Abstract
Background
The influence of different non-myeloablative conditioning regimens on clinical outcome remains undefined.
Material/Methods
We retrospectively analyzed the hematopoietic reconstitution, graft-versus-host disease (GVHD), and quality of life (QOL) in 56 patients with hematologic malignancies who underwent non-myeloablative stem cell transplantation (NST) with a conditioning regimen based on anti-thymocyte globulin (ATG), followed by donor lymphocyte infusion (n=24), or Fludarabine (FLU) (n=32). Hematopoietic stem cells were derived from low-resolution HLA-matched identical sibling donors.
Results
The blood type transformation and platelet reconstitution presented significantly earlier in the FLU group than the ATG group (P<0.05). Within 100 days post-transplantation, the incidence of grade I–IV acute GVHD was significantly lower in the ATG group than the FLU group (P<0.05). After 100 days post-transplant, extensive chronic GVHD (cGVHD) was more prevalent in the ATG group than the FLU group (P<0.05). There were lower cumulative risk of relapse and higher non-relapse-related mortality in the ATG group, but better QOL in the FLU group within 24 months, and no difference in 3-year disease-free survival (DFS) or overall survival (OS) between the 2 groups (P>0.05).
Conclusions
The FLU-based conditioning regimen improved hematopoietic reconstitution and decreased extensive cGVHD, but there was no difference in 3-year DFS or OS between the 2 groups.
Keywords: Graft vs Host Disease, Hematopoietic Stem Cell Transplantation, Hematopoietic System
Background
Hematopoietic stem cell transplantation (HSCT) is preceded by a myeloablative conditioning regimen of chemotherapy or irradiation to eliminate hematopoietic and immunologic function, suppress diseased immune tissue, and reduce graft-versus-host disease (GVHD), followed by donor hematopoietic stem cell engraftment, reconstitution of hemopoiesis, and immunology. However, complete ablation of the host hematopoietic system renders patients susceptible to infection. More recently, non-myeloablative conditioning regimens have been developed to reduce preparative regimen toxicity, widening the patient group in which HSCT can be safely applied [1]. Non-myeloablative HSCT (NST) employs a conditioning regimen of intensive immunosuppressants to promote donor cell implantation and eradicate abnormal hematopoietic and tumor clones [2–5]. Reduced-intensity conditioning regimens in NST lead to donor-recipient immunological tolerance and was found to be associated with delayed acute GVHD (aGVHD) [6,7]. GVHD is the major complication with chronic GVHD (cGVHD) as a major cause of death after allogeneic HSCT (allo-HSCT) [8,9].
Over the past decade, there have been few reports of clinical comparisons between non-myeloablative conditioning regimens or reduced-intensity conditioning (RIC) regimens. Patients receiving RIC today are often those for whom transplantation was not an option a decade ago [10], and few prospective comparative trials have been conducted in this population.
Recently, anti-thymocyte globulin (ATG) and the chemotherapeutic agent Fludarabine (FLU) have become the most commonly used immunosuppressants in non-myeloablative conditioning regimens. ATG has been administered as part of the conditioning regimen successfully combating GVHD in combination with post-transplant administration of glucocorticoids [11,12]. However, ATG can cause many adverse complications and, more recently, FLU has been employed to preserve the graft-versus-leukemia effect, and promote donor stem cell engraftment [13]. In a prospective randomized study, Blaise et al. compared 2 different popular conditioning regimens and determined that administration of FLU with oral busulfan and rabbit ATG was associated with greater disease control than FLU with total body irradiation [14]. However, due to the higher non-relapse mortality (NRM) associated with busulfan and ATG, this did not translate into better overall or progression-free survival [14].
Objective
In this study, we retrospectively compared hematopoietic reconstitution, GVHD, complications, primary disease relapse, survival, and quality of life (QOL) in patients treated with non-myeloablative conditioning regimens based on FLU or ATG. We observed that NST based on FLU conditioning resulted in earlier hematopoietic reconstitution, lower aGVHD and extensive cGVHD incidence, better QOL, higher relapse risk, and lower NRM in comparison with ATG-based conditioning NST followed by donor lymphocyte infusion.
Material and Methods
Clinical data of donors and recipients
We retrospectively studied patients undergoing allo-HSCT for hematologic malignancies in the Department of Hematology of Guangzhou First People’s Hospital, Guangzhou Medical University and the Department of Hematology of Nanfang Hospital, Southern Medical University between January 1, 1999 and May 31, 2011. No patient with liver, kidney, or heart dysfunction or older than 50 received myeloablative conditioning. In order to study a homogenous cohort, we also excluded patients who received allo-HSCT twice, and those who received chemotherapy for a relapse subsequent to allo-HSCT. Apart from chronic myeloid leukemia (chronic phase) and myelofibrosis, all patients underwent transplantation during their first complete hematological remission period. Patients with CML (chronic phase) and primary myelofibrosis were not treated before transplantation.
Donor hematopoietic stem cells were derived from low-resolution serological HLA-matched (6/6 or 5/6 loci) siblings. All donors and recipients were tested for antigen pp65 and antibody for cytomegalovirus (CMV). Before transplantation, there were 2 and 3 positive cases of CMV-IgG in ATG and Flu groups, respectively. Ganciclovir was used for prophylaxis of cytomegalovirus. Ganciclovir combined with human blood immunoglobulin was used to treat cytomegalovirus infection.
The study protocol received approval from the Ethics Committees at each center (Guangzhou First People’s Hospital, Guangzhou Medical University and Nanfang Hospital, Southern Medical University, 2012-SYL-077). Written informed consents were acquired from all participants or their families according to institutional guidelines.
Conditioning regimens
Fifty-six patients received non-myeloablative conditioning regimens based on immunosuppression with either ATG or FLU, combined with reduced-dose busulfan and cyclophosphamide. The preparative regimen consisted of rabbit anti-human thymocyte globulin (Pasteur Merieux) (3 mg/kg/d×3 d), or FLU (30–35 mg/m2/d×5 d), busulfan (injection; 1.6 mg/kg/d×4 d) or busulfan (capsule; 2 mg/kg/d×4 d), and cyclophosphamide (60 mg/kg/d×2 d).
Both regimens were used in both centers according to clinicians’ choices during treatment. In Guangzhou First People’s Hospital, ATG regimen was used for 18 cases while FLU regimen was used for 20 cases; in Nanfang Hospital 6 were treated with ATG regimen while 12 cases were treated with FLU regimen. No significant time period difference exists for the choice of regimen.
Mobilization and collection of stem cells
For bone marrow only graft, bone marrow was collected without G-CSF-mobilization and was infused. For peripheral blood graft, donors received subcutaneous injections of 5 μg/kg G-CSF once a day for 5 consecutive days. Peripheral blood stem cells were collected and infused on the fifth day. For graft with both bone marrow and peripheral blood, donors received consecutive subcutaneous injections of 5 μg/kg G-CSF once a day for 5 days. Bone marrow was collected and infused on the fourth day. Peripheral blood stem cells were then collected and infused on the fifth day.
GVHD prophylaxis
For prophylaxis of GVHD, CsA and methotrexate (MTX) were administered. MTX was administrated at 15 mg/m2 (+1d), and 10 mg/m2 (+3d, +6d, +11d). For patients receiving ATG, CsA was administered by intravenous infusion over 16 h at 3 mg/kg/d. When patients were able to eat, they were given 3–5 mg/kg/d CsA orally, divided into 2 doses per day, to maintain a trough plasma concentration of 0.1663–0.3326 μM (1 μM=1201.9 μg/L). In contrast, for patients receiving FLU, when the donor stem cells were engrafted at 3 weeks post-transplantation, CsA was reduced to a dose of 2.5 mg/kg/d, and then adjusted to maintain a trough plasma concentration of 0.0832–0.1633 μM. When aGVHD did not occur, the CsA dose was reduced after 2 weeks to 1 mg/kg/d, and then adjusted to maintain a trough concentration below 0.0832 μM. If GVHD did not occur, CsA dose was gradually tapered and stopped. When GVHD occurred, the CsA dose was increased or low-dose methylprednisolone (≤160 mg/d) or mycophenolate mofetil was added for 1–2 weeks.
Donor lymphocyte infusion
If mixed chimerism was achieved without GVHD in the ATG group, patients received the first donor lymphocyte infusion at 4 weeks post-transplant. According to the escalated-dose regimen, donor lymphocyte infusion was carried out at intervals of 20–30 days, 2 to 9 times. Before donor lymphocyte apheresis, donors received mobilization with G-CSF at 5 μg/kg/d for 3 consecutive days.
Methods of detecting chimerism
Whole peripheral mononuclear cells were used for chimera analysis. Donor cells were DNA fingerprinted, and sex chromosome and RBC typing was performed twice weekly post-transplantation. Recipient-to-donor cell ratio was quantitatively assessed by fluorescent short tandem repeat [15] weekly in the second and third month after transplantation, and then once every 2 weeks until patients achieved FDC.
Evaluation of hematopoietic recovery
We recorded the time at which patients achieved an ANC ≥0.5×109/L, and a platelet count ≥20×109/L and ≥50×109/L in peripheral blood. When the level of hemoglobin exceeded 70 g/L and the platelet count reached ≥20×109/L, infusions of red blood cells (RBC) and platelets were ceased. The infused volumes of RBC and platelets were calculated after transplantation conditioning. The profile of peripheral blood post-transplant was measured every day until ANC≥0.5×109/L and BPC≥50×109/L for hematologic recovery.
Evaluation of graft-versus-host disease
GVHD was evaluated according to classic and NIH criteria [15]. We first evaluated aGVHD according to the 1994 consensus conference on aGVHD grading [16]. cGVHD was further subcategorized as limited (involving only localized skin and/or hepatic dysfunction) or extensive, according to the Seattle criteria [17]. The latter category included generalized or localized skin and/or hepatic dysfunction; liver histology indicating chronic active hepatitis; bridging necrosis or cirrhosis; eye dryness: Schirmer test ≤5 mm; lip mucosa biopsies indicating mild dysfunction of salivary glands and oral mucosa; and other organ dysfunctions [17].
aGVHD may present after 3 months and manifestations of aGVHD and cGVHD can be present simultaneously [6,18]. We further analyzed GVHD 100 days post-transplant according to NIH criteria [19]. Briefly, aGVHD is defined as the absence of diagnostic or distinctive features of cGVHD. Therefore, aGVHD includes classic aGVHD occurring within 100 days after transplantation and persistent, recurrent, or late aGVHD (features of aGVHD occurring beyond 100 days). Categories of cGVHD include classic cGVHD (without features or characteristics of aGVHD) and an overlap syndrome in which diagnostic or distinctive features of cGVHD and aGVHD appear simultaneously.
Mouth ulcers caused by conditioning drugs and agranulocytosis at early stage after transplantation were rarely seen after 100 days post-transplant, and therefore was excluded due to our method of comparing GVHD complications in the ATG and FLU groups.
Follow-up and quality of life
We evaluated the relapse rate of primary disease, infection, survival, and QOL (according to the Karnofsky criteria) at 6, 12, and 24 months after stem cell transplantation.
Overall survival (OS) was defined as survival from first day after transplant to the final visit (follow-up term).
Statistical analysis
All statistical analyses were performed with SPSS15.0 software (IBM SPSS Inc., Chicago, US). Data found to be normally distributed are expressed as mean ±SD. For comparison between 2 groups, the independent samples t/t’-test was used. Non-normally distributed data were expressed as median, lower, and upper quartiles (P50, P25, P75). The non-parametric Mann-Whitney U test was used for comparison between groups. Categorical data are shown as frequency (f) and constituent ratio or percentage, analyzed by the Pearson chi-square test. Four-fold table data were switched to Fisher’s exact test method. Survival times are shown as mean and 95% confidence interval (CI). Kaplan-Meier survival curves were calculated for long-term survival, and compared using the log rank test. Data were censored for patients alive at their last follow-up visit. A P value ≤0.05 was considered significant.
Results
Baseline clinical characteristics of studied subjects
The demographic and clinical characteristics of all patients are presented in Table 1. Apart from those diagnosed with chronic myeloid leukemia and myelofibrosis, all patients underwent transplantation during their first complete hematological remission period. There was no significant difference in patient age, sex, diagnosis, HLA mismatch, sex match, or ABO match between patients receiving FLU and ATG.
Table 1.
Patient demographic and clinical characteristics.
| Characteristics | Immunosuppressant | P value | |
|---|---|---|---|
| ATG (n=24) | FLU (n=32) | ||
| Age (years, range) | 45 (10–55) | 44 (13–57) | 0.353 |
| Gender (n,%) | |||
| Male | 15 (62.5) | 14 (43.8) | 0.452 |
| Female | 9 (37.5) | 18 (56.2) | 0.764 |
| Diagnosis (n,%) | |||
| Acute leukemia | 13 (54.2) | 20 (62.5) | 0.724 |
| AML | 8 (33.33%) | 12 (37.50) | 0.785 |
| ALL | 5 (20.83%) | 8 (25.00) | 0.760 |
| Multiple myeloma | 3 (12.5) | 4 (12.5) | 0.645 |
| CML | 4 (16.7) | 3 (9.3) | 0.823 |
| NHL | 2 (6.25) | 2 (6.4) | 0.942 |
| Primary myelofibrosis | 2 (6.25) | 3 (9.3) | 0.424 |
| HLA-mismatch (n, %) | |||
| 0 locus mismatch | 22 (91.6) | 29 (90.6) | 0.525 |
| 1 locus mismatch | 2 (8.4) | 3 (9.4) | 0.697 |
| Donor-patient sex match (n, %) | |||
| Sex-match | 10 (41.7) | 12 (37.5) | 0.486 |
| Sex-mismatch | 14 (58.3) | 20 (62.5) | 0.649 |
| ABO match (n,%) | |||
| Match | 6 (25.0) | 10 (31.3) | 0.278 |
| Mismatch | 18 (75.0) | 22 (68.7) | 0.629 |
| MNCs in DLI (×108/kg, range) | 1.15 (0.80–3.75) | ||
| CD3+ cells in DLI (×108/kg, range) | 0.45 (0.12–2.15) | ||
| CD4+ cells in DLI (×108/kg, range) | 0.25 (0.08–0.85) | ||
| CD34+ cells in DLI (×108/kg, range) | 0.45 (0.05–5.75) | ||
| Sources of stem cells (n, %) | |||
| PB | 12 (50.0) | 15 (46.9) | 0.468 |
| BM | 3 (12.5) | 4 (12.5) | 0.376 |
| BM+PB | 9 (37.5) | 13 (40.6) | 0.287 |
| Stem cells infused (mean ±SD) | |||
| CD34+ cells infused (×106/kg) | 4.5±2.2 | 4.8±2.1 | 0.569 |
| MNCs infused (×108/kg) | 6.4±2.2 | 6.5±2.5 | 0.247 |
| Profile of peripheral blood post-transplant (mean ±SD) | |||
| Lowest WBC count (×109/L) | 0.151±0.095 | 0.095±0.085* | 0.0242 |
| ANC ≥0.5×109/L (d) | 14.5±6.4 | 13.5±6.5 | 0.5687 |
| BPC ≥20×109/L (d) | 10.6±4.6 | 11.4±5.6 | 0.5710 |
| BPC ≥50×109/L (d) | 14.6±6.6 | 18.5±4.5* | 0.0111 |
| Infused volume (U) | |||
| RBC | 6.3±4.5 | 4.2±2.5* | 0.0303 |
| Platelet | 9.2±3.5 | 6.4±2.5** | 0.0010 |
FLU – fludarabine; ATG – anti-thymocyte globulin; DLI – donor lymphocyte infusion; HSCT – hematopoietic stem cell transplantation; HLA – human leucocyte antigen; MNC – mononuclear cell; CML – chronic myeloid leukemia; PB – peripheral blood; BM – bone marrow; NHL – non-Hodgkin lymphoma; RBC – red blood cell; NSCT – non-myeloablative stem cell transplantation; BPC – blood platelet count; WBC – white blood cell; ANC – absolute neutrophil count.
0.05>P>0.01,
P< 0.01.
Post transplantation peripheral blood profile and infusion of blood components
The post-transplantation peripheral blood profiles of all patients and volume of infused RBCs and blood platelets are listed in Table 1. The lowest white blood cell count was significantly lower in the FLU group than in the ATG group (P=0.0242), the peripheral blood platelet count exceeded 50×109/L earlier in the FLU group than in the ATG group (P=0.0111), and the volumes of infused RBCs and platelets were lower in the FLU group than in the ATG group (P=0.0303 and P=0.0010). However, the time at which the absolute neutrophil count (ANC) exceeded 0.5×109/L post-transplantation did not differ significantly between the 2 groups (P=0.5687).
Transformation of blood type and chimerism
Median ABO blood type transformation occurred significantly earlier in the FLU group (45 days post-transplantation) than in the ATG group (90 days post-transplantation) (P=0.019) (Table 2).
Table 2.
The transformation of ABO Blood group (day) [P50 (P25, P75)].
| Groups | Transformation of ABO Blood group [day, P50 (P25, P75)] | FDC within 30 days (n,%) |
|---|---|---|
| ATG group (n=24) | 90 (45, 300) | 2 (8.3) |
| FLU group (n=32) | 45 (35, 60)* | 32 (100)** |
FDC – full donor chimerism. Note: In comparison to ATG group,
0.05>P>0.01,
P<0.001.
All patients achieved successful engraftment of donor stem cells, and we observed no conditioning-associated mortality within 100 days post-transplantation. All patients treated with FLU achieved a chimeric ratio of 95–100% donor-to-recipient cells within 30 days, and all achieved full donor chimerism (FDC) within 45 days post-transplant. However, 30 days post-transplant, patients treated with ATG achieved a chimerism ratio of only 50–80%, and the median time to achieve FDC was 120 (135±105) days (Table 2).
Graft-versus-host disease
As shown in Table 3, 4 (16.7%) patients in the ATG group and 16 (50.0%) patients in the FLU group were diagnosed with aGVHD within 100 days after transplantation (P<0.05, Table 3); however, after 100 days there was no significant difference in the fraction of patients in each group diagnosed with acute, classic cGVHD, or overlapping GVHD, according to the new NIH criteria (P>0.05) [19]. A significantly higher fraction of patients treated with ATG (37.5%) than FLU (9.4%) experienced extensive cGVHD (P=0.019), but fewer patients treated with ATG (12.5%) experienced oral complications of GVHD than patients treated with FLU (43.8%) (p=0.018).
Table 3.
Graft-versus-host disease following non-myeloablative stem cell transplantation.
| Classification of GVHD | ATG group (n=24) | FLU group (n=32) | P value |
|---|---|---|---|
| aGVHD within 100 days (n,%) | 4 (16.7) | 16 (50.0)* | 0.018 |
| Grade 1–2 | 4 (16.7) | 10 (31.25) | |
| Grade 3–4 | 0 (0.0) | 6 (18.75) | |
| GVHD post 100 d (n, %) | |||
| aGVHD | 8 (33.3) | 10 (31.2) | 0.969 |
| cGVHD | 12 (50) | 8 (25) | 0.274 |
| Mild | 4 | 3 | |
| Moderate | 4 | 3 | |
| Severe | 4 | 2 | |
| Classic cGVHD | 6 (25.0) | 4 (12.5) | 0.298 |
| Overlap GVHD | 6 (25.0) | 4 (12.5) | 0.298 |
| Extensive cGVHD (n,%) | 9 (37.5) | 3 (9.4)* | 0.019 |
| Oral GVHD complications post 100 d (n,%) | 3 (12.5) | 14 (43.8)* | 0.018 |
| Sever infection# | 12 (50.0) | 8 (25.0)* | 0.024 |
| Liver dysfunction | 10 (41.7) | 22 (68.8) | 0.058 |
| Elevated bilirubin | 12 (50.0) | 6 (18.8)* | 0.021 |
| PTLD | 2 (8.3) | 1 (3.1) | 0.565 |
GVHD – graft-versus-host disease; cGVHD – chronic GVHD; aGVGD – acute GVHD; PTLD – post transplant lymphoproliferative disease. Note: In comparison to ATG group,
P<0.05.
Sever infection including pulmonary aspergillosis, interstitial pneumonia, cytomegalovirus infection or sepsis.
Table 4.
Relapse, quality of life and mortality in the two groups.
| Paramater | ATG group (n=24) | FLU group (n=32) | P Value | |
|---|---|---|---|---|
| Rate of relapse and NRM (n (%)) | NRM | 8 (33.33) | 2 (6.25)* | 0.013 |
| Relapse rate | 1 (4.15) | 9 (28.13)* | 0.032 | |
| Quality of life after transplantation (mean ±SD) | 6 months | 75.6±14.6 | 78.5±16.5 | 0.4974 |
| 12 months | 65.6±15.6# | 80.5±17.5** | 0.0017 | |
| 24 months | 60.6±15.6## | 82.5±17.5** | 0.0001 | |
NRM – non-relapse-related mortality;
P<0.05 in comparison to ATG group;
P<0.01 in comparison to ATG group;
0.0265 and
P=0.0012 In comparison to 6 months.
Other complications
We observed the hepatic toxicity in the course of conditioning. As shown in Table 3, although the incidence of liver dysfunction was no statistical difference in the 2 groups (41.7% vs. 68.8%, p=0.058), the incidence of elevated total bilirubin level in the ATG group was significantly higher than that of the FLU group (50.0% vs. 18.8%, p=0.021<0.05). The incidence of sever infection, including pulmonary aspergillosis, interstitial pneumonia, cytomegalovirus infection or septicemia, in the ATG group was higher than that of the FLU group (50% vs. 25%, p=0.024). One (3.1%) and 3 (8.3%) patients had complicated EB virus-associated post-transplant lymphoproliferative disease (PTLD) in the FLU and ATG group, respectively, and there was not statistically significant difference (p=0.565).
Follow-up of survival
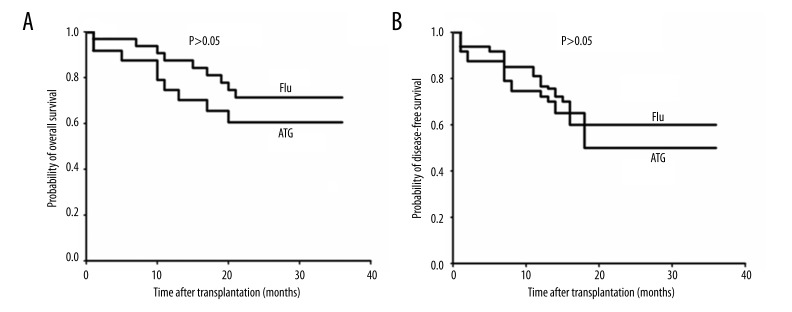
As shown in Figure 1, the overall survival (OS) expectation of 67.1 months (95% CI, 53.8–65.3) in the ATG group did not differ significantly from the 77.0 months (95%CI, 65.8–80.5) (P>0.05) in the FLU group. The 60.5 months (95% CI, 55.2–71.3) disease-free survival (DFS) expectation in the ATG group did not differ significantly from the 72.1 months (95% CI, 65.4–78.3) (P> 0.05) in the FLU group.
Figure 1.
(A, B) Overall survival (OS) and disease-free survival (DFS) following non-myeloablative stem cell transplantation (n=56). Twenty-eight patients with hematologic malignancies underwent non-myeloablative stem cell transplantation (NST) based on an ATG conditioning regimen followed by DLI, and the other 32 patients underwent reduced-intensity conditioning based on a FLU conditioning regimen followed by low-dose cyclosporine A.
Non-relapse-related mortality (NRM) was significantly less frequent in the FLU group than in the ATG group (P<0.05). The primary relapse rate was significantly higher in the FLU group than in the ATG group (P<0.05) (Table 4). NRM was mainly attributed to severe infections, including pulmonary aspergillosis, interstitial pneumonia, and cytomegalovirus infection.
In 10 recorded relapse cases, 1 case occurred in the ATG group at the stage of mixed chimerism. Complete remission and full donor chimerism was achieved after chemotherapy and donor lymphocyte infusion. All the other cases were in the FLU group. At the time of relapse, 3 cases had complete recipient chimera. Hematologic remission was achieved after chemotherapy. Another 3 cases had mixed chimerism. After chemotherapy and donor lymphocyte infusion, 1 case achieved complete hematologic remission and full donor chimerism. The other 2 cases failed to achieve remission.
We scored QOL according to the Karnofsky criteria at 6, 12, and 24 months after HSCT (Table 4). Although QOL scores did not differ significantly between the 2 groups at 6 months post-transplant, scores were higher in the FLU group at 12 and 24 months after transplantation (P<0.05, P<0.01), and QOL scores in the ATG group declined between 6 and 12 and 6 and 24 months (P<0.05, P<0.01) (Table 4).
Discussion
We retrospectively studied 56 patients who underwent allo-HSCT for hematologic malignancies and we compared hematopoietic reconstitution following immunosuppressant conditioning with either FLU or ATG, combined with reduced-dose busulfan and cyclophosphamide. We observed no regimen-related mortality. All patients achieved successful engraftment. At an early stage post-transplant, mixed and FDC were achieved in both the ATG and the FLU groups. The FLU regimen achieved earlier hematopoietic reconstitution, lower incidence of aGVHD within the first 100 days post-transplant, and extensive cGVHD, better QOL, higher relapse risk, and lower NRM in comparison with the ATG regimen. Although the FLU regimen achieved faster hematopoietic reconstitution, it was associated with a relatively high incidence of severe aGVHD within 100 days and a higher rate of relapse than the ATG regimen. In contrast, the ATG regimen was associated with higher NRM rate and therefore did not translate into better overall or disease-free survival.
During pretreatment, we discovered pretreatment-related toxicity. Although the incidence of liver dysfunction in the 2 groups was considerable, the incidence of elevated total bilirubin level in the ATG group was significantly higher than that of the FLU group.
RBC and platelet counts both decreased significantly in patients treated with ATG, but patients treated with FLU achieved recovery of platelet count earlier than those in the ATG group, and patients treated with FLU required infusion of less RBC and platelets. This observation may reflect FDC occurring at an earlier stage in patients treated with FLU, and may also be related to the previously described impact of ATG on the platelet and RBC requirements during RIC before allo-HSCT [20]. Although complete chimerism occurred far later in the ATG group, hematopoietic stem cells of both donor and recipient collaboratively performed hematopoiesis under conditions of immunological tolerance. As a result, there was no significant difference in the time taken for the peripheral blood profile to recover.
Mattsson et al. reported that severe aGVHD occurred less frequently in patients who gradually converted to FDC[21]. In our sample, aGVHD and severe aGVHD within the first 100 days post-transplant also occurred less frequently in the ATG group than in the FLU group, perhaps due to the early mixed chimerism achieved by the ATG group in contrast to the full chimerism observed in the FLU group.
Recently, other research groups have reported that although the incidence of grade II to IV aGVHD in NST was lower than in conventional HSCT, there was no significant difference in the incidence of GVHD after 100 days [6]. Mixed chimerism after NST was associated with primary disease relapse. When conventional donor lymphocyte infusion was used, primary disease relapse decreased. However, donor lymphocyte infusion after NST resulted in increased cGVHD pseudomorphs [22].
We analyzed the GVHD features 100 days after transplant and found that the incidence of aGVHD, classic cGVHD, and overlap GVHD did not significantly differ between patients treated with FLU and ATG; however, the incidence of oral GVHD was significantly higher in patients treated with FLU than ATG. The oral GVHD was resolved by increasing cyclosporine (CsA) dose and administration of glucocorticoid and mycophenolate mofetil.
One study indicated that the incidence of GVHD, especially cGVHD, correlated with the graft-versus-leukemia effect after allo-HSTC [23]. We observed a lower relapse rate in the ATG group, indicating that a strategy of NST followed by donor lymphocyte infusion achieved a stronger graft-versus-leukemia or tumor effect following administration of ATG than FLU.
The influence of cGVHD on NRM remains controversial. Vigorito et al. conducted a large-scale clinical study of allo-HSTC and reported that survival, NRM, and systemic immunotherapy were not correlated with the clinical features of cGVHD [24]. However, prolonged immunosuppressant use for late aGVHD was correlated with NRM. Persistent cGVHD or aGVHD beyond 100 days post-transplant were both independent predictors for poor survival [24]. Beyond 100 days after transplant, we observed no significant difference in the incidence of aGVHD or cGVHD between patients treated with ATG and FLU. There was also no difference detected in overall survival (OS) expectations between the 2 groups, as would be expected according to the similar persistent GVHD [25]. Refractory GVHD complicated by donor lymphocyte infusion resulting from prolonged immune suppression increased infection and mortality in the ATG group. In this study, NRM was more common in the ATG group than in the FLU group because of higher sever infection such as pulmonary aspergillosis, interstitial pneumonia, cytomegalovirus infection, or septicemia in the ATG group, but more patients died of primary diseases in the FLU group than in the ATG group. It is well known that EBV reactivation is frequently observed after ATG administration. As in the FLU group, the ATG group was also susceptible to EB virus-associated post-transplant lymphoproliferative disease (PTLD), but a statistically significant difference was not found because of the small sample size of this study. Three-year DFS and OS did not differ significantly between these groups, possibly as a result of the 2 risk factors offsetting one another.
Few previous reports have described long-term QOL of HSCT patients. Some studies suggested that the QOL of patients receiving RIC stem cell transplantation was superior to that of those who underwent myeloablative allogeneic and autologous HSCT [26–28], but systemic comparison of QOL in different NST strategies was not reported. In the present study we compared QOL between patients treated with ATG and FLU after stem cell transplantation according to Karnofsky scoring criteria. After 6 months, no statistically significant difference between the 2 groups was observed. Despite a lower incidence of primary disease relapse in the ATG group at 12 and 24 months, the QOL scores of patients in the FLU group were superior to those of the ATG group. In the ATG group, the higher incidence of severe infection, GVHD, and NRM may have been offset by the higher incidence of mortality resulting from primary disease relapse in the FLU group. This observation agrees with a previous study that reporting that refractory late aGVHD and cGCHD may have compromised attitude and behavior, increasing NRM and decreasing QOL scores at 12 and 24 months after transplantation [29]. We also observed lower scores at 12 and 24 months than at 6 months post-transplant in the ATG group.
Conclusions
The conclusions of this small experimental study are limited by the retrospective nature of the study and the limited sample size. Due to analysis of only a few patients at 2 sites, the study was insufficiently powered to make broad generalizations about the applicability of any observed differences between the groups to a larger sample. In summary, the ideal conditioning regimen before allo-HSCT has not yet been established. We attempted to develop novel RIC or non-myeloablative conditioning (NMAC) strategies capable of reducing the incidence and severity of GVHD without compromising the beneficial impact of graft-versus-leukemia activity or immune reconstitution after transplantation, and to improve long-term DFS and QOL in patients with hematologic malignancies. The FLU regimen achieved faster hematopoietic reconstitution, but was associated with a relatively high incidence of severe aGVHD and a higher rate of relapse than the ATG regimen. A recent report showed that, acute rejection rates, renal function, CMV infection, and the adverse effects of low-dose rabbit-ATG induction may be superior to high-dose rabbit-ATG induction therapy in renal transplantation [30]. Use of low-dose ATG effectively improved the survival rate for the partially-matched unrelated stem cell transplantation because the use of ATG lowered the cumulative incidence of sever acute GVHD chronic GVHD [31]. The sample size in these study was probably too small and lacked homogeneity. To explore a better balance between efficacy and complications of immune-suppressants in conditioning regimens, we conclude that further, larger clinical trials of improved regimens will be required to establish a successful conditioning regimen.
Footnotes
Source of support: This study was supported by the following grants: Project of Guangdong provincial Science and Technology (20011B031800053), Guangzhou Bureau of Science and Technology (20011Y100038-3 and 201300000100)
Conflict of interests
All authors declare that they have no conflict of interests.
References
- 1.Andersson BS, de Lima M, Thall PF, et al. Reduced-toxicity conditioning therapy with allogeneic stem cell transplantation for acute leukemia. Curr Opin Oncol. 2009;21(Suppl 1):S11–15. doi: 10.1097/01.cco.0000357469.83960.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Margolis J, Borrello I, Flinn IW. New approaches to treating malignances with stem cell transplantation. Semin Oncol. 2000;27(5):524–30. [PubMed] [Google Scholar]
- 3.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97(11):3390–400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 4.Resnick IB, Shapira MY, Slavin S. Nonmyeloablative stem cell transplantation and cell therapy for malignant and non-malignant diseases. Transpl Immunol. 2005;14(3–4):207–19. doi: 10.1016/j.trim.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Tauro S, Craddock C, Peggs K, et al. Allogeneic stem-cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease-free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. J Clin Oncol. 2005;23(36):9387–93. doi: 10.1200/JCO.2005.02.0057. [DOI] [PubMed] [Google Scholar]
- 6.Mielcarek M, Martin PJ, Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102(2):756–62. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 7.Mielcarek M, Storb R. Graft-vs-host disease after non-myeloablative hematopoietic cell transplantation. Leuk Lymphoma. 2005;46(9):1251–60. doi: 10.1080/10428190500125754. [DOI] [PubMed] [Google Scholar]
- 8.Pasquini MC, Wang Z, Horowitz MM, Gale RP. 2010 report from the Center for International Blood and Marrow Transplant Research (CIBMTR): current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin Transpl. 2010:87–105. [PubMed] [Google Scholar]
- 9.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(4):215–33. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 10.Blaise D, Castagna L. Do different conditioning regimens really make a difference? Hematology Am Soc Hematol Educ Program. 2012;2012:237–45. doi: 10.1182/asheducation-2012.1.237. [DOI] [PubMed] [Google Scholar]
- 11.Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10(9):855–64. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 12.Socie G, Schmoor C, Bethge WA, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117(23):6375–82. doi: 10.1182/blood-2011-01-329821. [DOI] [PubMed] [Google Scholar]
- 13.Li JM, Giver CR, Waller EK. Graft engineering using ex vivo methods to limit GVHD: fludarabine treatment generates superior GVL effects in allogeneic BMT. Exp Hematol. 2006;34(7):895–904. doi: 10.1016/j.exphem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Blaise D, Tabrizi R, Boher JM, et al. Randomized study of 2 reduced-intensity conditioning strategies for human leukocyte antigen-matched, related allogeneic peripheral blood stem cell transplantation: prospective clinical and socioeconomic evaluation. Cancer. 2013;119(3):602–11. doi: 10.1002/cncr.27786. [DOI] [PubMed] [Google Scholar]
- 15.Nollet F, Billiet J, Selleslag D, Criel A. Standardisation of multiplex fluorescent short tandem repeat analysis for chimerism testing. Bone Marrow Transplant. 2001;28(5):511–18. doi: 10.1038/sj.bmt.1703162. [DOI] [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–28. [PubMed] [Google Scholar]
- 17.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–17. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 18.Flowers ME, Parker PM, Johnston LJ, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002;100(2):415–19. doi: 10.1182/blood-2002-01-0011. [DOI] [PubMed] [Google Scholar]
- 19.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Granata A, Castagna L, Crocchiolo R, et al. Impact of antithymocyte globulin on the need for platelet transfusions during reduced-intensity conditioning administration before allogeneic stem cell transplantation. Exp Hematol. 2013;41(6):503–5. doi: 10.1016/j.exphem.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Mattsson J, Uzunel M, Remberger M, Ringden O. T cell mixed chimerism is significantly correlated to a decreased risk of acute graft-versus-host disease after allogeneic stem cell transplantation. Transplantation. 2001;71(3):433–39. doi: 10.1097/00007890-200102150-00017. [DOI] [PubMed] [Google Scholar]
- 22.Teshima T, Ferrara JL. Understanding the alloresponse: new approaches to graft-versus-host disease prevention. Semin Hematol. 2002;39(1):15–22. doi: 10.1053/shem.2002.29246. [DOI] [PubMed] [Google Scholar]
- 23.de Lima M, Bonamino M, Vasconcelos Z, et al. Prophylactic donor lymphocyte infusions after moderately ablative chemotherapy and stem cell transplantation for hematological malignancies: high remission rate among poor prognosis patients at the expense of graft-versus-host disease. Bone Marrow Transplant. 2001;27(1):73–78. doi: 10.1038/sj.bmt.1702726. [DOI] [PubMed] [Google Scholar]
- 24.Vigorito AC, Campregher PV, Storer BE, et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood. 2009;114(3):702–8. doi: 10.1182/blood-2009-03-208983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arora M, Nagaraj S, Witte J, et al. New classification of chronic GVHD: added clarity from the consensus diagnoses. Bone Marrow Transplant. 2009;43(2):149–53. doi: 10.1038/bmt.2008.305. [DOI] [PubMed] [Google Scholar]
- 26.Bevans MF, Marden S, Leidy NK, et al. Health-related quality of life in patients receiving reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;38(2):101–9. doi: 10.1038/sj.bmt.1705406. [DOI] [PubMed] [Google Scholar]
- 27.Diez-Campelo M, Perez-Simon JA, Gonzalez-Porras JR, et al. Quality of life assessment in patients undergoing reduced intensity conditioning allogeneic as compared to autologous transplantation: results of a prospective study. Bone Marrow Transplant. 2004;34(8):729–38. doi: 10.1038/sj.bmt.1704646. [DOI] [PubMed] [Google Scholar]
- 28.Wong R, Giralt SA, Martin T, et al. Reduced-intensity conditioning for unrelated donor hematopoietic stem cell transplantation as treatment for myeloid malignancies in patients older than 55 years. Blood. 2003;102(8):3052–59. doi: 10.1182/blood-2003-03-0855. [DOI] [PubMed] [Google Scholar]
- 29.Baker KS, Fraser CJ. Quality of life and recovery after graft-versus-host disease. Best Pract Res Clin Haematol. 2008;21(2):333–41. doi: 10.1016/j.beha.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Sohn SK, Moon JH. Favorable effects of low-dose anti-thymocyte globulin in a partially-mismatched HLA group in an unrelated allogeneic stem cell transplantation setting. Ann Transplant. 2015;20:7–15. doi: 10.12659/AOT.892298. [DOI] [PubMed] [Google Scholar]
- 31.Yang JW, Wang JN, Men TY, et al. Comparison of clinical outcome of low-dose and high-dose rabbit antithymocyte globulin induction therapy in renal transplantation: a single-center experience. Ann Transplant. 2014;19:277–82. doi: 10.12659/AOT.890069. [DOI] [PubMed] [Google Scholar]