derived DNA.
Importantly, this study adds to emerging work utilizing a longitudinal study design capable of measuring biological markers prior to disease onset as well as change between pre-disease and post-disease time points(Nieratschker et al., 2012; Rusiecki et al., 2012; Perroud et al., 2013; Rusiecki et al., 2013). Existing longitudinal studies have documented the importance of DNAm to mental health disorder risk, including differential change in DNAm of BDNF among individuals with vs. without borderline personality disorder(Perroud et al., 2013), increased DAT (SLC6A3) DNAm with age that may be driven by alcohol dependence(Nieratschker et al., 2012), and increasing SERT DNAm associated with bullying(Ouellet-Morin et al., 2013). Most relevant to the present study is work by Rusiecki et al.(Rusiecki et al., 2012) which provides evidence for increased global DNAm in controls, but not cases following trauma exposure, suggesting that resiliency is associated with increased global DNAm, potentially mediated by increased activity and expression of DNMTs. Indeed, our data presented here is consistent with this scenario, as DNAm of DNMT1 was observed to increase following trauma in cases, but not controls. In contrast, however, we observed an increase in DNMT3B DNAm following trauma in both cases and controls, and a pre-trauma association between higher DNAm pre-trauma and resiliency post-trauma. The presence of a CTCF binding site opens the possibility that increased DNAm at this locus is associated with increased gene expression because CTCF can act as either a transcriptional activator or repressor(Phillips and Corces, 2009), with strength of DNA binding inversely correlated with local DNAm(Barkess and West, 2012). If binding of CTCF to the DNMT3B locus results in transcriptional repression, then increased DNAm, and concurrent decreased CTCF binding, would be associated with increased, not decreased, gene expression. If true, this would put these findings in line with the previously published, longitudinal, trauma-associated epigenetic data: decreased DNAm in pre-trauma PTSD cases would result in tighter CTCF binding and reduced DNMT3B transcription and lower global DNAm levels, as reported by Ruisecki and colleagues(Rusiecki et al., 2012). Although DNMT1 is typically thought to maintain DNAm in adult tissues, evidence suggests that DNMT1 and DNMT3B cooperatively maintain DNAm, with one or the other, but not both, required for global DNAm(Rhee et al., 2002). More broadly, our data adds to the emerging evidence that longitudinal DNAm changes may contribute to the etiology of mental illness and can be taken as a proof of principle that locus-specific epigenetic variability both pre-exist and arise following disease-onset in biologically meaningful ways.
While our study is one of the first of its kind to compare pre- and post-trauma DNAm levels with regard to the development of PTSD, there is a minimum of four study limitations that should be kept in mind when interpreting our results. First, it is important to recognize that the epidemiological nature of our cohort precludes sample collection with a well-controlled experimental time course; times between pre-trauma data collection, trauma exposure, and post-trauma data collection differed between each test subject. As such, we are unable to resolve whether observed PTSD-associated post-trauma DNAm changes precede PTSD-development (i.e. occurred within the first four weeks following trauma). As DNMTs are involved in the global regulation of DNAm, it is tempting to conclude from our data that observed changes in DNMT DNAm are an upstream process of PTSD development, thereby having the potential to help explain differences in DNAm epigenome-wide reported elsewhere(Uddin et al., 2010; Smith et al., 2011; Rusiecki et al., 2012). However, it is also possible that the observed PTSD-associated DNAm changes are downstream effects of PTSD development, with no or little involvement in epigenetic modifications across the epigenome. Second, the nature of the epidemiological samples collected precluded the assessment of pre- and post-trauma gene expression differences and changes, as well as any analysis of blood cell composition. Third, the DNAm differences and effect sizes reported here are small; however, they are consistent with published work showing functional effects of DNAm variation(Tyrka et al., 2012). High intraclass correlation coefficients between experimental replicates for each of our assays increases confidence of the validity of observed DNAm differences. Indeed, our sample size and observed effect sizes are consistent with published work in the field(Perroud et al., 2011; Byrne et al., 2013). Fourth, our results across the multiple CpG sites within DNMT3B are not corrected for multiple testing. Although this is consistent with the current state of the science of DNAm variation in association with psychiatric endpoints(Perroud et al., 2011; Unternaehrer et al., 2012; Perroud et al., 2013; Rusiecki et al., 2013), we do report corrected results (Table 3) to assess the degree to which our findings might be attenuated by multiple hypothesis test correction. Accepting a stringent FDR of 0.05 requires that we reject several findings reported as significant in our study, notably pre-trauma DNAm differences between cases and controls at DNMT3B CpG 9. However, it also means that a significant association between DNAm and PTSD emerges as a result of correction, as a significant change in DNAm at DNMT3A following trauma is only seen in controls at this stringent FDR cutoff and would therefore be suggestive of a resiliency-associated change in DNAm (Table 3). While we have chosen to utilize a stringent FDR cut-off of 0.05, other DNAm analyses have accepted a cut-off as high as 0.20(Provencal et al., 2013). Overall, we stress the preliminary nature of these findings—both uncorrected and corrected for multiple hypothesis testing—and the importance of replication in an independent cohort.
Individuals exposed to trauma differ in their risk for subsequent PTSD. Our data suggest that variation in pre-trauma DNAm and post-trauma DNAm change may be part of the molecular underpinnings of PTSD risk and resiliency. Future research is needed to determine if the DNAm variation observed here is associated with functional changes that affect the long-term biology of individuals exposed to trauma. The identification of risk markers, including epigenetic markers, is an important step to understanding the biological underpinnings of PTSD risk and may lead to the development of tools to identify those individuals most at risk of developing PTSD as well as to develop evidence-based interventions.
Acknowledgments
We thank Dr. Chet Sherwood and Amy Bauernfeind from The George Washington University for contributions to this work. We thank the many Detroit residents who chose to participate in the DNHS.
This research was funded by NIH grants: 3R01DA022720-05, 3R01DA022720, and 1RC1MH088283-01 and NSF grant BCS-0827546. Levent Sipahi was funded by a Graduate Research Assistantship from the Wayne State University Office of the Vice President for Research, and a Grant-in-Aid of Research from Sigma Xi. Karestan Koenen is funded by R01MH093612, RC4MH092707, P51RR000165, U01OH010416, U01OH010407.
Footnotes
Conflict of interest
Sandro Galea is funded in part by a grant from Merck Pharmaceuticals for work unrelated to this project. Derek Wildman is funded in part from Lung, LLC for work unrelated to this project, and also reports receiving Honoraria from Elsevier, INC. Allison Aiello consults for SCA Tork for work unrelated to this study. Levent Sipahi, Karestan Koenen, Asad Abbas, and Monica Uddin declare no conflict of interest of interest.
References
- Aberg KA, Xie LY, Nerella S, Copeland WE, Costello EJ, van den Oord EJ. High quality methylome-wide investigations through next-generation sequencing of DNA from a single archived dry blood spot. Epigenetics. 2013:8. doi: 10.4161/epi.24508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acierno R, Ruggiero KJ, Galea S, Resnick HS, Koenen K, Roitzsch J, de Arellano M, Boyle J, Kilpatrick DG. Psychological sequelae resulting from the 2004 Florida hurricanes: implications for postdisaster intervention. American Journal of Public Health. 2007;97(Suppl 1):S103–108. doi: 10.2105/AJPH.2006.087007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews JA, Neises KD. Cells, biomarkers, and post-traumatic stress disorder: evidence for peripheral involvement in a central disease. Journal of Neurochemistry. 2012;120:26–36. doi: 10.1111/j.1471-4159.2011.07545.x. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and Statistical Manual of Mental Disorders: Fifth Edition (DSM-5) American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Barkess G, West AG. Chromatin insulator elements: establishing barriers to set heterochromatin boundaries. Epigenomics. 2012;4:67–80. doi: 10.2217/epi.11.112. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B. 1995;57:289–300. [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Journal of the American Medical Association. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. DNA methylation patterns and epigenetic memory. Genes & Development. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bourc’his D, Bestor TH. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature. 2004;431:96–99. doi: 10.1038/nature02886. [DOI] [PubMed] [Google Scholar]
- Bourc’his D, Xu GL, Lin CS, Bollman B, Bestor TH. Dnmt3L and the establishment of maternal genomic imprints. Science. 2001;294:2536–2539. doi: 10.1126/science.1065848. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Archives of General Psychiatry. 1991;48:216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Archives of General Psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Breslau N, Wilcox HC, Storr CL, Lucia VC, Anthony JC. Trauma exposure and posttraumatic stress disorder: a study of youths in urban America. Journal of Urban Health. 2004;81:530–544. doi: 10.1093/jurban/jth138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. Journal of Consulting and Clinical Psychology. 2000;68:317–336. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Byrne EM, Carrillo-Roa T, Henders AK, Bowdler L, McRae AF, Heath AC, Martin NG, Montgomery GW, Krause L, Wray NR. Monozygotic twins affected with major depressive disorder have greater variance in methylation than their unaffected co-twin. Translational Psychiatry. 2013;3:e269. doi: 10.1038/tp.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartharius K, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, Frisch M, Bayerlein M, Werner T. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics. 2005;21:2933–2942. doi: 10.1093/bioinformatics/bti473. [DOI] [PubMed] [Google Scholar]
- Chang SC, Koenen KC, Galea S, Aiello AE, Soliven R, Wildman DE, Uddin M. Molecular variation at the SLC6A3 locus predicts lifetime risk of PTSD in the Detroit Neighborhood Health Study. PLoS ONE. 2012;7:e39184. doi: 10.1371/journal.pone.0039184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congressionally Directed Medical Research Programs DoD. CDMRP Department of Defense Psychological Health/Traumatic Brain Injury Research Program. 2011. [Google Scholar]
- Consortium. A user’s guide to the encyclopedia of DNA elements (ENCODE) PLoS Biology. 2011;9:e1001046. doi: 10.1371/journal.pbio.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGrande L, Perrin MA, Thorpe LE, Thalji L, Murphy J, Wu D, Farfel M, Brackbill RM. Posttraumatic stress symptoms, PTSD, and risk factors among lower Manhattan residents 2–3 years after the September 11, 2001 terrorist attacks. Journal of Traumatic Stress. 2008;21:264–273. doi: 10.1002/jts.20345. [DOI] [PubMed] [Google Scholar]
- Dunn OJ. Multiple comparisons among means. Journal of the American Statistical Association. 1961;56:52–64. [Google Scholar]
- El-Sayed AM, Haloossim MR, Galea S, Koenen KC. Epigenetic modifications associated with suicide and common mood and anxiety disorders: a systematic review of the literature. Biology of Mood & Anxiety Disorders. 2012;2:10. doi: 10.1186/2045-5380-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg AP. Epigenetics at the epicenter of modern medicine. Journal of the American Medical Association. 2008;299:1345–1350. doi: 10.1001/jama.299.11.1345. [DOI] [PubMed] [Google Scholar]
- Feng J, Chang H, Li E, Fan G. Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system. Journal of Neuroscience Research. 2005;79:734–746. doi: 10.1002/jnr.20404. [DOI] [PubMed] [Google Scholar]
- Feng J, Fan G. The role of DNA methylation in the central nervous system and neuropsychiatric disorders. International Review of Neurobiology. 2009;89:67–84. doi: 10.1016/S0074-7742(09)89004-1. [DOI] [PubMed] [Google Scholar]
- Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, Silva AJ, Fan G. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nature Neuroscience. 2010;13:423–430. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea S, Ahern J, Tracy M, Hubbard A, Cerda M, Goldmann E, Vlahov D. Longitudinal determinants of posttraumatic stress in a population-based cohort study. Epidemiology. 2008;19:47–54. doi: 10.1097/EDE.0b013e31815c1dbf. [DOI] [PubMed] [Google Scholar]
- Goto K, Numata M, Komura JI, Ono T, Bestor TH, Kondo H. Expression of DNA methyltransferase gene in mature and immature neurons as well as proliferating cells in mice. Differentiation. 1994;56:39–44. doi: 10.1046/j.1432-0436.1994.56120039.x. [DOI] [PubMed] [Google Scholar]
- Hollegaard MV, Grauholm J, Norgaard-Pedersen B, Hougaard DM. DNA methylome profiling using neonatal dried blood spot samples: a proof-of-principle study. Molecular Genetics and Metabolism. 2013;108:225–231. doi: 10.1016/j.ymgme.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Inano K, Suetake I, Ueda T, Miyake Y, Nakamura M, Okada M, Tajima S. Maintenance-type DNA methyltransferase is highly expressed in post-mitotic neurons and localized in the cytoplasmic compartment. Journal of Biochem. 2000;128:315–321. doi: 10.1093/oxfordjournals.jbchem.a022755. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Okano M, Hata K, Sado T, Tsujimoto N, Li E, Sasaki H. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature. 2004;429:900–903. doi: 10.1038/nature02633. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, Sousa AM, Pletikos M, Meyer KA, Sedmak G, Guennel T, Shin Y, Johnson MB, Krsnik Z, Mayer S, Fertuzinhos S, Umlauf S, Lisgo SN, Vortmeyer A, Weinberger DR, Mane S, Hyde TM, Huttner A, Reimers M, Kleinman JE, Sestan N. Spatio-temporal transcriptome of the human brain. Nature. 2011;478:483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Kaneda M, Hata K, Kumaki K, Hisano M, Kohara Y, Okano M, Li E, Nozaki M, Sasaki H. Role of the Dnmt3 family in de novo methylation of imprinted and repetitive sequences during male germ cell development in the mouse. Human Molecular Genetics. 2007;16:2272–2280. doi: 10.1093/hmg/ddm179. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Wang PS. The descriptive epidemiology of commonly occurring mental disorders in the United States. Annual Review of Public Health. 2008;29:115–129. doi: 10.1146/annurev.publhealth.29.020907.090847. [DOI] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature Neuroscience. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Harley R, Lyons MJ, Wolfe J, Simpson JC, Goldberg J, Eisen SA, Tsuang M. A twin registry study of familial and individual risk factors for trauma exposure and posttraumatic stress disorder. The Journal of Nervous and Mental Disease. 2002;190:209–218. doi: 10.1097/00005053-200204000-00001. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Stellman JM, Stellman SD, Sommer JF., Jr Risk factors for course of posttraumatic stress disorder among Vietnam veterans: a 14-year follow-up of American Legionnaires. Journal of Consulting and Clinical Psychology. 2003;71:980–986. doi: 10.1037/0022-006X.71.6.980. [DOI] [PubMed] [Google Scholar]
- Kulka R, Schlenger W, Fairbank J, Hough R, Jordan BK, Marmar C, Weiss D, Grady D. Trauma and the Vietnam War Generation: Report of the findings from the National Vietnam Veterans Readjustment Study. Brunner/Mazel; New York: 1990. [Google Scholar]
- Kun P, Han S, Chen X, Yao L. Prevalence and risk factors for posttraumatic stress disorder: a cross-sectional study among survivors of the Wenchuan 2008 earthquake in China. Depression and Anxiety. 2009;26:1134–1140. doi: 10.1002/da.20612. [DOI] [PubMed] [Google Scholar]
- Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Logue MW, Baldwin C, Guffanti G, Melista E, Wolf EJ, Reardon AF, Uddin M, Wildman D, Galea S, Koenen KC, Miller MW. A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Molecular Psychiatry. 2013;18:937–942. doi: 10.1038/mp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu AT, Ogdie MN, Jarvelin MR, Moilanen IK, Loo SK, McCracken JT, McGough JJ, Yang MH, Peltonen L, Nelson SF, Cantor RM, Smalley SL. Association of the cannabinoid receptor gene (CNR1) with ADHD and post-traumatic stress disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics Part B. 2008;147B:1488–1494. doi: 10.1002/ajmg.b.30693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Suderman M, Sasaki A, Huang TC, Hallett M, Meaney MJ, Szyf M. Broad epigenetic signature of maternal care in the brain of adult rats. PLoS ONE. 2011;6:e14739. doi: 10.1371/journal.pone.0014739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Development. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Medicine Io. Treatment for Posttraumatic Stress Disorder in Military and Veteran Populations: Initial Assessment. National Academy of Sciences; Washington: 2012. [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Nieratschker V, Grosshans M, Frank J, Strohmaier J, von der Goltz C, El-Maarri O, Witt SH, Cichon S, Nothen MM, Kiefer F, Rietschel M. Epigenetic alteration of the dopamine transporter gene in alcohol-dependent patients is associated with age. Addiction Biology. 2012 doi: 10.1111/j.1369-1600.2012.00459.x. [DOI] [PubMed] [Google Scholar]
- NIMH. National Institute of Mental Health Strategic Plan. National Institutes of Health; Bethesda: 2008. [Google Scholar]
- Ooi SK, Qiu C, Bernstein E, Li K, Jia D, Yang Z, Erdjument-Bromage H, Tempst P, Lin SP, Allis CD, Cheng X, Bestor TH. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature. 2007;448:714–717. doi: 10.1038/nature05987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet-Morin I, Wong CC, Danese A, Pariante CM, Papadopoulos AS, Mill J, Arseneault L. Increased serotonin transporter gene (SERT) DNA methylation is associated with bullying victimization and blunted cortisol response to stress in childhood: a longitudinal study of discordant monozygotic twins. Psychological Medicine. 2013;43:1813–1823. doi: 10.1017/S0033291712002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud N, Paoloni-Giacobino A, Prada P, Olie E, Salzmann A, Nicastro R, Guillaume S, Mouthon D, Stouder C, Dieben K, Huguelet P, Courtet P, Malafosse A. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: a link with the severity and type of trauma. Translational Psychiatry. 2011;1:e59. doi: 10.1038/tp.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud N, Salzmann A, Prada P, Nicastro R, Hoeppli ME, Furrer S, Ardu S, Krejci I, Karege F, Malafosse A. Response to psychotherapy in borderline personality disorder and methylation status of the BDNF gene. Translational Psychiatry. 2013;3:e207. doi: 10.1038/tp.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JE, Corces VG. CTCF: master weaver of the genome. Cell. 2009;137:1194–1211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter MO, Du L, Weaver IC, Palkovits M, Faludi G, Merali Z, Szyf M, Anisman H. GABAA receptor promoter hypermethylation in suicide brain: implications for the involvement of epigenetic processes. Biological Psychiatry. 2008;64:645–652. doi: 10.1016/j.biopsych.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Provencal N, Suderman MJ, Caramaschi D, Wang D, Hallett M, Vitaro F, Tremblay RE, Szyf M. Differential DNA methylation regions in cytokine and transcription factor genomic loci associate with childhood physical aggression. PLoS One. 2013;8:e71691. doi: 10.1371/journal.pone.0071691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- Renaud S, Loukinov D, Abdullaev Z, Guilleret I, Bosman FT, Lobanenkov V, Benhattar J. Dual role of DNA methylation inside and outside of CTCF-binding regions in the transcriptional regulation of the telomerase hTERT gene. Nucleic Acids Research. 2007;35:1245–1256. doi: 10.1093/nar/gkl1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee I, Bachman KE, Park BH, Jair KW, Yen RW, Schuebel KE, Cui H, Feinberg AP, Lengauer C, Kinzler KW, Baylin SB, Vogelstein B. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature. 2002;416:552–556. doi: 10.1038/416552a. [DOI] [PubMed] [Google Scholar]
- Robertson KD, Uzvolgyi E, Liang G, Talmadge C, Sumegi J, Gonzales FA, Jones PA. The human DNA methyltransferases (DNMTs) 1, 3a and 3b: coordinate mRNA expression in normal tissues and overexpression in tumors. Nucleic Acids Research. 1999;27:2291–2298. doi: 10.1093/nar/27.11.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusiecki JA, Byrne C, Galdzicki Z, Srikantan V, Chen L, Poulin M, Yan L, Baccarelli A. PTSD and DNA Methylation in Select Immune Function Gene Promoter Regions: A Repeated Measures Case-Control Study of U.S. Military Service Members. Frontiers in Psychiatry. 2013;4:56. doi: 10.3389/fpsyt.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusiecki JA, Chen L, Srikantan V, Zhang L, Yan L, Polin ML, Baccarelli A. DNA methylation in repetitive elements and post-traumatic stress disorder: a case-control study of US military service members. Epigenomics. 2012;4:29–40. doi: 10.2217/epi.11.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seisenberger S, Peat JR, Hore TA, Santos F, Dean W, Reik W. Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers. Philosophical Transactions of the Royal Society B: Biological Sciences. 2013;368:20110330. doi: 10.1098/rstb.2011.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Siegmund KD, Connor CM, Campan M, Long TI, Weisenberger DJ, Biniszkiewicz D, Jaenisch R, Laird PW, Akbarian S. DNA methylation in the human cerebral cortex is dynamically regulated throughout the life span and involves differentiated neurons. PLoS ONE. 2007;2:e895. doi: 10.1371/journal.pone.0000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, Tang Y, Gillespie CF, Cubells JF, Ressler KJ. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2011;156:700–708. doi: 10.1002/ajmg.b.31212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. American Journal of Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- Sterner KN, Weckle A, Chugani HT, Tarca AL, Sherwood CC, Hof PR, Kuzawa CW, Boddy AM, Abbas A, Raaum RL, Gregoire L, Lipovich L, Grossman LI, Uddin M, Goodman M, Wildman DE. Dynamic gene expression in the human cerebral cortex distinguishes children from adults. PLoS ONE. 2012;7:e37714. doi: 10.1371/journal.pone.0037714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyokawa S, Uddin M, Koenen KC, Galea S. How does the social environment ‘get into the mind’? Epigenetics at the intersection of social and psychiatric epidemiology. Social Science and Medicine. 2012;74:67–74. doi: 10.1016/j.socscimed.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Archives of General Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PLoS ONE. 2012;7:e30148. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de Los Santos R, Goldmann E, Galea S. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proceedings of the National Academy of Sciences U S A. 2010;107:9470–9475. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Chang SC, Zhang C, Ressler K, Mercer KB, Galea S, Keyes KM, McLaughlin KA, Wildman DE, Aiello AE, Koenen KC. Adcyap1r1 genotype, posttraumatic stress disorder, and depression among women exposed to childhood maltreatment. Depression and Anxiety. 2013;30:251–258. doi: 10.1002/da.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unternaehrer E, Luers P, Mill J, Dempster E, Meyer AH, Staehli S, Lieb R, Hellhammer DH, Meinlschmidt G. Dynamic changes in DNA methylation of stress-associated genes (OXTR, BDNF ) after acute psychosocial stress. Translational Psychiatry. 2012;2:e150. doi: 10.1038/tp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldic M, Caruncho HJ, Liu WS, Davis J, Satta R, Grayson DR, Guidotti A, Costa E. DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proceedings of the National Academy of Sciences U S A. 2004;101:348–353. doi: 10.1073/pnas.2637013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldic M, Guidotti A, Maloku E, Davis JM, Costa E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proceedings of the National Academy of Sciences U S A. 2005;102:2152–2157. doi: 10.1073/pnas.0409665102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Maurano MT, Qu H, Varley KE, Gertz J, Pauli F, Lee K, Canfield T, Weaver M, Sandstrom R, Thurman RE, Kaul R, Myers RM, Stamatoyannopoulos JA. Widespread plasticity in CTCF occupancy linked to DNA methylation. Genome Research. 2012;22:1680–1688. doi: 10.1101/gr.136101.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wong N, Morley R, Saffery R, Craig J. Archived Guthrie blood spots as a novel source for quantitative DNA methylation analysis. Biotechniques. 2008;45:423–424. 426. doi: 10.2144/000112945. 428 passim. [DOI] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35:1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Goldberg J, Bremner JD, Vaccarino V. Association between promoter methylation of serotonin transporter gene and depressive symptoms: a monozygotic twin study. Psychosomatic Medicine. 2013;75:523–529. doi: 10.1097/PSY.0b013e3182924cf4. [DOI] [PMC free article] [PubMed] [Google Scholar]