Abstract
Background
Transgelin is supposed to be a tumor suppression gene and it is down-regulated in a variety of human cancers. However, the role of transgelin in different cancers is still very controversial. In addition, currently little information is available the relationship between transgelin and Oral Squamous Cell Carcinoma (OSCC).
Material/Methods
Western Blotting was performed to test the transgelin protein expression level in OSCC tissues and adjacent normal tissues. Real-time PCR was used to examine the expression level of transgelin mRNA in tissue, serum and saliva of OSCC patients and negative controls. The correlation between tissue and salivary transgelin mRNA expression level with a variety of clinical parameters was further studied.
Results
Transgelin protein expression was increased in OSCC patients compared with healthy individuals. Similarly, the expression level of both tissue and salivary transgelin mRNA were increased significantly in patients with OSCC in comparison with normal controls. However, little difference of serum transgelin mRNA expression was found between the OSCC patients and healthy controls. In addition, overexpression of tissue or salivary transgelin was closely associated with various clinical parameters including poorer overall survival. Furthermore, our results showed that tissue and salivary transgelin mRNA were independent prognosis factors for OSCC.
Conclusions
The expressions level of tissue mRNA and protein were increased in OSCC patients. Both tissue and salivary transgelin mRNA were closely correlated with various important clinicopathological parameters and were independent prognosis factors for OSCC, indicating they might serve promising biomarkers for OSCC.
MeSH Keywords: Mouth Neoplasms; RNA, Messenger; Saliva; Serum
Background
Oral squamous cell carcinoma (OSCC), with a 5-year survival rate at approximately 50%, is the most frequent entity in head and neck squamous cell carcinoma [1]. The occurrence and development of OSCC are multi-stage processes, which involve in a variety of changes in genes expression level and signal transduction pathways. The underlying mechanisms that promote OSCC development remain unknown. Clinically, large-scale surgery and radiation therapy are the treatments of choice. However, the current state of identification of useful OSCC prognostic factors, which serves as important guidance for clinical doctors, is not promising. It is an urgent and important task.
Transgelin, also known as smooth muscle protein 22, is a 201-amino acid protein which plays important roles in podosome formation and myocyte migration, vascular and visceral smooth muscle cell differentiation. Transgelin is believed to be tumour-suppressive gene, as it is down-regulated in a variety of cancers. Moreover, Loss of transgelin expression in transformed cells is closely correlated with oncogenesis [2]. Prasad et al. showed that both transgelin mRNA and protein was down-regulated in prostate cancer tissue and most prostate cancer cell lines [3]. Similarly, the expression level of transgelin was reduced in gallbladder cancer tissues [4]. Transgelin was reported be a suppressor of MMP-9, which is an important protein for cancer metastasis [5]. In addition, depletion of transgelin promotes cell survival and cancer metastasis potential [6]. However, the tumor suppressive role of transgelin is controversial, it seems that the role of transgelin is different types is various. Even contradictory findings about transgelin have been reported in the same kind of cancer. Lee et al. reported that the expression level of transgelin was significantly increased in cancer stem cells than non-tumorigenic cells. Moreover, transgelin was showed to be a positive regulator of cancer cell invasion capacity [7]. Huang et al. revealed the transgelin expression level increased significantly in gastric adenocarcinoma compared with normal tissues [8].
Currently, little information is available the relationship between transgelin and OSCC. In the present study, we assessed the expression level of tissue/serum/saliva transgelin level in OSCC patients and normal controls to find out whether they could be serve potential biomarkers for early detection and prediction of OSCC.
Material and Methods
Patients and samples
This study was approved by the Ethics Committee of the Chinese People’s Liberation Army General Hospital. All patients and health controls who were recruited for participation in this study gave their informed consent before sample collection (serum, saliva, and tissue). OSCC tumor tissues and neighboring normal tissues were obtained by surgical excision from 78 cancer patients in the Department of Stomatology, Chinese People’s Liberation Army General Hospital between 2009 and 2013. The saliva and serum of these patients were also collected before surgery.
Western blotting
Tissue lysate was isolated and separated by SDS-PAGE. The proteins were then transferred from the gel to nitrocellulose membrane. The membrane was then incubated with rabbit anti-transgelin (1:500; ProteinTech Group Inc., Wuhan, China). This was followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (Thermo Scientific Pierce, Rockford, IL, USA). The bands were detected using SuperSignal (R) West Femto Maximum Sensitivity Substrate (Thermo Scientific Pierce). The analysis was performed in triplicate.
Real-time PCR
Tissue RNA was extracted using TRIzol (Takara, Dalian, China) according to the manufacturer’s instructions. Salivary and serum RNA were isolated from 560 μL of saliva supernatant and sera with QIAamp Viral RNA kit (Qiagen, Valencia, CA, USA) respectively. First-strand cDNA was generated using the PrimeScript TM RT reagent Kit (Takara). The cDNA was amplified with SYBR Premix Dimer Eraser TM (Takara). The PCR reaction was performed at the Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Gene expression was normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Primer sequences for are as follows:
Forward transgelin 5′-GTTCCAGACTGTTGACCTCTT-3′
Reverse transgelin 5′-CTGCGCTTTCTTCATAAACC-3′
Forward GAPDH 5′-TGTTCGTCATGGGTGTGAAC-3′
Reverse GAPDH 5′-ATGGCATGGACTGTGGTCAT-3′
Statistical analysis
The expression levels of tissue/serum/saliva transgelin mRNAs in OSCC patients and controls were compared using Mann-Whitney U-test. The χ2-test was used to analyze the correlation of tissue/salivary transgelin mRNA with clinical parameters. The overall survival of OSCC patients was measured using the Kaplan-Meier method. The Cox proportional hazards regression model was employed for univariate and multivariate analyses to estimate the prognostic factors for OSCC. Two-tailed p values of <0.05 were considered statistically significant. The software of SPSS version 13.0 for Windows was used for statistical analysis (SPSS Inc., Chicago, IL, USA).
Results
Over-expression of transgelin mRNA and protein in OSCC tissues
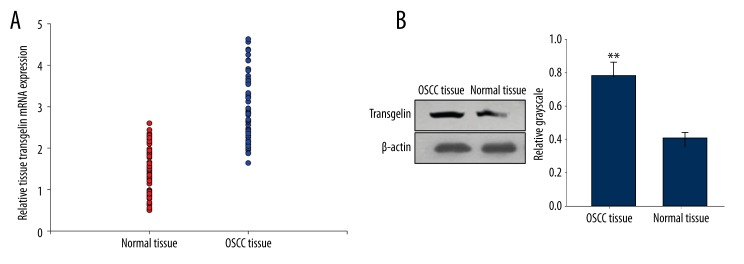
The expression levels of transgelin mRNA and protein were measured by real-time PCR and Western Blot respectively. Transgelin mRNA expressed significantly higher in OSCC tissues compared with adjacent normal tissues (p<0.01, Figure 1A). Our results also showed that transgelin protein was increased in patients with OSCC (p<0.01, Figure 1B)
Figure 1.
The expression level of tissue transgelin mRNA and protein in OSCC patients and healthy controls.
Expression levels of transgelin mRNAs in serum and saliva
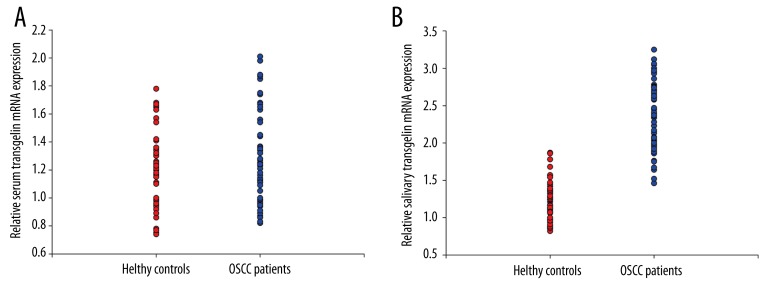
The expression levels of transgelin mRNAs in serum and saliva were examined by real-time PCR. Statistically difference was observed in the salivary transgelin mRNA expression level between OSCC patients and health controls (p<0.01), while no difference was found between the expression level of serum transgelin mRNA between the two groups (p>0.05) (Figure 2).
Figure 2.
The expression level of transgelin mRNA in serum and saliva.
Association of tissue/salivary transgelin mRNA with clinical parameters
The median expression level of tissue transgelin mRNA (2.87 fold) and salivary transgelin mRNA (2.35 fold) were used as the cut-off points to define the high expression level or low expression level. The correlations of the expression levels of tissue/salivary transgelin mRNAs with clinical parameters were shown in Table 1. Statistically difference was found between tissue transgelin mRNA expression and N stage (p=0.021), TNM stage (p<0.000), differentiation (p=0.048), tumor depth (p=0.028), extracapsular spread of lymph node (LN ECS) (p=0.003). However, there was no correlation between tissue transgelin mRNA expression and age (p=0.512), sex (p=0.452), T stage (p=0.086). As to salivary transgelin mRNA, significant correlations were found between salivary transgelin mRNA expression and T stage (p=0.030), N stage (p=0.004), TNM stage (p<0.000), differentiation (p=0.014), LN ECS (p=0.002); and no statistically difference was found between salivary transgelin mRNA expression and age (p=0.906), sex (p=0.801), tumor depth (p=0.224).
Table 1.
The correlation of tissue/salivary transgelin mRNA with clinical parameters.
| Parameter | Tissue transgelin mRNA high expression | Saliva transgelin mRNA high expression | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | No (%) | Yes (%) | p | No (%) | Yes (%) | P | |
| Age | |||||||
| >60 | 44 | 20 (25.6) | 24 (30.8) | 0.512 | 20 (25.6) | 24 (30.8) | 0.906 |
| ≤60 | 34 | 18 (23.1) | 16 (20.5) | 15 (19.2) | 19 (24.4) | ||
|
| |||||||
| Sex | |||||||
| Male | 48 | 25 (32.1) | 23 (29.5) | 0.452 | 21 (26.9) | 27 (34.6) | 0.801 |
| Female | 30 | 13 (16.7) | 17 (21.8) | 14 (17.9) | 16 (20.5) | ||
|
| |||||||
| T stage | |||||||
| T1–T2 | 50 | 28 (35.9) | 22 (28.2) | 0.086 | 27 (34.6) | 23 (29.5) | 0.030 |
| T3–T4 | 28 | 10 (12.8) | 18 (23.1) | 8 (10.3) | 20 (25.6) | ||
|
| |||||||
| N stage | |||||||
| N=0 | 54 | 31 (39.7) | 23 (29.5) | 0.021 | 30 (38.5) | 24 (30.8) | 0.004 |
| N>0 | 24 | 7 (9.0) | 17 (21.8) | 5 (6.4) | 19 (24.4) | ||
|
| |||||||
| TNM stage | |||||||
| I–II | 46 | 30 (38.5) | 16 (20.5) | 0.000 | 29 (37.2) | 17 (21.8) | 0.000 |
| III–IV | 32 | 8 (10.3) | 24 (30.8) | 6 (7.7) | 26 (33.3) | ||
|
| |||||||
| Differentiation | |||||||
| Well | 51 | 29 (37.2) | 22 (28.2) | 0.048 | 28 (35.9) | 23 (29.5) | 0.014 |
| Moderate/poor | 27 | 9 (11.5) | 18 (23.1) | 7 (9.0) | 20 (25.6) | ||
|
| |||||||
| Tumor depth >10 mm | |||||||
| Yes | 28 | 9 (11.5) | 19 (24.4) | 0.028 | 10 (12.8) | 18 (23.1) | 0.224 |
| No | 50 | 29 (37.2) | 21 (26.9) | 25 (32.1) | 25 (32.1) | ||
|
| |||||||
| LN ECS | |||||||
| Yes | 25 | 6 (7.7) | 19 (24.4) | 0.003 | 5 (6.4) | 20 (23.1) | 0.002 |
| No | 53 | 32 (41.0) | 21 (26.9) | 30 (38.5) | 23 (29.5) | ||
|
| |||||||
| Total | 78 | 38 (48.7) | 40 (51.3) | 35 (44.9) | 43 (55.1) | ||
Survival analysis
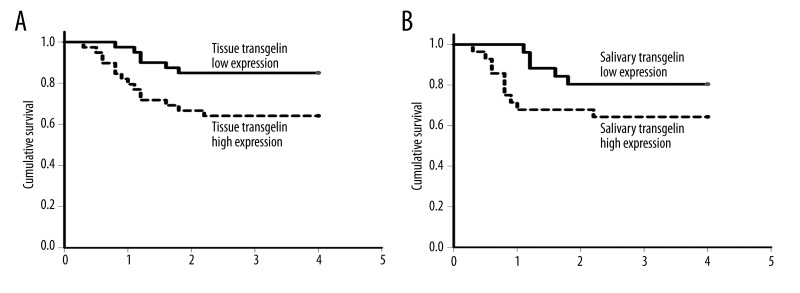
The association between tissue/salivary transgelin mRNAs expression levels and the overall survival of OSCC patients were measured using the Kaplan-Meier method. Our results showed that OSCC patients who had a higher expression level of tissue transgelin mRNA (p=0.004, Figure 3A) or salivary transgelin mRNA (p=0.011, Figure 3B) suffered a poorer overall survival rate.
Figure 3.
The association between tissue/salivary transgelin mRNA expression level and the overall survival of OSCC patients.
Univariant analysis and multivariant analysis of prognostic factors in OSCC
As showed in Table 2, N stage (p=0.037), TNM stage (p<0.001), differentiation (p=0.024), LN ECS (p=0.003), tissue transgelin mRNA (p=0.006), salivary transgelin mRNA (p=0.016) were significant prognostic indicators for OSCC.
Table 2.
Univariant analysis of prognostic factors in OSCC.
| Parameter | Hazard ratio | p value |
|---|---|---|
| Age (>60/≤60) | 1.387 | 0.298 |
| Sex (male/female) | 0.955 | 0.875 |
| T stage (T3–T4/T1–T2) | 1.199 | 0.567 |
| N stage (N>0/N=0) | 1.934 | 0.037 |
| TNM stage (III–IV/I–II) | 4.450 | <0.001 |
| Differentiation (moderate and poor/well) | 2.147 | 0.024 |
| Tumor depth >10 mm (yes/no) | 1.308 | 0.376 |
| LN ECS (yes/no) | 2.987 | 0.003 |
| Tissue transgelin mRNA (high/low) | 2.694 | 0.006 |
| Salivary transgelin mRNA (high/low) | 2.396 | 0.016 |
Our multivariant analysis revealed that TNM stage (p=0.006), LN ECS (p=0.014), tissue transgelin mRNA (p=0.046), salivary transgelin mRNA (p= 0.034) were independent prognostic factors for OSCC (Table 3).
Table 3.
The independent prognostic factors of OSCC in multivariant analysis model.
| Parameter | Hazard ratio | p value |
|---|---|---|
| TNM stage | 3.274 | 0.006 |
| LN ECS | 2.541 | 0.014 |
| Tissue transgelin mRNA | 1.852 | 0.046 |
| Salivary transgelin mRNA | 2.145 | 0.034 |
Discussion
OSCC is a malignant disease that causes high morbidity and mortality. In addition, in most cases the lesion only develops in the oral cavity, thus it is difficult to discover until it has progressed to advanced stage, when the treatment options are very limited [9]. The TNM system and histological grading are important guidance for clinical doctors when treating OSCC; however, they cannot help doctors detect the diseases at an early stage. More importantly, these clinical parameters fail to provide the information about status of patients in real time. Even though many new OSCC biomarkers have been identified in recent years [10,11], much effort is still needed. It is important and urgent to discover sensitive and accurate biomarkers that can help early diagnosis and predict the prognosis of OSCC.
In this study, we found that the expression levels of both tissue and salivary transgelin mRNAs were increased significantly in patients with OSCC in comparison with normal controls. However, little difference of transgelin mRNA expression was found between the OSCC patients and healthy controls. In addition, overexpression of tissue or salivary transgelin was closely correlated with various clinical parameters including poorer survival rate. Furthermore, our results showed that tissue and salivary transgelin mRNA were independent prognosis factors for OSCC.
Consistent with our results about overexpression of tissue transgelin mRNA and protein in OSCC, Wang et al. used DIGE method to screen the different protein expression profiles between OSCC tissue and control normal tissues, they found that the expression level of cancer tissue transgelin was 2.1 fold higher than that of normal tissues [12]. Wu et al. showed that transgelin protein overexpression was associated with TNM stage, lymph node metastasis and poor differentiation in lung adenocarcinoma patients [13]. Similarly, Overexpression of transgelin protein was observed in colorectal cancer patients with lymph node metastasis. In addition, down-regulation of transgelin inhibited proliferation, invasion, and anoikis resistance of colorectal cancer cell lines, indicating transgelin played an important role in regulation of epithelial-mesenchymal transition program and cancer metastasis [14]. However, Li et al. showed that the expressions of transgelin in colorectal carcinoma tissues and LoVo cells were significantly decreased compared with normal controls. In addition, transgelin was proved to be a suppressor of MMP-9 expression, indicating that transgelin might be a negative regulator of cancer metastasis [15]. The contradictory finding about transgelin in colorectal carcinoma suggested the complex role of transgelin in regulation of cancer progression. Further and larger scale research is urgently needed to pinpoint the role of transgelin in colorectal carcinoma.
Even though large amount of tissue protein are proved to be closely correlated with the prognosis of OSCC, we cannot detect the expression level of them in vivo and in real time. Testing the expression level of potential biomarkers in the body fluid such as serum, saliva and urine is an effective way to solve this problem. In this way we can not only use the biomarkers to diagnosis the cancer at an early stage, but also can monitor the whole treatment process. Moreover, these techniques are less invasive than biopsy and easily accessible. Our results showed that no difference was found in serum transgelin mRNA expression level between OSCC patients and health people. There may be several reasons for this finding. First, it is possible that OSCC cells secret little transgelin mRNA into the serum. Second, there may be large intra- and inter-individual variation of serum transgelin mRNA under normal conditions, thus the sample size in this study is not large enough to detect the difference. Moreover, it is also possible that the secreted transgelin mRNA in the blood is recaptured or digested by other cell types to maintain the normal expression level of transgelin mRNA in the serum.
Saliva has been become a popular body fluid for diagnosis of various diseases in recent years (cancer, Sjögren’s syndrome, chronic diseases etc.). The development from normal to OSCC might cause altered expression of protein, and miRNA and mRNA markers in saliva [16]. Our results showed that OSCC patients had a higher expression of transgelin mRNA in saliva, and the salivary transgelin mRNA was related with various important clinical parameters including T stage, N stage, TNM stage, differentiation, LN ECS and overall survival. More importantly, the expression of salivary transgelin mRNA was showed be an independent prognosis factor for OSCC, suggesting that salivary transgelin mRNA might be a promising biomarker for early detection of OSCC and predicting the prognosis for OSCC patients. The enhanced expression level of saliva transgelin in patients with OSCC may be due to the close anatomical position between oral cancer tissues and salivary glands, and OSCC cells might secret more transgelin into the saliva. To our best knowledge, this is the first time to test the potential clinical relationship between the salivary transgelin mRNA and cancer. However, further and larger scale studies are needed to be carried out to confirm the potential of salivary transgelin mRNA as a biomarker for OSCC.
Conclusions
The expression levels of tissue transgelin mRNA and protein were increased in OSCC patients. In addition, tissue and salivary transgelin mRNAs expression level were closely correlated with various important clinicopathological parameters. Higher tissue and salivary transgelin mRNAs expression predicted worse overall survival rates and they were independent prognosis factors for OSCC, indicating that tissue and salivary transgelin mRNA might be promising biomarkers for OSCC.
Footnotes
Conflict of interest
We declare that we have no conflicts of interest.
Source of support: The study is supported by the Natural Science Foundation of Beijing City (No.7122161) and the PLA General Hospital Technological Innovation Nursery Foundation (No.11KMM11)
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Shields JM, Rogers-Graham K, Der CJ. Loss of transgelin in breast and colon tumors and in RIE-1 cells by Ras deregulation of gene expression through Raf-independent pathways. J Biol Chem. 2002;277:9790–99. doi: 10.1074/jbc.M110086200. [DOI] [PubMed] [Google Scholar]
- 3.Prasad PD, Stanton JA, Assinder SJ. Expression of the actin-associated protein transgelin (SM22) is decreased in prostate cancer. Cell Tissue Res. 2010;339:337–47. doi: 10.1007/s00441-009-0902-y. [DOI] [PubMed] [Google Scholar]
- 4.Sahasrabuddhe NA, Barbhuiya MA, Bhunia S, et al. Identification of prosaposin and transgelin as potential biomarkers for gallbladder cancer using quantitative proteomics. Biochem Biophys Res Commun. 2014;446:863–69. doi: 10.1016/j.bbrc.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chunhua L, Donglan L, Xiuqiong F, et al. Apigenin up-regulates transgelin and inhibits invasion and migration of colorectal cancer through decreased phosphorylation of AKT. J Nutr Biochem. 2013;24:1766–75. doi: 10.1016/j.jnutbio.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Thompson O, Moghraby JS, Ayscough KR, Winder SJ. Depletion of the actin bundling protein SM22/transgelin increases actin dynamics and enhances the tumourigenic phenotypes of cells. BMC Cell Bio. 2012;13:1. doi: 10.1186/1471-2121-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee EK, Han GY, Park HW, et al. Transgelin promotes migration and invasion of cancer stem cells. J Proteome Res. 2010;9:5108–17. doi: 10.1021/pr100378z. [DOI] [PubMed] [Google Scholar]
- 8.Huang Q, Huang Q, Chen W, et al. Identification of transgelin as a potential novel biomarker for gastric adenocarcinoma based on proteomics technology. J Cancer Res Clin Oncol. 2008;134:1219–27. doi: 10.1007/s00432-008-0398-y. [DOI] [PubMed] [Google Scholar]
- 9.Timmons SR, Nwankwo JO, Domann FE. Acetaldehyde activates Jun/AP-1 expression and DNA binding activity in human oral keratinocytes. Oral Oncol. 2002;38:281–90. doi: 10.1016/s1368-8375(01)00056-2. [DOI] [PubMed] [Google Scholar]
- 10.Lei W, Liu YE, Zheng Y, Qu L. MiR-429 inhibits oral squamous cell carcinoma growth by targeting ZEB1. Med Sci Monit. 2015;21:383–89. doi: 10.12659/MSM.893412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang D, Ni Z, Xu X, Xiao J. MiR-32 functions as a tumor suppressor and directly targets EZH2 in human oral squamous cell carcinoma. Med Sci Monit. 2014;20:2527–35. doi: 10.12659/MSM.892636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Jiang L, Huang C, et al. Comparative proteomics approach to screening of potential diagnostic and therapeutic targets for oral squamous cell carcinoma. Mol Cell Proteomics. 2008;7:1639–50. doi: 10.1074/mcp.M700520-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu X, Dong L, Zhang R, et al. Transgelin overexpression in lung adenocarcinoma is associated with tumor progression. Int J Mol Med. 2014;34:585–91. doi: 10.3892/ijmm.2014.1805. [DOI] [PubMed] [Google Scholar]
- 14.Lin Y, Buckhaults PJ, Lee JR, et al. Association of the actin-binding protein transgelin with lymph node metastasis in human colorectal cancer. Neoplasia. 2009;11:864–73. doi: 10.1593/neo.09542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Shi R, Wang Y, Niu X. TAGLN suppresses proliferation and invasion, and induces apoptosis of colorectal carcinoma cells. Tumour Biol. 2013;34:505–13. doi: 10.1007/s13277-012-0575-0. [DOI] [PubMed] [Google Scholar]
- 16.Brinkmann O, Kastratovic DA, Dimitrijevic MV, et al. Oral squamous cell carcinoma detection by salivary biomarkers in a Serbian population. Oral Oncol. 2011;47:51–55. doi: 10.1016/j.oraloncology.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]