SUMMARY
BACKGROUND
Human immunodeficiency virus (HIV) exposed infants are at high risk of Mycobacterium tuberculosis exposure, have high rates of progression to tuberculosis (TB) disease and are at significant risk of bacille Calmette-Guérin (BCG) induced adverse events.
OBJECTIVE
To evaluate a delayed BCG vaccination strategy in HIV-exposed infants.
DESIGN
A randomised trial of routine BCG vaccination given at birth compared to 14 weeks of age in HIV-exposed non-infected and non-HIV-exposed infants to investigate longitudinal BCG-induced immune responses using a 7-day whole blood interferon-gamma (IFN-γ) enzyme-linked immunosorbent assay.
RESULTS
A significantly higher proportion of infants had positive responses to M. tuberculosis purified protein derivative (PPD) and BCG at 14 weeks in the birth vs. delayed vaccination groups (P = 0.001 for both). This difference was no longer apparent at weeks 24 or 52. Among infants vaccinated at birth, the 14-week IFN-γ response to M. tuberculosis PPD was lower among HIV-exposed than non-exposed infants (276.5 pg/ml vs. 790.2, P = 0.048). Among all infants, there were significant correlations between the magnitude of IFN-γ responses to BCG, M. tuberculosis PPD, TB 10.4 and culture filtrate protein 10/early secreted antigenic target 6.
CONCLUSIONS
The timing of vaccination had limited effect on BCG-induced IFN-γ responses, which waned considerably over 1 year despite initial vigorous responses in both vaccination groups. The lower responses in HIV-exposed non-infected infants suggest potentially altered mycobacterial immunity early in life.
Keywords: TB, randomised vaccine trial, IFN-γ, response, ELISA
Infants born to human immunodeficiency virus (HIV) infected women (HIV-exposed infants) have a high risk of exposure to Mycobacterium tuberculosis1 and, even in the absence of HIV infection, are at high risk of developing active tuberculosis (TB).2 The bacille Calmette-Guérin (BCG) vaccination protects young, non-HIV-infected children against disseminated forms of TB.3,4 In contrast to responses to most other early-life vaccinations, BCG induces robust T-helper 1 (Th-1) type immune responses in infants when measured 3 months after birth vaccination.5–9 Although there is no validated immune marker for BCG vaccine-induced protection,10–14 Th-1 CD4+ T-cells and interferon-gamma (IFN-γ) appear to be critical in the containment of mycobacterial infection.15–17
Due to the well-characterised risk of serious BCG-related adverse events, including 1% disseminated BCG disease in HIV-infected infants in the absence of antiretroviral therapy,18,19 HIV is a relative contraindication to BCG vaccination. However, HIV status is typically not known at birth; BCG vaccination of HIV-infected infants therefore continues to occur in high TB and HIV burden settings. Delaying BCG vaccination in HIV-exposed infants until HIV infection is ruled out may be an effective strategy to reduce the risk of live vaccination.19,20 Data characterising the effect of delayed BCG vaccination or the influence of maternal HIV infection on infant immune responses against mycobacteria are limited; however, altered BCG immunogenicity has been described in HIV-exposed non-infected infants following routine BCG vaccination at birth.21,22 BCG-induced immunity in infants is important in settings where there is a high risk of M. tuberculosis exposure early in life, and even more so in settings with high maternal HIV prevalence, which poses a risk of both vertical HIV transmission and M. tuberculosis exposure.23,24
The present study aimed to investigate the effect of delaying BCG vaccination until 14 weeks of age, and the effect of maternal HIV status on infant cellular immune responses to mycobacterial antigens.
MATERIALS AND METHODS
This was an individual, open-label, exploratory randomised Phase 2 clinical trial investigating immunological and clinical effects of early and delayed BCG vaccination in HIV-exposed and non-exposed infants (trial number DOH-27-1106-1520).19 Pregnant HIV-positive and -negative women were recruited and randomised at an antenatal clinic in Khayelitsha, Cape Town, South Africa, where there is a well-established prevention of mother-to-child HIV transmission programme (PMTCT). Maternal HIV prevalence was 32.7% (95% confidence interval [CI] 29.5–35.9) in 2006; HIV testing was opt-out. During the study period, dual treatment for PMTCT was provided for mothers and infants. Mothers were counselled to either exclusively breastfeed or formula feed their infants. A single infant HIV DNA polymerase chain reaction test (Amplicor, Roche Molecular Diagnostics, Pleasanton, CA, USA) was routinely offered at 12 weeks of age. Reported vertical HIV transmission rates were 4.5–5.4% (unpublished data, Western Cape Department of Health).
Infants were randomised to receive either routine Danish strain BCG (0.05 ml, Statens Serum Institute [SSI], Copenhagen, Denmark, 1331) on day 1 of life (birth group) or delayed BCG vaccination at 14 weeks of age (delayed group) in the right deltoid region, according to the manufacturer’s specifications. Enrolment was stratified by maternal HIV status to ensure that two thirds of infants were HIV-exposed and one third non-exposed, using two computer-generated randomisation lists. Women of ≥32 weeks’ gestation with known HIV status were screened for eligibility. Written informed consent was obtained in the local language (isiXhosa). Women who intended to move out of the district within the first year of the infant’s life, those with proven or suspected active TB or a current known household TB contact were excluded. Infants were excluded for stillbirth, birth weight <1.6 kg, severe congenital malformation, asphyxia or other severe illness at birth. Infants excluded during the first week postpartum were replaced by additional randomised subjects. All women with a negative antenatal HIV test result underwent HIV testing again 2 weeks postpartum.
Whole blood assay
One ml of blood was collected into pre-heparinised syringes at 14, 24 and 52 weeks of age. Samples were processed within 2 h of collection. Whole blood was diluted 1:10 in Roswell Park Memorial Institute medium and 200 µl was incubated with each antigen in duplicate for 7 days at 36°C, 5% carbon dioxide.20 On day 7, supernatants were removed and stored at −80°C for batched IFN-γ enzyme-linked immunosorbent assay (ELISA).
Antigens
M. tuberculosis purified protein derivative (PPD) for in vitro use (SSI) was added at a final concentration of 5 µg/ml. Lyophilised BCG vaccine (SSI) was tested at a final density of 5×105 colony-forming units (cfu)/ml. Early secreted antigenic target 6/culture filtrate protein 10 (ESAT-6/CFP-10) fusion protein (Leiden University Medical College, Leiden, The Netherlands) and TB10.4 peptide pool (SSI) were both used at a final concentration of 10 µg/ml. Staphylococcus enterotoxin B (SEB) (Sigma, St Louis, MO, USA) was used as a positive control at a final concentration of 1 µg/ml, and medium alone (unstimulated cells) was used as negative control.
Interferon-gamma ELISA
The IFN-γ ELISA was performed as previously described.20 Laboratory personnel were blinded to all clinical data. The mean IFN-γ concentration detected in duplicate wells was used to measure antigen-induced immune responses. The negative control IFN-γ value was subtracted from the mean antigen and positive control-induced IFN-γ values. Following background subtraction, a positive response cut-off was defined as 62 pg/ml.
Data and statistical analysis
Analysis of antigen responses was based on both the magnitude and the proportion of infants with positive IFN-γ responses (≥62 pg/ml). Primary comparisons investigated the proportion of positive IFN-γ responses between arms, measured at 14, 24 and 52 weeks of age. Secondary analyses examined the effect of HIV exposure on the proportion of responders and the magnitude of IFN-γ responses at 14, 24 and 52 weeks of age in the group that received vaccination at birth.
CONSORT (Consolidated Standards of Reporting Trials) guidelines for reporting randomised clinical trials and a per-protocol analysis were followed.21 Protocol violators were infants randomised to delayed vaccination but who were given routine BCG at birth. Longitudinal responses at each time point were treated as dependent measures. Both the effect of calendar time since vaccination and age at vaccination were considered in the analyses. Non-parametric analytical approaches were used for non-normal data. Response magnitudes were summarised using medians with interquartile ranges (IQRs). The effect of vaccination group on the proportion of responders was compared using the χ2 or Fisher’s exact tests; effect estimates (odds ratios [ORs] and 95%CI) were calculated. Paired analyses were conducted for comparison of individual responses over time, stratified by vaccination group. Overall differences between the three timepoints were assessed using the Friedman test statistic. Responses to antigens between weeks 14 and 24 and between weeks 24 and 52 were then compared using the Wilcoxon signed rank test.
RESULTS
Of the 180 total randomised infants, 148 infants completed phlebotomy at week 14: 84 in the birth group and 64 in the delayed BCG group; 95 (64.2%) infants were HIV-exposed and 53 (35.8%) were non-exposed (Figure 1). Of the infants who completed phlebotomy at week 14, 11 were protocol violators. Baseline characteristics between protocol violators and other infants were comparable (data not shown), and were therefore included in the birth analyses. Overall, 117 infants completed phlebotomy at week 24: 67 in the birth and 50 in the delayed group; 71 (60.7%) were HIV-exposed and 46 (39.3%) non-exposed. Infants lost to follow-up had similar baseline characteristics compared to infants included in the analysis. Immune response data on a subset of 67 infants (32 HIV-exposed) who underwent phlebotomy at week 52 are reported: 41 in the birth and 26 in the delayed vaccination group. Infants with assay results at 1 year had similar characteristics compared to the total study sample at enrolment. Two HIV-infected infants (transmission 1.7%) were excluded from the analysis.
Figure 1.
Overview of study cohort investigated for immunological measures based on actual vaccination status at birth (per protocol analysis). HIV = human immunodeficiency virus; BCG = bacille Calmette-Guérin.
Magnitude of IFN-γ responses between vaccination groups
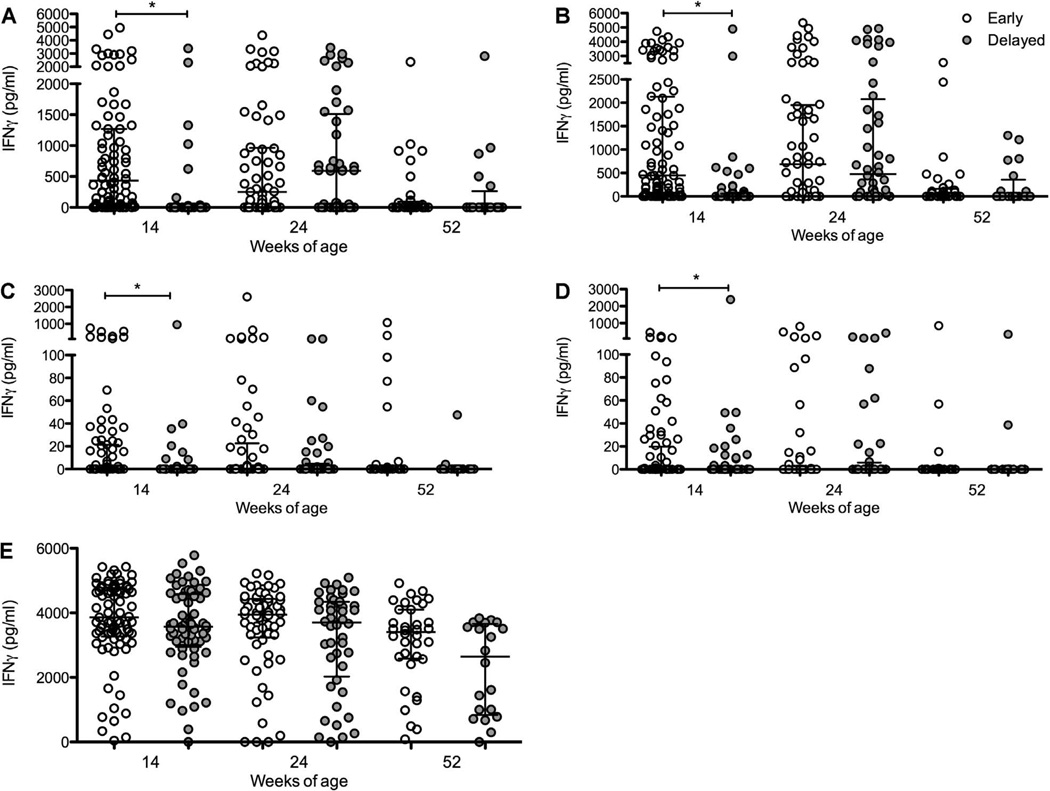
The majority of the infants (146/148, 98.6%) had positive responses to SEB at 14 weeks of age. Both of the SEB non-responders were able to respond to other antigens and were therefore included in the analysis. The mean coefficient of variation across all duplicate ELISA results was 3.26%. In infants vaccinated at birth, marked IFN-γ responses were seen at 14 weeks of age after stimulation with M. tuberculosis PPD and BCG (Figure 2). Considerable responses against these two antigens were still present at 24 weeks of age, followed by a substantial decline in cytokine production at 52 weeks of age (Figure 2). In infants vaccinated at 14 weeks, responses to M. tuberculosis PPD and BCG increased significantly from 14 to 24 weeks of age, followed by a decrease at 52 weeks of age. The IFN-γ response to SEB was robust at all three timepoints in both groups.
Figure 2.
IFN-γ responses (pg/ml) measured by ELISpot in response to A) M. tuberculosis PPD, B) BCG, C) TB 10.4, D) ESAT-6/CFP-10 and E) SEB in infants vaccinated at birth (early, open circles) vs. at 14 weeks (delayed, grey circles) measured at 14, 24 and 52 weeks of age. Lines represent medians and interquartile ranges. * P < 0.05. † P < 0.001. IFN-γ = interferon-gamma; PPD = purified protein derivative; BCG = bacille Calmette-Guérin; ESAT-6 = early secreted antigenic target 6; CFP-10 = culture filtrate protein 10; SEB = staphylococcus enterotoxin B.
Proportion of positive IFN-γ responses between vaccination groups
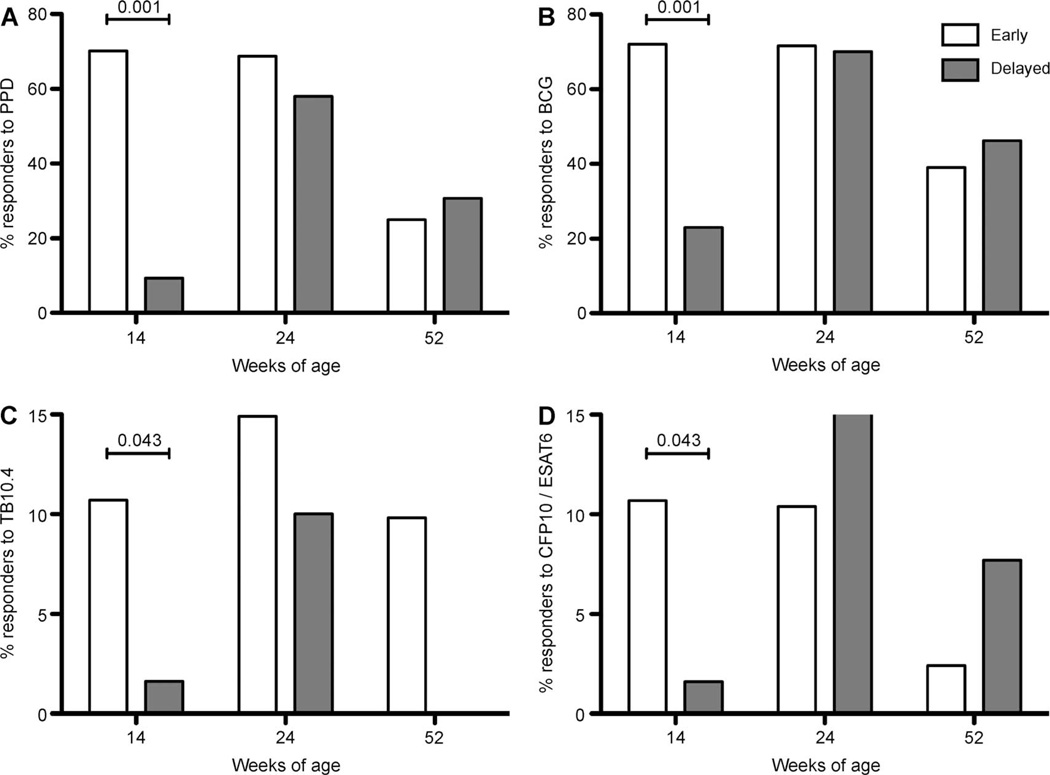
The proportion of infants with a positive IFN-γ response to M. tuberculosis PPD in the group vaccinated at birth was 70.2% at 14 weeks, 68.7% at 24 weeks and 25% at 52 weeks of age; 72.6% of the infants vaccinated at birth had positive responses to BCG at 14 weeks, 71.6% at 24 weeks and 39% at 52 weeks of age. In contrast, the proportion of infants with a positive response to M. tuberculosis PPD in the delayed vaccination group was 9.4% at 14 weeks, 58.0% at 24 weeks and 30.8% at 52 weeks of age; 23.4% of these infants had a positive response to BCG at 14 weeks, 70.0% at 24 weeks and 46.2% at 52 weeks of age (Figure 3).
Figure 3.
Proportion of infants vaccinated at birth (early) or at 14 weeks of age (delayed) with positive IFN-γ responses (≥62 pg/ml) to A) M. tuberculosis PPD, B) BCG, C) TB 10.4, D) ESAT-6/CFP-10 in infants vaccinated at birth (early, white bars) vs. at 14 weeks (delayed, grey bars), measured at 14 (n = 148, all antigens), 24 (n = 105, all antigens) and 52 weeks (n = 57, all antigens) of age. IFN-γ = interferon-gamma; PPD = purified protein derivative; BCG = bacille Calmette-Guérin; ESAT-6 = early secreted antigenic target 6; CFP-10 = culture filtrate protein 10.
As expected, a significantly higher proportion of infants had positive responses to M. tuberculosis PPD (OR 22.81, 95%CI 8.72–59.69, P ≤ 0.001) and to BCG (OR 8.66, 95%CI 4.09–18.37, P ≤ 0.001) at 14 weeks of age in the birth vaccination group. This effect was no longer apparent at 24 or 52 weeks of age (Figure 3). At 14 weeks of age, infants in the birth group were also more likely to have positive responses to ESAT-6/CFP-10 (OR 7.56, 95%CI 0.93–61.30, P = 0.043) and TB 10.4 (OR 7.56, 95%CI 0.93–61.30, P = 0.043) than those in the delayed group, implying some cross-reactivity between these antigens. At 24 and 52 weeks of age, this effect was no longer observed. No infant with either a positive ESAT-6/CFP-10 or TB 10.4 response at 14 weeks of age had a history of exposure to a TB source case or any suggestive symptoms.
The proportion of infants with a positive response to M. tuberculosis PPD at 14 weeks of age in the vaccination at birth group was compared to the proportion of responders at 24 weeks of age in the delayed group (i.e., at 14 weeks vs. 10 weeks following vaccination), and was found to be similar (70.2% vs. 58%, OR 1.78, 95%CI 0.80–3.97, P = 0.122). Responses to BCG were also similar at these time points (70.2% in the birth group at week 14 and 70.0% in the delayed group at week 24; OR 1.14, 95%CI 0.49–2.63, P = 0.51). At week 52, similar proportions of infants responded to M. tuberculosis PPD in the delayed group (30.8%) compared to the birth group (25.0%; OR 0.75, 95%CI 0.25–2.25).
Results for the proportion of M. tuberculosis PPD responders per vaccination group were similar when protocol violators were excluded from analysis (data not shown). In the birth group, the median responses to M. tuberculosis PPD declined with age (Table). In the delayed group, median responses to M. tuberculosis PPD were higher at 24 weeks than at 14 weeks of age, as expected (594.81, IQR 0–1752.29 vs. 0, IQR 0–0, P = 0.001), but then declined by 52 weeks of age (0, IQR 0–385.40 vs. 594.81, IQR 0–1752.29, P = 0.006).
Table.
Within-infant analysis of interferon-γ responses (pg/ml) to BCG, M. tuberculosis PPD, TB 10.4, ESAT-6/CFP-10 and SEB comparing weeks 14, 25 and 52, and stratified by vaccination group
| Week 14 (n = 148) |
Week 24 (n = 117) |
Week 52 (n = 67) |
||||
|---|---|---|---|---|---|---|
| Response Median [IQR] |
Response Median [IQR] |
Response Median [IQR] |
Friedman P value weeks 14, 24 and 52 |
P value week 14 vs. 24* |
P value week 24 vs. 52* |
|
| BCG at birth group | ||||||
| BCG† | 450.2 [29.8–2130.3] | 692.5 [11.0–1968.2] | 0 [0–135.6] | <0.001 | 0.50 | <0.001 |
| M.tuberculosis PPD‡ | 435.4 [37.3–1267.7] | 214.9 [0–938.8] | 0 [0–67.9] | <0.001 | 0.047 | <0.001 |
| TB 10.4 | 0 [0–21.29] | 0 [0–19.30] | 0 [0–0] | 0.002 | 0.39 | 0.017 |
| ESAT-6/CFP-10 | 0 [0–19.75] | 0 [0–2.28] | 0 [0–0] | 0.023 | 0.91 | 0.016 |
| SEB | 3859.6 [3265.2–4777.5] | 3947.1 [3156.7–4418.3] | 3328.2 [2583.7–4099.7] | 0.008 | 0.22 | 0.006 |
| Delayed BCG group | ||||||
| BCG† | 0 [0–56.83] | 544 [0–2860.8] | 0 [0–547.7] | <0.001 | <0.001 | 0.001 |
| M.tuberculosis PPD‡ | 0 [0–0] | 594.8 [0–1752.3] | 0 [0–385.4] | 0.006 | 0.001 | 0.010 |
| TB 10.4 | 0 [0–0] | 0 [0–8.23] | 0 [0–0] | 0.067 | 0.520 | 0.026 |
| ESAT-6/CFP-10 | 0 [0–0] | 0 [0–16.57] | 0 [0–0] | 0.091 | 0.170 | 0.041 |
| SEB | 3572 [2948–4585.7] | 3764.1 [2243.7–4361.7] | 3195.7 [1005.4–3730.1] | 0.001 | 0.183 | 0.044 |
Wilcoxon sign test.
Whole live BCG vaccine, Danish strain (1331).
M. tuberculosis PPD for in vitro use.
BCG = bacille Calmette-Guérin; PPD = purified protein derivative; ESAT-6 = early secreted antigenic target 6; CFP-10 = culture filtrate protein 10; IQR = interquartile range; SEB = staphylococcus enterotoxin B (positive control).
Effect of HIV exposure on IFN-γ responses in infants vaccinated at birth
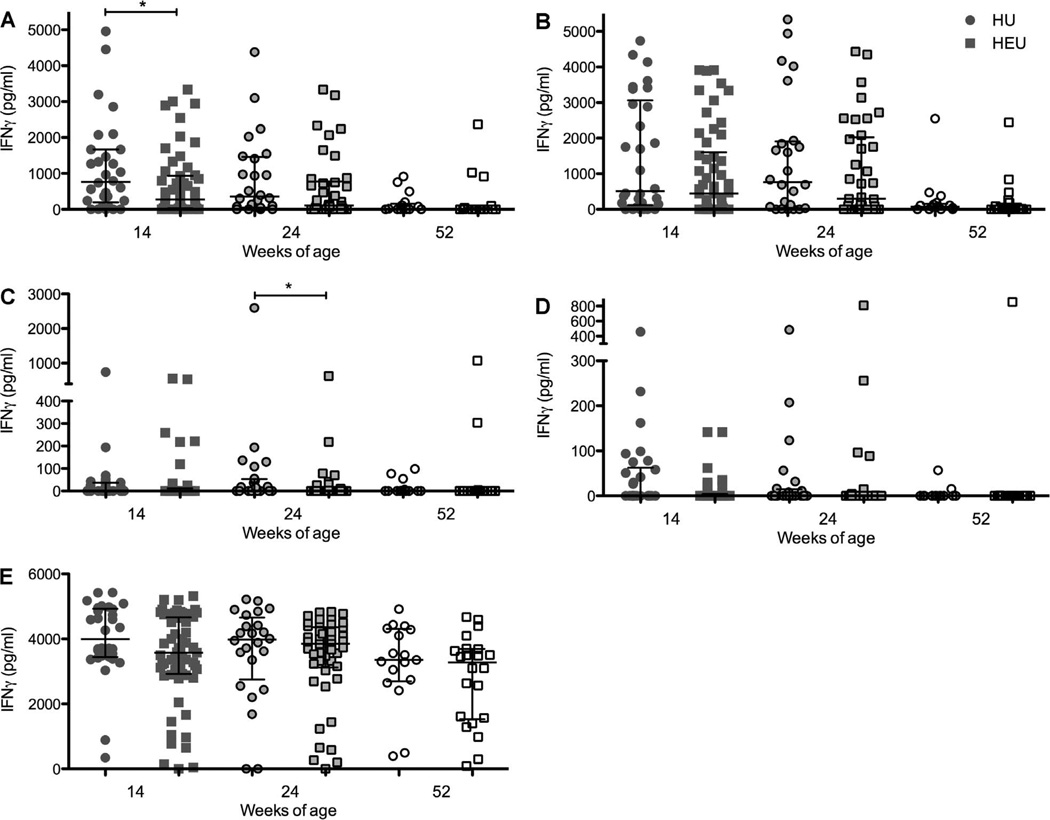
Among the infants vaccinated at birth, the median IFN-γ responses to M. tuberculosis PPD at week 14 were lower in HIV-exposed than in non-exposed infants (276.49, IQR 30.55–935.0 vs. 790.17, IQR 238.56–1668.01, P = 0.048; Figure 4). Responses to ESAT-6/CFP-10 demonstrated a similar pattern (0, IQR0–4.22 vs. 0, IQR 0–58.21, P = 0.060). Responses to BCG, TB10.4 and SEB did not differ between HIV-exposed and non-exposed infants at week 14. At week 24, results for HIV-exposed and non-exposed infants were similar in the birth group for M. tuberculosis PPD, BCG and ESAT-6/CFP-10, but HIV-exposed infants had a lower response to TB 10.4 (P = 0.044). At week 52, the responses were comparable between HIV-exposed and non-exposed infants for all antigens tested. Of the HIV-exposed infants in the birth group, 64.8% produced a positive IFN-γ response to M. tuberculosis PPD at week 14 compared to 80.0% of non-exposed infants (P = 0.145). A significantly lower proportion of HIV-exposed infants had a positive ESAT-6/CFP-10 response at week 14 (P = 0.009).
Figure 4.
IFN-γ responses (pg/ml) to A) M. tuberculosis PPD, B) BCG, C) TB 10.4, D) ESAT-6/CFP-10 and SEB at 14 weeks of age in HIV-exposed (circles) and non-exposed (squares) infants who received BCG at birth. Lines represent medians and interquartile ranges. * P < 0.05. IFN-γ = interferon-gamma; PPD = purified protein derivative; BCG = bacille Calmette-Guérin; ESAT-6 = early secreted antigenic target 6; CFP-10 = culture filtrate protein-10; SEB = staphylococcus enterotoxin B; HIV = human immunodeficiency virus.
Among the infants who received delayed BCG vaccination at 14 weeks of age, there were no significant differences in responses to M. tuberculosis PPD or BCG between HIV-exposed and non-exposed infants at 24 and 52 weeks of age. At 24 weeks of age (10 weeks post-vaccination), median responses to M. tuberculosis PPD were 49.77, IQR 0–1704 in the HIV-exposed infants vs. 613.5, IQR 0–1364 in the non-exposed infants; and median responses to BCG were 354.5, IQR 26.46–1512 in the HIV-exposed and 634.9, IQR 0–2286 in the non-exposed infants. At 52 weeks of age (38 weeks post-vaccination), the median response to M. tuberculosis PPD was 0, IQR 0–0 in the HIV-exposed infants and 0.71, IQR 0–737.9 in the non-exposed infants, P = 0.199. Similarly, median responses to BCG were respectively 0, IQR 0–342.6 and 26, IQR 0–284.7 in the HIV-exposed and non-exposed infants.
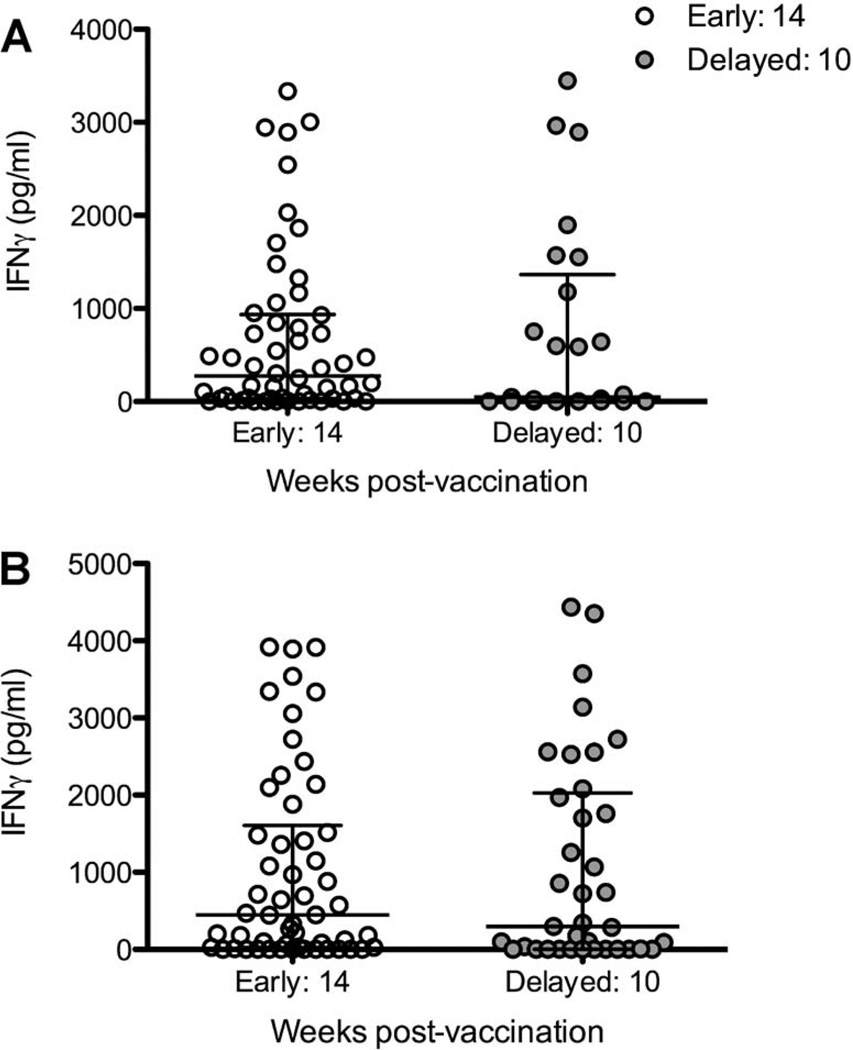
To evaluate the effect of delaying vaccination in HIV-exposed infants until after HIV status is determined, the median IFN-γ response to BCG and M. tuberculosis PPD at 14 weeks of age in the birth group was compared to the median IFN-γ responses at 24 weeks of age following delayed vaccination in the HIV-exposed infants only (i.e., at 14 vs. 10 weeks following vaccination) (Figure 5). There were no significant differences in responses to M. tuberculosis PPD (median 435, IQR 38.5–953.99 in the early group vs. 589.56, IQR 0–147.47 in the delayed group, P = 0.370) nor BCG (median 450.18, IQR 30.8–2120.26 in the early vaccinated infants at 14 weeks vs. 476.22, IQR 14.2–2002.26 in the delayed group at 24 weeks of age, P = 0.839), implying that delayed vaccination does not reduce BCG immunogenicity.
Figure 5.
IFN-γ responses (pg/ml) to A) M. tuberculosis PPD, B) BCG, in HIV-exposed infants vaccinated at birth (early, open circles) at 14 weeks of age, 14 weeks post-vaccination, were compared to HIV-exposed infants vaccinated at 14 weeks of age (delayed, grey circles) measured at 24 weeks of age, 10 weeks post-vaccination. Lines represent medians and interquartile ranges. IFN-γ = interferon-gamma; PPD = purified protein derivative; BCG = bacille Calmette-Guérin; HIV = human immunodeficiency virus.
DISCUSSION
As expected, infants who received BCG vaccination at birth had higher IFN-γ responses against mycobacterial antigens at 14 weeks of age than unvaccinated infants. However, the magnitude of cytokine responses at both 24 and 52 weeks of age were similar in those vaccinated at birth and at 14 weeks, although responses in both arms declined considerably by 52 weeks. IFN-γ responses measured at 14 weeks of age in the group vaccinated at birth were also similar to responses measured at 24 weeks of age in the delayed vaccination group.
Although studies have evaluated delayed BCG vaccination in non-HIV-exposed infants,25–27 the number of published studies on the effect of BCG vaccination timing on infant immune responses to mycobacteria in HIV-exposed infants are limited. We have previously demonstrated increased Th1 cytokine production to BCG at 14 weeks in HIV-exposed infants vaccinated with BCG at 8 weeks of age vs. at birth.28 This current study shows that Th1 cytokine production at 52 weeks is equivalent, regardless of HIV exposure or vaccination age. These differences may be due to the duration of follow-up, differences in assays used or differences in age at vaccination in the two studies. Nevertheless, delaying BCG vaccination in HIV-exposed infants does not appear to be detrimental to vaccine responses.
Several studies have described non-specific effects of in utero HIV exposure on immune responses in HIV-negative infants,21,22,29,30 the long-term significance of which is still unknown. In the present study, HIV-exposed infants vaccinated at birth had lower IFN-γ responses to M. tuberculosis PPD and ESAT-6/CFP-10 at 14 weeks of age compared to non-HIV-exposed infants. Van Rie et al., using the identical whole blood assay, also found lower IFN-γ responses to M. tuberculosis PPD in 6-week-old South African HIV-exposed compared to non-exposed infants.21 In contrast, Mansoor et al., using multiparameter flow cytometry, demonstrated that T-cell responses to BCG during the first year of life were not altered by HIV exposure.6 In the present study, effects relating to HIV exposure were lost by 52 weeks of age, suggesting possible early, transient T-cell immune suppression as a result of HIV exposure. In addition, when infants were vaccinated at 14 weeks of age, there were no differences in the responses according to HIV exposure status, which further confirms a short-term effect of HIV exposure. Although the clinical relevance is unclear, our data suggest increased immunological susceptibility to TB in HIV-exposed infants during the first 3 months of life, when they may also experience a high level of exposure to M. tuberculosis.
Acknowledgements
The authors thank B Magwegwe, N Dziba, E Mhlamba and S Masimini, S Le Roux and the personnel at the Site B Midwife Obstetric Unit and the Site B Comprehensive Health Centre for their help and support with this study. We would also like to thank Cape Town City Health and the Western Cape Department of Health.
This work was supported by a Thrasher Research Fund (Salt Lake City, UT, USA) award [NR-0006] and an Elizabeth Glazer Pediatrics (Washington, DC, USA) AIDS Foundation International Leadership award [Sub Grant ZQ-00-8-520-0123-0-00] to ACH. HBJ is supported in part by the National Institute of Allergy and Infectious Diseases (Bethesda, MD, USA) K08 HD 069201.
Footnotes
Conflict of interest: none declared.
References
- 1.Cotton MF, Schaaf HS, Lottering G, Weber HL, Coetzee J, Nachman S. Tuberculosis exposure in HIV-exposed infants in a high-prevalence setting. Int J Tuberc Lung Dis. 2008;12:225–227. [PubMed] [Google Scholar]
- 2.Madhi S, Nachman S, Violari A, et al. Primary isoniazid prophylaxis against tuberculosis in HIV-exposed children. N Engl J Med. 2011;365:29–39. doi: 10.1056/NEJMoa1011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22:1154–1158. doi: 10.1093/ije/22.6.1154. [DOI] [PubMed] [Google Scholar]
- 4.Trunz B, Fine P, Dye C, et al. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 5.Hussey GD, Watkins MLV, Goddard EA, et al. Neonatal mycobacterial specific cytotoxic T-lymphocyte and cytokine profiles in response to distinct BCG vaccination strategies. Immunology. 2002;105:314–324. doi: 10.1046/j.1365-2567.2002.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansoor N, Abel B, Scriba TJ, et al. Significantly skewed memory CD8(+) T cell subsets in HIV-1 infected infants during the first year of life. Clin Immunol. 2008;130:280–289. doi: 10.1016/j.clim.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lalor MK, Ben-Smith A, Gorak-Stolinska P, et al. Population differences in immune responses to bacille Calmette-Guérin vaccination in infancy. J Infect Dis. 2009;199:795–800. doi: 10.1086/597069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weir RE, Gorak-Stolinska P, Floyd S, et al. Persistence of the immune response induced by BCG vaccination. BMC Infect Dis. 2008;8:9. doi: 10.1186/1471-2334-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorak-Stolinska P, Weir RE, Floyd S, et al. Immunogenicity of Danish-SSI 1331 BCG vaccine in the UK: comparison with Glaxo-Evans 1077 BCG vaccine. Vaccine. 2006;24:5726–5733. doi: 10.1016/j.vaccine.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Kagina BM, Abel B, Scriba TJ, et al. other members of the South African Tuberculosis Vaccine Initiative. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guérin vaccination of newborns. Am J Respir Crit Care Med. 2010;182:1073–1079. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forbes EK, Sander C, Ronan EO, et al. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol. 2008;181:4955–4964. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittrücker H, Steinhoff U, Köhler A, et al. Poor correlation between BCG vaccination-induced T-cell responses and protection against tuberculosis. Proc Natl Acad Sci U S A. 2007;104:12434–12439. doi: 10.1073/pnas.0703510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day CL, Abrahams DA, Leruma L, et al. Functional capacity of Mycobacterium tuberculosis-specific T-cell responses in humans is associated with mycobacterial load. J Immunol. 2011;187:2222–2232. doi: 10.4049/jimmunol.1101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jouanguy E, Altare F, Lamhamedi S, et al. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N Engl J Med. 1996;335:1956–1961. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 15.Scriba TJ, Kalsdorf B, Abrahams D-A, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T-cell subsets contribute to the human antimycobacterial immune response. J Immunol. 2008;180:1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutherland JS, Adetifa IM, Hill PC, Adegbola RA, Ota MOC. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur J Immunol. 2009;39:723–729. doi: 10.1002/eji.200838693. [DOI] [PubMed] [Google Scholar]
- 17.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hesseling AC, Johnson LF, Jaspan H, et al. Disseminated bacille Calmette-Guerin disease in HIV-infected South African infants. Bull World Health Organ. 2009;87:505–511. doi: 10.2471/BLT.08.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hesseling AC, Caldwell J, Cotton MF, et al. BCG vaccination in South African HIV-exposed infants: risks and benefits. S Afr Med J. 2009;99:88–91. [PMC free article] [PubMed] [Google Scholar]
- 20.Hesseling AC, Cotton MF, Marais BJ, et al. BCG and HIV reconsidered: moving the research agenda forward. Vaccine. 2007;25:6565–6568. doi: 10.1016/j.vaccine.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 21.Van Rie A, Madhi SA, Heera JR, et al. Gamma interferon production in response to Mycobacterium bovis BCG and Mycobacterium tuberculosis antigens in infants born to human immunodeficiency virus-infected mothers. Clin Vaccine Immunol. 2006;13:246–252. doi: 10.1128/CVI.13.2.246-252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kidzeru EB, Hesseling AC, Passmore JA, et al. In-utero exposure to maternal HIV infection alters T cell immune responses to vaccination in HIV-uninfected infants. AIDS. 2014;28:1421–1430. doi: 10.1097/QAD.0000000000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta A, Nayak U, Ram M, et al. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002–2005. Clin Infect Dis. 2007;45:241–249. doi: 10.1086/518974. [DOI] [PubMed] [Google Scholar]
- 24.Kali PBN, Gray GE, Violari A, Chaisson RE, McIntyre JA, Martinson NA. Combining PMTCT with active case finding for tuberculosis. J Acquir Immune Defic Syndr. 2006;42:379–381. doi: 10.1097/01.qai.0000218434.20404.9c. [DOI] [PubMed] [Google Scholar]
- 25.Kagina B, Abel B, Hussey GD, et al. Delaying BCG vaccination from birth to 10 weeks of age may result in an enhanced memory CD4 T-cell response. Vaccine. 2009;27:5488–5495. doi: 10.1016/j.vaccine.2009.06.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ota M, Vekemans J, Schlegel-Haueter SE, et al. Influence of Mycobacterium bovis bacillus Calmette-Guerin on antibody and cytokine responses to human neonatal vaccination. J Immunol. 2002;168:919–925. doi: 10.4049/jimmunol.168.2.919. [DOI] [PubMed] [Google Scholar]
- 27.Burl S, Adetifa UJ, Cox M, et al. Delaying bacillus Calmette-Guerin vaccination from birth to 4 1/2 months of age reduces postvaccination Th1 and IL-17 presponses but leads to comparable myobacterial responses at 9 months of age. J Immunol. 2010;185:2620–2628. doi: 10.4049/jimmunol.1000552. [DOI] [PubMed] [Google Scholar]
- 28.Tchakoute CT, Hesseling AC, Kidzeru EB, et al. Delaying BCG vaccination until 8 weeks of age results in robust BCG-specific T-cell responses in HIV-exposed infants. J Infect Dis. 2015;211:338–346. doi: 10.1093/infdis/jiu434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones C, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. Infants exposed to HIV at birth but not infected may have lower antibody levels against certain diseases. JAMA. 2011;305:535–634. doi: 10.1001/jama.2011.100. [DOI] [PubMed] [Google Scholar]
- 30.Kuhn L, Coutsoudis A, Moodley D, et al. Interferon-gamma and interleukin-10 production among HIV-1-infected and uninfected infants of HIV-1-infected mothers. Pediatr Res. 2001;50:412–416. doi: 10.1203/00006450-200109000-00018. [DOI] [PubMed] [Google Scholar]