Abstract
The delivery of pharmaceutical aerosols to infants receiving mechanical ventilation is extremely challenging due to small diameter flow passages, low tidal volumes, and frequent exhalation of the aerosol. The use of small charged particles is proposed as a novel method to prevent deposition in ventilator components and foster deposition in the lower infant airways. The objective of this study was to compare the performance of multiple new devices for generating small charged particles that are expected to maximize respiratory drug delivery in ventilated infants. Criteria used to select a leading device included production of a charged aerosol with a mass median aerodynamic diameter (MMAD) ≤ approximately 1.8 μm; low device depositional loss of the aerosol (<20%); particle charge in the range of the Rayleigh limit/100; and high drug output with low performance variability. Proposed new devices were a wick electrospray (WES) system with accelerated cross-flow air; a condensational vapor (CV) system with a charged solution and strong field gradient; and a low flow - induction charger (LF-IC) designed to operate with a modified commercial mesh nebulizer. Based on infant ventilation conditions, flow rates through the devices were in a range of 2–5 L/min and the devices were assessed in terms of depositional drug loss and emitted drug mass; droplet size distribution (DSD) using a Mini-MOUDI; and DSD and net charge with a modified ELPI. Considering the WES, primary limitations were (i) low and variable aerosol production rates and (ii) high device depositional losses. The CV device produced a high quality aerosol with a MMAD of 0.14 μm and a drug delivery rate of 25 μg/min. However, the device was excluded because it failed to produce a charged aerosol. In contrast, the LF-IC produced a 1.6 μm aerosol with high net charge, low device depositional loss (<15% based on recovery), and low variability. In the ELPI size fraction bin nearest the MMAD, the LF-IC produced >100 elementary charges per particle, which was an order of magnitude increase compared to the case of zero charging voltage. In conclusion, the LF-IC was selected as a leading system that is expected to improve aerosol delivery efficiency in ventilated infants through the use of small charged particles.
Keywords: wick electrospray, condensational aerosol generator, induction charger, mesh nebulizer, respiratory drug delivery, infants, mechanical ventilation
Graphical Abstract
1. Introduction
Infants receiving mechanical ventilation can frequently benefit from pharmaceutical aerosols with the goal of improving lung function while minimizing systemic exposure to the medication and associated side effects (Fink 2004; Mazela and Polin 2011; Rubin and Fink 2001). However, the delivery of aerosolized medication to infants is a significant challenge with lung delivery efficiencies reported in the range of <1–14% (Dubus et al., 2005; Fink 2004; Fok et al., 1996; Mazela and Polin 2011; Sidler-Moix et al., 2013). Impediments to effective aerosol delivery include small diameter connective ventilator tubing, small tidal volumes, short inhalation periods (Fink 2004; Rubin and Fink 2001), and ventilation components that are designed for gas delivery and not aerosol administration (Longest et al., 2014a; Longest et al., 2014b). Low delivery efficiency wastes valuable medications and makes the administration of high dose drugs very challenging. For example, surfactant delivery with a mesh nebulizer was reported in one study to require a 3 hour period for the administration of a 72 mg dose (Finer et al., 2010). Furthermore, low delivery efficiency is typically associated with high variability in the delivered dose, which makes the use of medications with a narrow therapeutic window difficult. Both low delivery efficiency and high variability are implicated as reasons for the clinical failure of some aerosolized medications delivered to infants (Mazela and Polin 2011; Shah et al., 2007). Improved dose delivery is needed for ventilated infants in order to maximize the clinical benefit of existing medications and to help develop new inhaled therapies (Brion et al., 2006; Cole et al., 1999; Fink 2004; Rubin and Williams 2014; Shah et al., 2007).
Several recent studies have proposed strategies for improving pharmaceutical aerosol delivery to ventilated infants. The use of vibrating mesh nebulizers and synchronization of nebulization with inhalation was reported to deliver approximately 12–14% of the aerosolized dose in a macaque animal model of ventilated infants (Dubus et al., 2005). Mazela et al. (2014) describes a newly designed Y-connector, referred to as the VC connector (also known as Afectair), that separates nebulizer and ventilation gas bias flows thereby improving the lung delivery efficiency of a jet nebulizer when used with infants. Based on an in vitro model of ventilated premature infants, Mazela et al. (2014) reported delivery efficiency at the exit of a commercial Y-connector to be approximately 1% or less vs. a maximum of approximately 7% with the VC connector. In a previous study, Longest et al. (2014a) evaluated optimal aerosol delivery conditions for a newborn full-term infant receiving mechanical ventilation through an endotracheal tube (ETT) using in vitro, CFD, and whole-lung modeling techniques. With a mesh nebulizer, commercial Y-connector, and a 3 mm ETT, the predicted lung deposition fraction was found to range from 6.8–13.5%, which was similar to the in vivo animal model predictions of Dubus et al. (2005) with a lung deposition fraction range of 12.6–14%. Aerosol deposition in infant lungs was then optimized by Longest et al. (2014a) for mesh nebulized aerosols with the use of a new streamlined (SL) Y-connector, delivery of the aerosol during only a portion of inspiration, and selection of optimal droplet sizes. The study of Longest and Tian (2015) evaluated the delivery of a submicrometer excipient enhanced growth (EEG) aerosol through a port on top of a new streamlined Y-connector. With appropriate timing of delivery, a maximum tracheobronchial deposition fraction of 56% was achieved and a method of growing an initially small dry powder aerosol was demonstrated.
A new strategy to target the deposition of pharmaceutical aerosols within the lungs of ventilated infants is the use of small charged droplets generated from an initial solution or suspension liquid formulation. In this approach, the droplet size is sufficiently small to allow for effective penetration through the delivery system and upper infant airways without significant depositional drug loss, which likely requires an aerosol mass median aerodynamic diameter (MMAD) of approximately 1.8 μm or less (Longest et al., 2014a). As described by Longest et al. (2014a), exhalation of the aerosol dose is a significant problem with ventilated infants. To prevent the exhalation of large dose fractions, charge on the pharmaceutical aerosol will foster deposition within the lung airways and potentially allow for targeting the site of delivery. An optimal level of charge is required to minimize deposition in the delivery system or extrathoracic airways, where velocities are relatively high, and then maximize deposition in the tracheobronchial and/or alveolar airspace. The ability to tune the charge level is an important design factor of the delivery technology. While a number of studies in adults have considered the use of aerosol charge to target deposition in the lungs (Bailey 1997; Bailey et al., 1998; Balachandran et al., 1997; Chan et al., 1978), previous studies have not applied this approach to infants and have not attempted to use initially small particles to minimize extrathoracic or ventilation system deposition.
A number of devices are available for the production of charged micrometer and submicrometer aerosols. Corona charging is frequently used to provide high charge to existing aerosols (Hinds 1999); however, ozone from both positive and negative corona makes this method incompatible with respiratory drug delivery. Electrospray is a well known method to generate particles with high charge (Chen et al., 1995; de Juan and De la Mora 1997). However, output mass is typically low compared with the needs of pharmaceutical delivery unless advanced techniques such as the use of liquid sheets or multiple nozzles are applied (Lee et al., 2011; Park et al., 2011). Furthermore, it is not known if the high charges associated with electrospray aerosols will be detrimental to efficient lung delivery. The use of corona needles for electrospray discharge (Ijsebaert et al., 2001) is associated with ozone formation (Hinds 1999), making them impractical for use in inhalers. Vibrating orifice charged particle generators are limited by very low mass output (Azhdarzadeh et al., 2014a; Azhdarzadeh et al., 2014b; Reischl et al., 1977).
While not typically associated with charged aerosols, devices known to produce high doses of submicrometer aerosols are capillary aerosol generation (CAG) and vibrating mesh nebulizers coupled with evaporation-type devices. The CAG process pumps a solution containing dissolved drug through a heated capillary producing a vapor or vapor and droplet spray, which exits a nozzle at high pressure and velocity (Hindle 2004; Hindle et al., 2004; Shen et al., 2004). Condensation in the vicinity of the nozzle produces an aerosol with properties that depend on the evaporating/condensing vehicle and delivery rate. The CAG approach was later considered in a series of studies to better understand the underlying physics and optimize operation in an inhaler (Hindle and Longest 2013; Longest and Hindle 2009; Longest et al., 2007).
Vibrating mesh nebulizers typically produce aerosols with MMADs in the size range of 2–6 μm (Dhand 2002). Longest et al. (2012; 2013b) demonstrated the use of a new mixer-heater device to evaporate these aerosols and produce high concentrations of submicrometer pharmaceutical aerosols suitable for direct inhalation. Loss within the mixer-heater system was <10% and production of submicrometer aerosols required significant heating of the flow stream at the nebulizer’s commercially available output rate.
A recent advance of electrospray aerosols is the introduction of inexpensive and disposable spray elements to feed the liquid solution and produce a Taylor cone (Tepper and Kessick 2008; Tepper and Kessick 2009; Tepper et al., 2007). Electrosprays have been produced with polymer wicks, toothpicks, and shaped paper (Hu et al., 2011; Liu et al., 2010; Tepper and Kessick 2009; Yang et al., 2012). An advantage of this approach is the replacement of the expensive spray pump control unit with a self-feeding and disposable unit. Considering condensational aerosols, Ouyang (1995) has previously demonstrated the production of charged condensational aerosols with the addition of high voltage charging.
The objective of this study is to compare the performance of multiple new devices for generating small charged particles delivered at flows that are that are suitable to maximize respiratory drug delivery in ventilated infants. Evaluation criteria of the devices include:
Production of a charged aerosol with a MMAD ≤ approximately 1.8 μm;
Low device depositional loss of the aerosol (< 20%);
Net Particle charge in the range of the Rayleigh limit/100; and
High drug output (>10 μg) with low performance variability (<10% coefficient of variation for emitted dose).
Based on the study of Longest et al. (2014a), aerosols with MMADs ≤ 1.8 μm are expected to efficiently penetrate the infant ventilation system. Initial estimates, calculated from non-dimensional quantities set forth by Finlay (2001), indicate that aerosols with a charge in the range of the Raleigh limit/100 will not have significantly increased deposition in the large diameter ventilation system connectors and will have improved retention in the lungs due to the image attraction force and reduced velocities and airway diameters within the alveolar region. Furthermore, high drug output is required for the delivery of high dose medications like inhaled surfactants and antibiotics. Three new devices are developed and prototyped for in vitro aerosol characterization. Aerosol performance testing is evaluated in terms of the delivered dose (and reproducibility), aerosol droplet size distribution (DSD), and net charge per particle. Based on this analysis, the best performing device is selected for future study in terms of emitted dose from an infant ventilation system and infant lung delivery.
2. Methods
2.1. Overview of devices
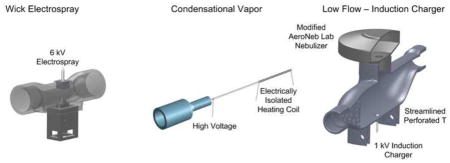
In this study, three new devices for the production of charged small (≤1.8 μm) pharmaceutical aerosols are developed and compared. Each device begins with an existing aerosol generation platform and includes significant modifications in order to attain the desired aerosol characteristics. Each of the devices is operated at air flow rates consistent with infant ventilation conditions (2–5 LPM) to deliver aerosol out of the device. The first device implements the paper wick electrospray platform (Fig. 1a), which has the advantages of low cost and no moving parts. However, low aerosol emission is expected due to the high charging field. Modifications include the addition of a narrow flow channel intended to accelerate the cross-flow air and remove the aerosol from the electrical field region prior to deposition on the charged electrode. The second device employs the CAG approach and is termed the condensational vapor (CV) device (Fig. 1b). In this method, a drug solution is pumped through a heated capillary to produce a vapor. Nearly complete vaporization of the solution is used to eliminate the need for a nozzle at the capillary tip, which may reduce the potential for device clogging. A high voltage charge is also applied to the capillary, which is electrically isolated from the heating element. The space inside the metal capillary represents a region of high field gradient with zero field strength at the center of the capillary in the evaporation zone. This high field gradient is expected to potentially charge the evaporating liquid filaments and droplets. It was not known if this potential charge would influence the evaporation or condensation processes and potentially produce a charged aerosol, as with the condensational vapor strategy of Ouyang et al. (1995). Modifying the size of a recovered condensational aerosol or producing a charged aerosol by charging the base solution was seen as a potentially advantageous development. Finally, a commercial mesh nebulizer (Aeroneb Lab; Aerogen, Galway, Ireland) was employed as a convenient aerosol source in the low flow - induction charger (LF-IC; Fig. 1c). Modifications included altering the vibration amplitude to reduce liquid output, thereby enabling evaporation to near submicrometer size at the low flow rates required for infant ventilation, and including an electrode positioned below the mesh to charge the aerosol by induction charging. Other studies have used induction charging with single orifice plates (Azhdarzadeh et al., 2014a; Azhdarzadeh et al., 2014b), but the application of induction charging to a vibrating mesh system with low airflow is a new advancement. Further details in device development and experimental testing methods for these new systems are described in the following sections.
Figure 1.
Three new pharmaceutical aerosol generators developed and tested in this study including the (a) wick electrospray (WES), (b) condensational vapor (CV), and (c) low flow - induction charger (LF-IC) devices. These devices are based on existing platforms with important new modifications made in this study in order to produce appropriately charged small (≤1.8 μm) aerosols with the goal of improving the delivery of aerosolized medications to ventilated infants.
2.2. Overview of test systems
A schematic of the three experimental setups that were employed to generate albuterol sulfate (Spectrum Chemicals, New Brunswick, NJ; model drug commonly used as a bronchodilator) aerosols is provided in Figure 2. To quantify performance, options considered for each system were assessing aerosol depositional drug loss in the device, collection of the emitted drug mass on an aerosol filter, determination of aerosol droplet size distribution (DSD) with the Mini-MOUDI (MSP Corp., Shoreview, MN) at a flow of 2 LPM, and determination of net charge on separate aerosol size fraction bins and DSD with a modified electrical low pressure impactor (ELPI; Dekati, Finland) at a flow rate of 30 LPM. Separate flow rates were required by the Mini-MOUDI and ELPI based on internal calibrations by the respective manufacturers. Sampling at 2 LPM provides for an accurate view of the aerosol DSD emitted from the device because makeup air is not required. In contrast, sampling at 30 LPM will result in some evaporation of aerosol droplets; however, the ELPI device allows for quantification of the net aerosol charge within 12 different aerosol size fraction bins. In a bipolar aerosol, the ELPI measures the net charge of the combined positive and negative charges for each stage bin. Selection of an appropriate series of tests depended in part on operation of the individual devices.
Figure 2.
Schematic diagrams of the three systems including the experimental setup and evaluation components. Increasing complexity can be observed with progression from (a) WES, (b) CV, and (c) LF-IC. It is noted that the filter setup is used for assessing mass output and then replaced with the impactor for determining droplet size distribution (Mini-MOUDI) or net electrostatic charge (ELPI) where warranted.
Both the WES and LF-IC contain internal flow passages such that depositional drug loss was evaluated. In these experiments, the device was operated for a specified amount of time and then disassembled. Device components were washed in distilled deionized water. Drug mass recovered was quantified using a previously validated High Performance Liquid Chromatography (HPLC) method for albuterol sulfate (AS). Device deposition was not assessed for the CV system (only delivered dose was determined) because an enclosure housing was not constructed. This was because, as described later, this device was not selected for future study and an enclosure system was not required.
Delivered dose testing was conducted by operating the device at its specified flow rate and collecting the delivered dose on a high-efficiency respiratory filter (Pulmoguard II; Quest Medical, Brockton, MA). An airflow of 10 LPM was maintained through the filter using a vacuum pump. HPLC analysis was again used to determine the drug mass delivered to the filter. If the filter drug mass was below a typical infant drug delivery mass (10 μg for albuterol sulfate) or had a coefficient of variation greater than 10%, then the device was considered not to be a viable option and testing with additional methods was not pursued.
Impactor measurements consisted of employing either the Mini-MOUDI or modified ELPI at the manufacturer specified flow rates of 2 or 30 LPM, respectively. The aerosol generation devices operated in a flow rate range of 2–5 LPM. During sampling of the aerosol, room-condition makeup air was used to satisfy the various flow rate requirements of the generation devices and impactors. The Mini-MOUDI was implemented as received from the manufacturer and drug mass on each stage was determined by washing with deionized water before HPLC analysis quantified AS recovery. The ELPI was operated in a modified mode as described by Kotian et al. (2009) in order to determine both aerosol size and net charge. In this approach, the corona charger of the ELPI was removed. Drug mass deposition on individual stages was determined by HPLC analysis and used to determine the DSD at 30 LPM. The electrical reading of each stage was used to determine the net aerosol charge within each respective size fraction bin. Further details of these testing methods describing how they were applied to each aerosol generation system are provided in the following sections.
Table 1 provides a comparison of device parameters selected for the performance characterization experiments. Considering the number of possible variables with three charged aerosol generation systems, device optimization can be a difficult task. To address this issue, preliminary screening experiments were conducted to guide parameter selection and maximize device performance. As a result, the devices selected for comparison and the parameters defined in Table 1 represent the end product of a large amount of preliminary optimization work. For example, the minimum stable operating voltage for an electrospray was found to be 6 kV with the WES system. In contrast, the LF-IC system was found to effectively emit charged aerosols with a charging voltage of 1 kV. The CV system was limited to 1 kV applied voltage due to the dielectric material coating the heating wire breaking down at higher voltages. Aerosolization rates in Table 1 report liquid volume emitted from each system determined by weighing the devices before and after use. Due to the low aerosolization rate of the WES (μL/hour), compared with the CV and LF-IC (mL/hour), the drug concentration in the WES device was increased to 5%w/v, which resulted in a highly conductive solution and enhanced electrospray performance. In contrast, producing small aerosols with CV and LF-IC require using <1% w/v solution concentration. WES was enhanced with a surfactant (0.02% w/v poloxamer 188 (Leutrol F68) donated from BASF Corporation, Florham Park, NJ) to reduce surface tension of the solution, whereas NaCl (Sigma Chemical Company, St. Louis, MO) was used as a charging excipient in the CV and LF-IC devices. Finally, typical inhalation waveforms used with full term neonates have a mean tracheal flow rate of approximately 2–5 LPM (Walsh and DiBlasi 2010). The lower end of this range was found to generate a sufficiently small aerosol with the WES and CV systems. In contrast, a combination of 5 LPM flow and modification of the nebulizer output signal were important to produce a near submicrometer aerosol with the LF-IC device, as described further in Section 2.5.
Table 1.
Experimental parameters of the three aerosol generation systems. A voltage of 6kV was required to generate an aerosol with the WES system, but could be reduced to 1kV with the CV and LF-IC systems. Concentration of albuterol sulfate (AS) was maximized in WES to address the expected low aerosolization rate. Excipients were selected to maximize aerosolization and charging performance of each system. The range of supply air flowing through the devices and to the infant for effective ventilation was 2–5 LPM.
| Parameters | WES | CV | LF-IC |
|---|---|---|---|
| Charging Voltage | 6 kV | 1 kV | 1 kV |
| Aerosolization Rate | 0.54 μL/hour* | 0.6 mL/hour | 2 mL/hour |
| Formulation | 5% w/v AS | 0.25% w/v AS | 0.25% w/v AS |
| Excipients | 0.02% w/v Poloxamer 188 | 0.25% w/v NaCl | 0.25% w/v NaCl |
| Supply Air | 2 LPM | 2 LPM | 5 LPM |
For the specific wick and solution combination
2.3. Wick electrospray (WES)
The WES device was composed of a streamlined airspace that connects a grounded electrode and a liquid reservoir containing a triangular tipped paper wick submerged in the drug solution at a high voltage, as seen in Figure 1a. In the device, the grounded electrode is positioned above the paper wick. The paper wick is made of Whatman Grade 3MM Cellulose Chromatography Paper cut to a width of 5 mm, a length of 15 mm and a 60 degree angle tip was formed to emit the electrospray. Air flows over the top of the wick, perpendicular to the direction of the electrical field and aerosol spray. The intent of this design was to create a highly charged aerosol via electrospray and then remove the aerosol from the electrostatic field using a crossing airflow before the droplets deposit on the grounded electrode. The distance between the paper wick tip and the grounded electrode was a constant 3 mm. Filtered air was connected to the inlet of the WES shell and regulated to flow at 2 LPM as confirmed upstream by an inline flow meter.
The formulation drug solution used was 5 %w/v AS and 0.2 % w/v Poloxamer 188 in distilled water as shown in Table 1. Poloxamer 188 is a surfactant that was added to reduce the surface tension of the solution and improve spraying. The solution was sonicated for at least thirty minutes to ensure complete dissolution. The solution was then pipetted into the WES reservoir to the maximum fill line of 400 microliters after the wick was inserted vertically with the sharp tip oriented toward the grounded electrode.
A high voltage power supply (CZE1000R; Spellman High Voltage Electronics Corporation, Hauppauge, NY, USA) operating in negative mode was connected to the wick reservoir, while the ground of the power supply was connected to the electrode. The high voltage power supply can be limited by either current or voltage. To operate in current limiting mode, the voltage limit was set to a value higher than the measured voltage. The current limit was set to 3 microamperes and slowly decreased to 0.6 microamperes to maintain the desired operating current with an appropriate voltage. An electrical resistor was used in series with the grounded electrode to determine the functionality of the device and the mode of electrospray achieved. Voltage across this resistor was recorded once per second for each experiment using a digital multimeter (Fluke Corporation, Everett, WA) with data-logging software. The voltage across the resistor was observed during the run to determine the electrospray current and operating mode of the system. After the one hour run time was completed, the high voltage power supply was discharged by turning off the power supply and then connecting the high voltage lead to ground.
For assessing device drug deposition and drug delivery, a 30 cm length of 10 mm (diameter) ventilator tubing was connected to the WES device. The aerosol generated at a flow rate of 2 LPM was collected on a Pulmoguard II filter that was positioned at the outlet of the tubing. An airflow of 10 LPM was maintained through the filter using a vacuum pump. The increased filter flow rate was used to ensure complete entrainment and capture of the aerosol exiting the tubing.
Performance of the electrospray system was assessed by quantifying the drug deposition in each relevant component of Figure 2a. To determine drug deposition in the WES system, the counter electrode, tubing and filter were all washed in deionized water and the AS concentrations of the resulting solutions determined by a validated HPLC method. Recovered drug mass was determined by multiplication of AS concentration by the dilution volume. In order to determine the percentage AS recovery, known volumes of the formulation solution were also diluted to accurately quantify the concentration of the formulation.
2.4. Condensational vapor (CV)
The CV device was expected to function similar to CAG with an aqueous drug solution formulation (Longest et al., 2007) but with differences of (i) increased energy per volume of solution to achieve near full vaporization, (ii) use of a straight tip capillary, and (iii) charging of the solution prior to vaporization and condensation. Increased energy input was intended to fully vaporize the solution and form a more consistent nano-scale condensational aerosol. Drug molecules are expected to be contained in hydrated ion clusters or un-evaporated nano-droplets. By providing sufficient energy to vaporize the solution, the capillary nozzle can be eliminated, which was a source of significant spray momentum (Longest et al., 2008) and potentially system clogging and malfunction. Charging the solution is expected to create a charged aerosol or charged evaporated droplets during the vaporization process through conduction charging (Zhao et al., 2005). In the vicinity of vaporization, high charge is expected on the walls of the capillary and fluid phase with zero charge in the vaporized core. The gradient between the charged and unchanged regions during liquid boiling-breakup was expected to create charged discrete droplet elements. The evaporation of these charged droplets could then produce charged ions. Both the presence of charge and generated ions could influence the evaporation and condensation processes altering the final aerosol size and/or producing charged condensational droplets.
Control of the capillary temperature was adjusted and monitored using electrically insulated heating wire and a thermocouple. 40 gauge (0.0031 inch) NiCr heating wire with DuPont’s heavy polymide 240C enamel insulation was obtained from WireTronic Inc. (Volcano, CA). The wire was wrapped around the capillary approximately 35 times to form the heating element, as shown in Figure 1b. The resistance of this heating wire was measured before and after each experiment and replaced when it was not between 10 – 20 ohms. A thermocouple temperature probe was placed on the capillary approximately 1 cm away from the tip to measure the capillary wall temperature. A DC power supply (HY3005F-3, Mastech, San Jose, CA) provided approximately 1.5 Watts to the 40 gauge heating wire to obtain heating that varied the capillary temperature (1 cm from the tip) between 100 and 160 °C. Control experiments were also performed in the absence of charging to determine the effect on both DSD and net particle charge.
The formulation drug solution used in the CV system was 0.25 % w/v AS and 0.25 %w/v NaCl in distilled water as shown in Table 1. The solution was sonicated for at least thirty minutes to ensure complete dissolution. 0.7 mL of solution was loaded into a 1 mL syringe and connected to the heated capillary. A syringe pump (NE – 300, New Era Pump Systems Inc. Farmingdale, NY) was used to pump the solution to the heated capillary at a constant flow rate of 0.6 mL/hour. The loaded syringe was inverted, tapped, and depressed to ensure there were no air bubbles in the syringe before each experiment.
The DSD was determined for the CV device using the Mini-MOUDI impactor for a 20 minute run. A vacuum pump provided the pressure drop needed to sample 2 LPM through the Mini-MOUDI impactor (Fig 2b). A flow meter was attached to the inlet of the impactor prior to aerosol generation to verify 2 LPM through the Mini-MOUDI and removed before generating an aerosol. Potential losses were minimized by spraying directly from the heated capillary to the impactor inlet to avoid deposition in connecting tubing.
The DSD and net particle charge were determined simultaneously using the ELPI at 30 LPM for a 20 minute sample time. The aerosol was sprayed directly into a streamlined connector (Figure 3) attached to the inlet of the ELPI. A downstream vacuum pump was used to draw 30 LPM of room-condition air containing the aerosol through the impactor. Drug aerosol deposition was recovered by washing the inlet and each stage of the impactor and quantified using a validated HPLC technique.
Figure 3.
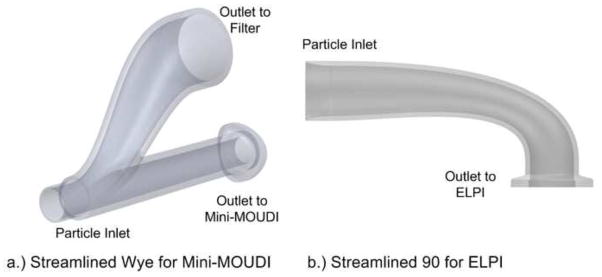
Custom streamlined impactor connectors that were fabricated in order to sample the charged aerosols with the (a) Mini-MOUDI or (b) ELPI instruments and provide minimal depositional loss.
With the ELPI, electrometer current data was collected for each of the 12 stages and recorded to a .CSV file using Dekati software. Using the midpoint method, this data was then numerically integrated with respect to time to give the net charge collected per stage. The HPLC-determined drug mass per stage was used with the midpoint diameter between the corresponding stages’ cut sizes to determine the number of particles depositing on each stage. The net stage charge was then divided by the total number of particles per stage to determine the average number of charges per particle.
2.5. Low flow-induction charger (LF-IC)
The LF-IC was based on the commercially available Aeroneb Lab vibrating mesh nebulizer. The nebulizer was positioned in the streamlined perforated T-connector (SLpT) design previously developed by Longest et al. (2013a) as shown in Figure 1c. This connector was shown to reduce depositional loss by a factor of 4.5x compared with the commercial design of the T-connector. Two modifications of this system include adjusting the driving signal of the mesh nebulizer and the addition of a charged electrode. As described below, the driving signal of the mesh nebulizer was adjusted to reduce the mass output by a factor of 10x in order to allow for evaporation of the aerosol. The desired target was evaporation to a 1.8 μm MMAD with 5 LPM of gas flow. The charged electrode was positioned below the mesh on the opposite side of the SLpT (distance of 47.6 mm) to produce a charged aerosol by induction charging at an applied voltage of 1 kV. Induction charging with a vibrating orifice generator was previously described by Reischl et al. (1977) and implemented by Azhdarzadeh et al. (2014a; 2014b). Our group (Golshahi et al., 2015) has recently developed an induction charger with a mesh nebulizer that operates at high flow rates (30–90 LPM). This study is the first to report the functioning of a low flow (5LPM) induction charger system with a mesh nebulizer. Use of the mesh provides a substantial increase in output compared with vibrating single orifice devices, such that pharmaceutically relevant doses of medication can be delivered.
One tenth of the aerosol output by mass with the Aeroneb Lab nebulizer was achieved by reducing the amplitude of the applied sinusoidal driving voltage while maintaining the commercial frequency of 128.2 kHz. A Rigol DG10220 function waveform generator (Beaverton, OR) produced the desired signal and was amplified using a TS200 modulated power supply from Accel Instruments (Irvine, CA). This signal was continuously monitored using a Tektronix TDS 210 oscilloscope (Beaverton, OR). This reduced output should enable adequate drying at 5 LPM as opposed to the study of Golshahi et al. (2015), where a flow rate of 90 LPM was required to dry the aerosol. 5 LPM of filtered air was directed through the LF-IC, drying and entraining the charged aerosol before it was exposed to atmospheric pressure and directed to a collection filter or an impactor. Control experiments were also performed in the absence of charging using 0 kV to determine the effect on both DSD and net particle charge.
Dose variability and device deposition were determined directly from the impactor studies. In all cases, the device was operated for 5 minutes. To determine device deposition, the system was disassembled after the aerosol generation time period and components were washed with deionized water. As with the previous systems, drug mass was determined with a validated HPLC method.
The nominal dose of each run was calculated by determining the mass of formulation nebulized. After each run, the inner wall of the nebulizer was wiped before being weighed and subtracted from the initial mass of the nebulizer. A solution density of 1 g/cm3 was assumed and the difference between the measured masses was multiplied by the formulation AS concentration to calculate the nominal mass of AS that was aerosolized.
To determine the DSD from the LF-IC, 2 LPM of the 5 LPM airstream from the LF-IC was sampled into the Mini-MOUDI. A streamlined connector used for sampling the 5 LPM flow stream and providing 2 LPM to the impactor is displayed in Figure 3a. This connector interfaces with the SLpT, the Mini-MOUDI, and a Pulmoguard II filter that was used to capture excess flow. Mass of drug on the connector, inlet, and each stage of the impactor was determined using washings and HPLC analysis.
To calculate charge per particle, the ELPI was again employed as with the CV device. The 5 LPM airstream from the LF-IC was directed into the streamlined connector added to the ELPI (Figure 3b), which also pulled in 25 LPM of makeup room-condition air based on operation of a downstream vacuum pump. Current measurement data was recorded using the ELPI’s electrometers and software for 30 seconds prior to aerosol generation. The nebulizer and the induction charger were turned on simultaneously. After the run the current data was recorded for 30 seconds beyond when the generator was turned off. For each experiment, the generator and charger were both operated for 5 minutes. The collected current data was processed as with the CV device. Only the current that was collected during the generation/charging time was included in the calculation of charge per particle.
Droplet particle sizing with the ELPI was expected to report smaller droplet size compared with the Mini-MOUDI due to particle evaporation at a higher airflow rate. However, evaporation is not expected to alter the charging profile. It is assumed that droplets do not evaporate beyond a critical Rayleigh limit, which would cause breakup due to electrostatic effects. Measured net charge on the droplets can then be used to approximate charge on the aerosol exiting the LF-IC device at 5 LPM.
3. Results
3.1. Wick electrospray
The WES emitted mass results captured on the filter showed high variability and high device losses within a group of four replicate experiments. As shown in Table 2, the WES device aerosolized a mean of about 27 μg of drug in the 1 hour period, of which about 12 μg was deposited on the device and this was associated with high variability (coefficient of variation = 106%). Device deposition ranged from approximately 1 to 25 μg of drug. Similar variability was observed for the emitted dose (109%) with the mean emitted dose of 15 ug in 1 hr. This variability was too great considering that the system needs to provide safe, reproducible, and reliable drug delivery. Also, the mean delivery rate of 15 μg per hour to the filter was a hindrance to further development because it is lower than typical electrospray systems with micropump controllers and the system was observed to not be stable over the long delivery times that would be necessary for pharmaceutical use. The measured current provided by the electrospray was observed to decrease over time, which indicated a change in spray mode. The paper wick was also observed to be physically damaged after approximately 20 minutes of spraying. This was likely because of high temperatures associated with corona effects at the sharp wick tip as well as spraying fragments of paper. As a result of these observations, the WES approach was determined to not be effective for respiratory drug delivery to ventilated infants and was not considered further.
Table 2.
Summary of the AS aerosol delivery characteristics for the WES device (n = 4) with 1 hour of operation. WES Device and Emitted Dose represent the mass of drug in micrograms remaining in the device flow pathway and emitted from the device, respectively. For the WES, AS device loss was greater than the emitted dose. Moreover, high coefficients of variation in both WES device deposition and emitted dose indicate highly variable performance.
| Mean AS mass (μg) | Coefficient of Variation (%) | Range (μg) | |
|---|---|---|---|
| WES Device | 11.80 | 106.0 | 0.90 – 25.2 |
| Emitted Dose | 15.05 | 109.1 | 0.71 – 29.9 |
| Total Recovered Dose | 26.85 | 107.4 | 1.87 – 55.1 |
3.2. Charged vaporization
Unlike the WES device, in which the delivered dose was determined by the wick characteristics, the delivered dose from the pump controlled CV device was determined and controlled by the pump flow rate. Given these fixed delivery conditions, the emitted dose of the CV device was determined using the impactor testing rather than the filter capture apparatus. With the Mini-MOUDI system, the mean (standard deviation; SD) drug mass emitted from the device and deposited in the impactor (including the inlet) was 257.6 (24.6) μg over a period of 20 minutes. The expected dose based on the pumping rate and solution concentration was 500 μg, which indicates that some drug mass remained in the capillary or escaped from the impactor inlet. Variability was lower than observed with the WES device, with a coefficient of variation of 9.5%, which was expected with this pump based system. The mean (error bars are SD) DSD of the CV aerosol measured with the Mini-MOUDI is illustrated in Fig. 4 with a mean (SD) MMAD of 0.14 (0.015) μm. Based on measurements in the ELPI, the aerosol had a negligible amount of net charge. For example, experiments were performed with and without the high voltage charge being applied. There was only a small difference observed in the charged and uncharged elementary charge units on Stage 3 (cut size midpoint = 0.13 μm), with values of 0.21 e and 0.5 e, respectively. Furthermore, both the aerosols generated with and without the application of high voltage had an MMAD of approximately 0.14 μm. As a result, it was observed that charging the solution had no effect on formation of the condensation aerosol. Due to the generation of relatively unchanged particles that would easily be exhaled due to their small size, the CV device was not selected for further evaluation.
Figure 4.
Droplet size distribution measured with the Mini-MOUDI for the CV device at 2 LPM and 1 kV charging voltage (MMAD = 0.14 ± 0.015 μm; n = 3).
3.3. Low flow - induction charger
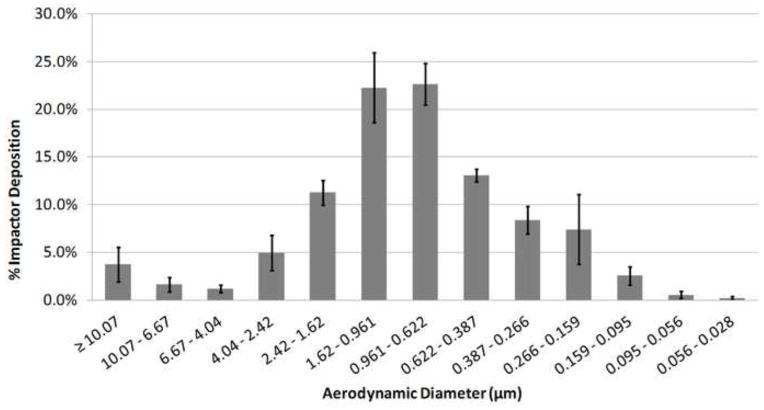
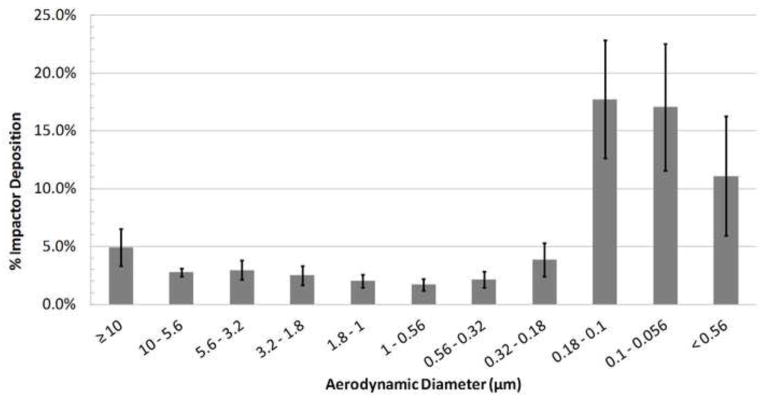
For the LF-IC operated with 1 kV for 5 minutes, aerosol characterization with the Mini-MOUDI resulted in a mean (SD) emitted dose of 913.8 (45.1) μg. Considering the run time of 5 minutes, the LF-IC could potentially deliver 182.8 μg/min of charged AS particles to a patient with low emitted dose variability with a coefficient of variation of 4.9%. Based on a nominal dose of 1065.8 (38.4) μg, the mean (SD) depositional loss in the nebulizer and device was 14.3 (1.7)%. The overall recovery of drug was 96.9 (2.7)% of the nominal dose indicating good mass balance in this study. The mean DSD for the LF-IC is displayed in Figure 5 based on the Mini-MOUDI measurements and results in a mean (SD) MMAD of 1.57 (0.1) μm. This result demonstrates that an appropriate amount of evaporation was achieved at the airflow rate of 5 LPM with the modified nebulizer signal. Using the ELPI, the measured mean DSD is displayed in Figure 6. The measured MMAD was 0.88 (0.08) μm, indicating that the aerosol was composed of droplets at a flow rate of 5 LPM and that further size reduction of the aerosol was possible when the aerosol was exposed to the higher flow rate employed with the EPLI.
Figure 5.
Droplet size distribution measured with the Mini-MOUDI for the LF-IC device at 2 LPM and 1 kV charging voltage (MMAD = 1.57 ± 0.10 μm; n = 3).
Figure 6.
Droplet size distribution measured with the ELPI for the LF-IC device at 30 LPM and 1 kV charging voltage (MMAD = 0.882 ± 0.078 μm; n = 3).
For particles with a mean size of 0.79 μm (midpoint size of Stage 7 which is nearest to the MMAD of the aerosol when sampled at 30 LPM), the uncharged LF-IC (0 kV) was shown to provide an average of 13.17 elementary charges for every particle collected based on ELPI measurements. This small amount of charging is expected within a mesh nebulizer due to the difference between positive and negative ion mobility within the solution. Distilled water is expected to have a net negative charge of a similar magnitude for this reason (Finlay 2001). Increasing the induction voltage to 1 kV increased the charge by a factor of 12.9x, resulting in 169.94 elementary charges per particle. The charge per particle vs. charging voltage results are summarized in Table 3 along with results from other stages in the ELPI. It is observed on stages with a majority of aerosol drug mass deposition (Stages 5–10), charging at 1 kV produces conditions similar to the Rayleigh limit/100, which can be considered a high level of charge that will have an effect on lung deposition. Net charges on other stages in Table 3 were excluded because deposition on these stages was below the limit of detection for the HPLC assay, and it is estimated that this mass represents < 3% of the drug deposited in the impactor.
Table 3.
Elementary charges (e) per particle produced by the LF-IC device and measured in the ELPI. Drug depositions on other stages (not shown) were below the HPLC assay limit of detection. 1 kV charging was observed to increase the net charges per particle for all stages (sizes) beyond Stage 4. At the aerosol MMAD (~0.79 μm in the ELPI), 1kV charging increases e by a factor of 12.9x. Furthermore, 1kV charging produces values consistent with Rayleigh limit charging/100, which is viewed as advantageous for enhanced lung deposition.
| ELPI | Midpoint Diameter (μm) | eRayleigh/100 | 1 kV(e) | 0 kV(e) |
|---|---|---|---|---|
| Stage 4 | 0.21 | 43.55 | 2.13 | 14.82 |
| Stage 5 | 0.33 | 83.43 | 25.91 | 0.94 |
| Stage 6 | 0.50 | 159.88 | 79.88 | 12.93 |
| Stage 7 | 0.79 | 313.59 | 169.94 | 13.17 |
| Stage 8 | 1.29 | 653.72 | 513.83 | 34.61 |
| Stage 9 | 2.02 | 1280.96 | 1590.35 | 126.82 |
| Stage 10 | 3.23 | 2590.08 | 9646.40 | 1822.96 |
Discussion
This study has developed and compared three new systems intended to produce charged small aerosols with the ultimate goal of integrating a lead device into an infant ventilation system and significantly improving lung delivery efficiency. The developed devices were compared in terms of emitted dose, aerosolization characteristics, and net aerosol charge. Selection criteria for the best performing device were described in the introduction. Based on these evaluations, strengths and weaknesses of each device have emerged leading to the selection of a system for further testing.
Considering the WES device, primary limitations were (i) low and variable aerosol delivery rates and (ii) high device depositional losses. This study considered the new approach of inexpensive spray elements to replace the micropump controller. It was anticipated that known low aerosol production rates with electrospray could be counteracted by longer spray times or continuous delivery. Furthermore, controlled slow delivery of the inhaled medication may be advantageous for immature infant lungs. Unfortunately, the paper spray elements quickly broke down and had highly variable aerosol production. More robust elements like polymer wicks (Tepper and Kessick 2008; Tepper and Kessick 2009; Tepper et al., 2007) may spray for a longer time, but high variability is still expected. Electrospray delivery could be improved through the use of a traditional micropump controlled system. However, the second major limitation of this approach is the very strong electrical field compared with the available air flow rate to remove the particles at 2–5 LPM. Spraying required an initializing voltage of 6 kV for a stable ion current. It is known that the typical charge on electrospray droplets is very near the Rayleigh limit (Ganan-Calvo et al., 1997). Insufficient airflow is available to reduce deposition of this aerosol on the charged electrode without deionization. As described, use of a corona needle is not acceptable for inhalation systems. Fu et al. (2011) described the use of radioactive spot ionizers in an electrospray system. However, insufficient space is available in the system to include these units. Increasing the device volume is not practical due to the limited tidal volume used in infant ventilation. Based on these observations, it now appears that the WES system or electrospray in general may not be effective at improving lung delivery efficiency in a ventilated infant system.
Considering the CV device, a high quality submicrometer and almost nanometer scale aerosol was formed with low variability. Heating of the capillary effectively produced a condensation aerosol with some residue of drug remaining on the capillary walls. By increasing the energy input per solution volume compared with previous CAG systems, the need for a capillary nozzle was removed and a submicrometer aerosol was formed with an aqueous solution. The thermocouple near the tip of the capillary was used to ensure temperature in the range of 100–160 °C, which may be damaging to some therapeutic molecules. However, previous analysis has indicated that a number of common respiratory medications have low thermal degradation when aerosolized using the CAG device (Hindle et al., 2004). The primary disadvantage of CV was the absence of charge on the condensed aerosol. Previous studies have demonstrated conduction charging with sprays (Zhao et al., 2005) and effects of ions on condensational aerosol formation (Ouyang et al., 1995). However, the charging approach that was selected in the CV study failed to produce a charged aerosol or alter the final aerosol size. This is likely because the system failed to provide an effective field gradient in the region of phase change or the direction of droplet separation. Furthermore, 100 nm particles lack sufficient Brownian motion (Xi and Longest 2008) to foster deposition over the short infant inhalation times. Primarily due to the failure to produce a charged aerosol, the CV device was not selected for further consideration.
The previous study of Golshahi et al. (2015) demonstrated that induction charging coupled with a mesh nebulizer was effective at high flow rates. In this study it was determined that (i) the mesh nebulizer output rate could be reduced to allow for evaporation and formation of a 1.6 μm aerosol and (ii) high delivery efficiency could be achieved at a flow rate of 5 LPM through the device with a charging voltage of 1 kV. Device depositional losses were <15% based on recovered dose, which includes losses on the nebulizer body, SLpT, and charger counter electrode. Perhaps as important, the variability associated with the delivered aerosol dose was low, with a coefficient of variation of only 4.9%. The previous study of Longest et al. indicated that a 1.8 μm aerosol had only minor losses in a 3 mm endotracheal tube at a flow rate of 5 LPM; however, particle charge effects were not considered in that study. Considering the analysis of Finlay (2001) on electrostatic effects in the lungs, 100 elementary charge units on a 1 μm particle is expected to have a significant impact on deposition. The LF-IC produced >100e with a charging voltage of 1 kV, whereas in the absence of the charging voltage, spray charging from the nebulizer produced only ~10e.
While the LF-IC produces the targeted aerosol size and charge, integration into an infant ventilation system will require addressing several additional challenges. Infant ventilation requires very small tidal volumes. As a result, dead volume in the system may need to be reduced together with connection of the SLpT very near the Y-connector. Inspiration only occurs over approximately 0.5 s with a 1 or 2 s exhalation. Therefore, synchronization of aerosol generation with inspiration will also be necessary. Safety of the system can be ensured by covering the charged counter electrode with a plastic film. Common dielectric materials block electrical conduction but permit the passage of electrical fields.
In conclusion, the LF-IC system was found to produce a targeted aerosol size of approximately 1.6 μm with low device loss, low variability in emitted dose and what is expected to be the correct net particle charge to enhance lung deposition and reduce exhalation of the dose. In contrast, the WES system had both poor emitted dose and high variability. The CV device provided a high quality submicrometer aerosol but negligible net particle charge. Future studies are needed to integrate the LF-IC device into the infant ventilation system, evaluate aerosol transmission through the endotracheal tube and assess lung deposition and delivery. Selection and development of the LF-IC is a first step toward using small charged aerosols to enhance the delivery of inhaled pharmaceuticals to infants in future studies.
Highlights.
Small charged particles are proposed to improve pharmaceutical aerosol delivery for ventilated infants.
Three new devices are developed to generate an aerosol which is appropriately sized and charged with low device losses.
Droplet size distribution and net number of charges per particle are quantified using a Mini-MOUDI impactor at 2 LPM and an electrical low pressure impactor at 30 LPM.
The low flow – induction charger device is found to be superior to wick electrospray and condensational vapor devices.
Acknowledgments
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R21HD073728.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azhdarzadeh M, Olfert JS, Vehring R, Finlay WH. Effect of electrostatic charge on oral-extrathoracic deposition for uniformly charged monodisperse aerosols. Journal of Aerosol Science. 2014a;68:38–45. doi: 10.1089/jamp.2013.1118. [DOI] [PubMed] [Google Scholar]
- Azhdarzadeh M, Olfert JS, Vehring R, Finlay WH. Effect of induced charge on deposition of uniformly charged particles in a pediatric oral-extrathoracic airway. Aerosol Science and Technology. 2014b;48(5):508–514. [Google Scholar]
- Bailey AG. The inhalation and deposition of charged particles within the human lung. Journal of Electrostatics. 1997;42:25–32. [Google Scholar]
- Bailey AG, Hashish AH, Williams TJ. Drug delivery by inhalation of charged particles. Journal of Electrostatics. 1998;44:3–10. [Google Scholar]
- Balachandran W, Machowski W, Gaura E, Hudon C. Control of drug aerosol in human airways using electrostatic forces. Journal of Electrostatics. 1997;40&41:579–584. [Google Scholar]
- Brion LP, Primhak RA, Yong W. Aerosolized diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database Of Systematic Reviews. 2006;(3) doi: 10.1002/14651858.CD001694.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TL, Lippmann M, Cohen V, Schlesinger RB. Effect of electrostatic charges on particle deposition in a hollow cast of the human larynx-tracheobronchial tree. Journal of Aerosol Science. 1978;9:463–468. [Google Scholar]
- Chen DR, Pui DYH, Kaufman SL. Electrospraying of conducting liquids for monodisperse aerosol generation in the 4 nm to 1.8 um diameter range. Journal of Aerosol Science. 1995;26(6):963–977. [Google Scholar]
- Cole CH, Colton T, Shah BL, Abbasi S, MacKinnon BL, Demissie S, Frantz ID. Early inhaled glucocorticoid therapy to prevent bronchopulmonary dysplasia. New England Journal Of Medicine. 1999;340(13):1005–1010. doi: 10.1056/NEJM199904013401304. [DOI] [PubMed] [Google Scholar]
- de Juan L, De la Mora JF. Charge and size distribution of electrospray drops. Journal of colloid and interface science. 1997;186:280–293. doi: 10.1006/jcis.1996.4654. [DOI] [PubMed] [Google Scholar]
- Dhand R. Nebulizers that use a vibrating mesh or plate with multiple apertures to generate aerosol. Respiratory Care. 2002;47(12):1406–1416. [PubMed] [Google Scholar]
- Dubus JC, Vecellio L, De Monte M, Fink JB, Grimbert D, Montharu J, Valat C, Behan N, Diot P. Aerosol deposition in neonatal ventilation. Pediatric Research. 2005;58(1):10–14. doi: 10.1203/01.PDR.0000156244.84422.55. [DOI] [PubMed] [Google Scholar]
- Finer NN, Merritt TA, Bernstein G, Job L, Mazela J, Segal R. An open label, pilot study of Aerosurf combined with nCPAP to prevent RDS in preterm neonates. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2010;23(5):303–309. doi: 10.1089/jamp.2009.0758. [DOI] [PubMed] [Google Scholar]
- Fink JB. Aerosol delivery to ventilated infant and pediatric patients. Respiratory Care. 2004;49(6):653–665. [PubMed] [Google Scholar]
- Finlay WH. The Mechanics of Inhaled Pharmaceutical Aerosols. Academic Press; San Diego: 2001. [Google Scholar]
- Fok TF, Monkman S, Dolovich M, Gray S, Coates G, Paes B, Rashid F, Newhouse M, Kirpalani H. Efficiency of aerosol medication delivery from a metered dose inhaler versus jet nebulizer in infants with bronchopulmonary dysplasia. Pediatric Pulmonology. 1996;21(5):301–309. doi: 10.1002/(SICI)1099-0496(199605)21:5<301::AID-PPUL5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Fu HJ, Patel AC, Holtzman MJ, Chen DR. A new electrospray aerosol generator with high particle transmission efficiency. Aerosol Science And Technology. 2011;45(10):1176–1183. doi: 10.1080/02786826.2011.582899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganan-Calvo AM, Davila J, Barrero A. Current and droplet size in the electrospraying of liquids. Scaling laws. J Aerosol Sci. 1997;28(2):249–275. [Google Scholar]
- Golshahi L, Longest PW, Holbrook L, Snead J, Hindle M. Produciton of highly charged pharmaceutical aerosols using a new aerosol induction charger. Pharmaceutical Research. 2015 doi: 10.1007/s11095-015-1682-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindle M. Soft mist inhalers: A review of current technology. The Drug Delivery Companies Report Autumn/Winter. 2004:31–34. [Google Scholar]
- Hindle M, Gupta R, Cox KA. Adding pharmaceutical flexibility to the capillary aerosol generator. In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ, editors. Respiratory Drug Delivery IX. DHI Publishing; River Grove, IL: 2004. pp. 247–254. [Google Scholar]
- Hindle M, Longest PW. Quantitative analysis and design of a spray aerosol inhaler. Part 2: Improvements in mouthpiece performance. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2013;26(5):237–247. doi: 10.1089/jamp.2012.0995. [DOI] [PubMed] [Google Scholar]
- Hinds WC. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. John Wiley and Sons; New York: 1999. [Google Scholar]
- Hu B, So PK, Chen H, Yao ZP. Electrospray ionization using wooden tips. Analytical chemistry. 2011;83(21):8201–8207. doi: 10.1021/ac2017713. [DOI] [PubMed] [Google Scholar]
- Ijsebaert JC, Geerse KB, Marijnissen JCM, Lammers JWJ, Zanen P. Electro-hydrodynamic atomization of drug solutions for inhalation purposes. Journal of Applied Physiology. 2001;91:2735–2741. doi: 10.1152/jappl.2001.91.6.2735. [DOI] [PubMed] [Google Scholar]
- Kotian R, Peart J, Bryner J, Byron PR. Calibration of the modified electrical low-pressure impactor (ELPI) for use with pressurized pharmaceutical aerosols. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2009;22(1):55–65. doi: 10.1089/jamp.2008.0683. [DOI] [PubMed] [Google Scholar]
- Lee YH, Zhang JJ, Chen DR. Note: Electrohydrodynamic atomization of liquid sheet. Review Of Scientific Instruments. 2011;82(2) doi: 10.1063/1.3553400. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang H, Manicke NE, Lin JM, Cooks RG, Ouyang Z. Development, characterization, and application of paper spray ionization. Analytical chemistry. 2010;82(6):2463–2471. doi: 10.1021/ac902854g. [DOI] [PubMed] [Google Scholar]
- Longest PW, Azimi M, Hindle M. Optimal delivery of aerosols to infants during mechanical ventilation. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2014a;27(5):371–385. doi: 10.1089/jamp.2013.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longest PW, Golshahi L, Hindle M. Improving pharmaceutical aerosol delivery during noninvasive ventilation: Effects of streamlined components. Annals of Biomedical Engineering. 2013a;41(6):1217–1232. doi: 10.1007/s10439-013-0759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longest PW, Hindle M. Quantitative analysis and design of a spray aerosol inhaler. Part 1: Effects of dilution air inlets and flow paths. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2009;22(3):271–283. doi: 10.1089/jamp.2008.0739. [DOI] [PubMed] [Google Scholar]
- Longest PW, Hindle M, Das Choudhuri S, Byron PR. Numerical simulations of capillary aerosol generation: CFD model development and comparisons with experimental data. Aerosol Science and Technology. 2007;41(10):952–973. [Google Scholar]
- Longest PW, Hindle M, Das Choudhuri S, Xi J. Comparison of ambient and spray aerosol deposition in a standard induction port and more realistic mouth-throat geometry. Journal of Aerosol Science. 2008;39(7):572–591. [Google Scholar]
- Longest PW, Mandana A, Golshahi L, Hindle M. Improving aerosol drug delivery during invasive mechanical ventilation with redesigned components. Respiratory Care. 2014b;59(5):686–698. doi: 10.4187/respcare.02782. [DOI] [PubMed] [Google Scholar]
- Longest PW, Spence BM, Holbrook LT, Mossi KM, Son YJ, Hindle M. Production of inhalable submicrometer aerosols from conventional mesh nebulizers for improved respiratory drug delivery. Journal of Aerosol Science. 2012;51:66–80. doi: 10.1016/j.jaerosci.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longest PW, Tian G. Development of a new technique for the efficient delivery of aerosolized medications to infants on mechanical ventilation. Pharmaceutical Research. 2015;32:321–336. doi: 10.1007/s11095-014-1466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longest PW, Walenga RL, Son YJ, Hindle M. High efficiency generation and delivery of aerosols through nasal cannula during noninvasive ventilation. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2013b;26(5):266–279. doi: 10.1089/jamp.2012.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazela J, Chmura K, Kulza M, Henderson C, Gregory TJ, Moskal A, Sosnowski TR, Florek E, Kramer L, Keszler M. Aerosolized albuterol sulfate delivery under neonatal ventilatory conditions: In vitro evaluation of a novel ventilator circuit patient interface connector. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2014;27(1) doi: 10.1089/jamp.2012.0992. [DOI] [PubMed] [Google Scholar]
- Mazela J, Polin RA. Aerosol delivery to ventilated newborn infants: historical challenges and new directions. Eur J Pediatr. 2011;170:433–444. doi: 10.1007/s00431-010-1292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang JT, Hui HX, Zhang Y, Cui M. Generation of charged aerosol from superheated steam in laval nozzle. J Aerosol Sci. 1995;26(4):559–562. [Google Scholar]
- Park I, Kim W, Kim SS. Multi-jet model electrospray for non-conducting fluids using two fluids and a coxail grooved nozzle. Aerosol Science and Technology. 2011;45:629–634. [Google Scholar]
- Reischl G, John W, Devor W. Uniform electrical charging of monodisperse aerosols. Journal of Aerosol Science. 1977;8(1):55–65. [Google Scholar]
- Rubin BK, Fink JB. Aerosol therapy for children. Respir Care Clin N Am. 2001;7(2):175–213. doi: 10.1016/s1078-5337(05)70030-7. [DOI] [PubMed] [Google Scholar]
- Rubin BK, Williams RW. Emerging aerosol drug delivery strategies: From bench to clinic. Advanced drug delivery reviews. 2014;75:141–148. doi: 10.1016/j.addr.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Shah V, Ohlsson A, Halliday HL, Dunn MS. Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates. Cochrane Database Of Systematic Reviews. 2007;(4) doi: 10.1002/14651858.CD001969.pub2. [DOI] [PubMed] [Google Scholar]
- Shen X, Hindle M, Byron PR. Effect of energy on propylene glycol aerosols using the capillary aerosol generator. International Journal of Pharmaceutics. 2004;275(1–2):249–258. doi: 10.1016/j.ijpharm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Sidler-Moix AL, Dolci U, Berger-Gryllaki M, Pannatier A, Cotting J, Di Paolo ER. Albuterol Delivery in an In Vitro Pediatric Ventilator Lung Model: Comparison of Jet, Ultrasonic, and Mesh Nebulizers. Pediatric Critical Care Medicine. 2013;14(2):E98–E102. doi: 10.1097/PCC.0b013e3182712783. [DOI] [PubMed] [Google Scholar]
- Tepper G, Kessick R. A study of ionization and collection efficiencies in electrospray-based electrostatic precipitators. Aerosol Science. 2008;39:609–617. [Google Scholar]
- Tepper G, Kessick R. Nanoelectrospray aerosols from microporous polymer wick sources. Applied Physics Letters. 2009;94:084106. [Google Scholar]
- Tepper G, Kessick R, Pestov D. An electrospray-based, ozone-free air purification technology. Journal Of Applied Physics. 2007;102(11) [Google Scholar]
- Walsh BK, DiBlasi RM. Mechanical ventilation of the neonate and pediatric patient. In: Walsh BK, Czervinske MP, DiBlasi RM, editors. Perinatal and Pediatric Respiratory Care. Saunders Elsevier; St. Louis: 2010. pp. 325–347. [Google Scholar]
- Xi J, Longest PW. Effects of oral airway geometry characteristics on the diffusional deposition of inhaled nanoparticles. ASME Journal of Biomechanical Engineering. 2008;130:011008. doi: 10.1115/1.2838039. [DOI] [PubMed] [Google Scholar]
- Yang Q, Wang H, Maas JD, Chappell WJ, Manicke NE, Cooks RG, Ouyang Z. Paper spray ionization devices for direct, biomedical analysis using mass spectrometry. International journal of mass spectrometry. 2012;312:201–207. doi: 10.1016/j.ijms.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Castle GSP, Adamiak K. Comparison of conducting and induction charging in liquid spraying. Journal of Electrostatics. 2005;63:871–876. [Google Scholar]