Abstract
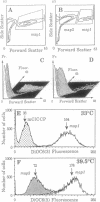
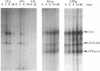
Rodent embryo cells immortalized with temperature-sensitive mutants of simian virus 40 large tumor (T) antigen have a proliferative potential that depends on temperature. At the restrictive temperature, heat-inactivation of large T antigen causes p53 release, growth arrest, and cell death. Morphological and molecular analysis indicate that the induced cell death corresponds to apoptosis. Flow cytometric analysis using a combination of forward light scatter and side scatter allows a discrimination of cells committed to apoptosis within the whole population. These cells display a reduction in cell size and a higher cellular density, confirming the apoptotic nature of the cell death. When cells exhibiting the morphological features of apoptosis were stained with a fluorescent probe of the mitochondrial membrane potential, a decreased accumulation of the dye was recorded. Measures of cellular respiration, performed with whole-cell populations, showed that the lower mitochondrial membrane potential (delta psi m) correlates, as expected, with an uncoupling of electron transport from ATP production and is linked to the induction of apoptosis. We also show that this decrease in delta psi m is associated with a decrease in the rate of mitochondrial translation. These events are detected at early stages of the apoptotic process, when most of the cells are not irreversibly committed to death, suggesting that mitochondria could be a primary target during apoptosis.
Full text
PDF
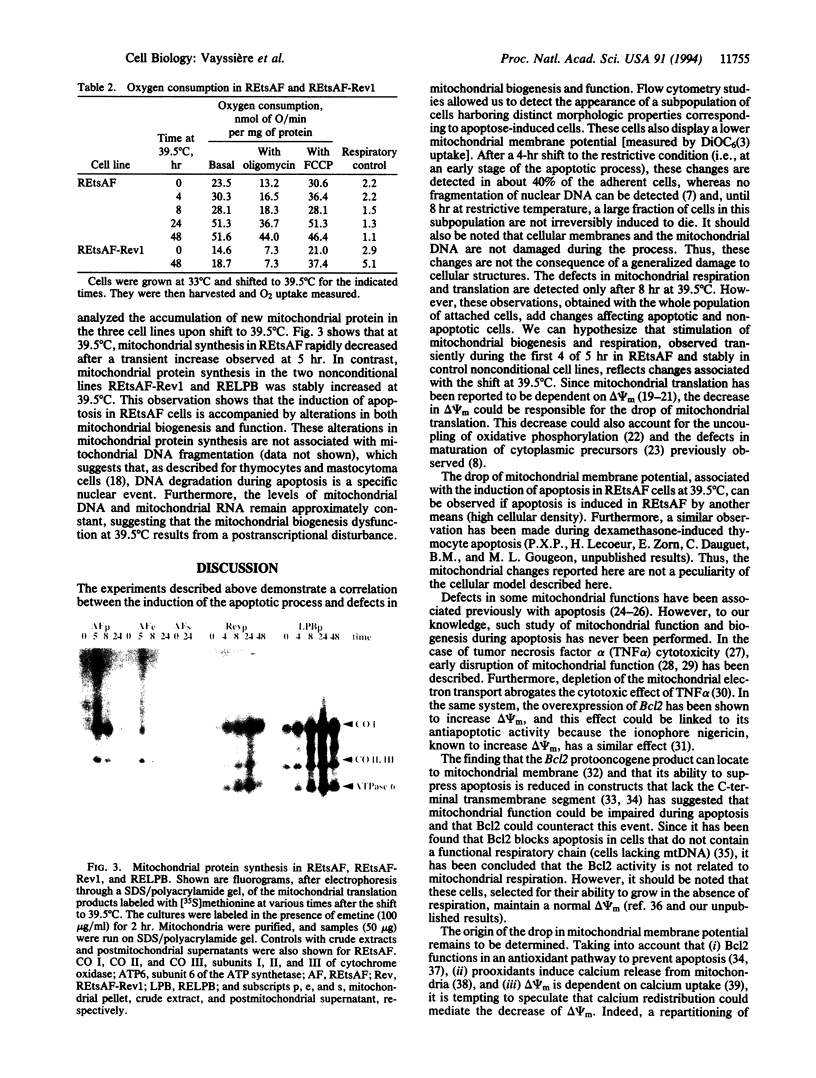

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnemri E. S., Robertson N. M., Fernandes T. F., Croce C. M., Litwack G. Overexpressed full-length human BCL2 extends the survival of baculovirus-infected Sf9 insect cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7295–7299. doi: 10.1073/pnas.89.16.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardi G., Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- Baffy G., Miyashita T., Williamson J. R., Reed J. C. Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem. 1993 Mar 25;268(9):6511–6519. [PubMed] [Google Scholar]
- Bursch W., Oberhammer F., Schulte-Hermann R. Cell death by apoptosis and its protective role against disease. Trends Pharmacol Sci. 1992 Jun;13(6):245–251. doi: 10.1016/0165-6147(92)90077-j. [DOI] [PubMed] [Google Scholar]
- Cordeau-Lossouarn L., Vayssière J. L., Larcher J. C., Gros F., Croizat B. Mitochondrial maturation during neuronal differentiation in vivo and in vitro. Biol Cell. 1991;71(1-2):57–65. doi: 10.1016/0248-4900(91)90051-n. [DOI] [PubMed] [Google Scholar]
- Côté C., Boulet D., Poirier J. Expression of the mammalian mitochondrial genome. Role for membrane potential in the production of mature translation products. J Biol Chem. 1990 May 5;265(13):7532–7538. [PubMed] [Google Scholar]
- Darzynkiewicz Z., Bruno S., Del Bino G., Gorczyca W., Hotz M. A., Lassota P., Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13(8):795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- Glab N., Petit P. X., Slonimski P. P. Mitochondrial dysfunction in yeast expressing the cytoplasmic male sterility T-urf13 gene from maize: analysis at the population and individual cell level. Mol Gen Genet. 1993 Jan;236(2-3):299–308. doi: 10.1007/BF00277126. [DOI] [PubMed] [Google Scholar]
- Glick B., Schatz G. Import of proteins into mitochondria. Annu Rev Genet. 1991;25:21–44. doi: 10.1146/annurev.ge.25.120191.000321. [DOI] [PubMed] [Google Scholar]
- Gunter T. E., Pfeiffer D. R. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990 May;258(5 Pt 1):C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- Hennet T., Bertoni G., Richter C., Peterhans E. Expression of BCL-2 protein enhances the survival of mouse fibrosarcoid cells in tumor necrosis factor-mediated cytotoxicity. Cancer Res. 1993 Mar 15;53(6):1456–1460. [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Jacobson M. D., Burne J. F., King M. P., Miyashita T., Reed J. C., Raff M. C. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993 Jan 28;361(6410):365–369. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- Jacquemin-Sablon H., Ganz L., Feunteun J. Transfer of immortality by transfection of genomic DNA from SV40 established cell lines into rat embryo fibroblasts. Biol Cell. 1990;68(3):227–230. doi: 10.1016/0248-4900(90)90312-q. [DOI] [PubMed] [Google Scholar]
- Jat P. S., Sharp P. A. Cell lines established by a temperature-sensitive simian virus 40 large-T-antigen gene are growth restricted at the nonpermissive temperature. Mol Cell Biol. 1989 Apr;9(4):1672–1681. doi: 10.1128/mcb.9.4.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane D. J., Sarafian T. A., Anton R., Hahn H., Gralla E. B., Valentine J. S., Ord T., Bredesen D. E. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993 Nov 19;262(5137):1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- Korchak H. M., Rich A. M., Wilkenfeld C., Rutherford L. E., Weissmann G. A carbocyanine dye, DiOC6(3), acts as a mitochondrial probe in human neutrophils. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1495–1501. doi: 10.1016/s0006-291x(82)80076-4. [DOI] [PubMed] [Google Scholar]
- Lancaster J. R., Jr, Laster S. M., Gooding L. R. Inhibition of target cell mitochondrial electron transfer by tumor necrosis factor. FEBS Lett. 1989 May 8;248(1-2):169–174. doi: 10.1016/0014-5793(89)80454-5. [DOI] [PubMed] [Google Scholar]
- Laster S. M., Wood J. G., Gooding L. R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988 Oct 15;141(8):2629–2634. [PubMed] [Google Scholar]
- Mignotte B., Larcher J. C., Zheng D. Q., Esnault C., Coulaud D., Feunteun J. SV40 induced cellular immortalization: phenotypic changes associated with the loss of proliferative capacity in a conditionally immortalized cell line. Oncogene. 1990 Oct;5(10):1529–1533. [PubMed] [Google Scholar]
- Murgia M., Pizzo P., Sandoná D., Zanovello P., Rizzuto R., Di Virgilio F. Mitochondrial DNA is not fragmented during apoptosis. J Biol Chem. 1992 Jun 5;267(16):10939–10941. [PubMed] [Google Scholar]
- Nicoletti I., Migliorati G., Pagliacci M. C., Grignani F., Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991 Jun 3;139(2):271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K., Young D. A. Glucocorticoid action on rat thymic lymphocytes. Experiments utilizing adenosine to support cellular metabolism lead to a reassessment of catabolic hormone actions. J Biol Chem. 1976 Dec 10;251(23):7295–7303. [PubMed] [Google Scholar]
- Petit C. A., Gardes M., Feunteun J. Immortalization of rodent embryo fibroblasts by SV40 is maintained by the A gene. Virology. 1983 May;127(1):74–82. doi: 10.1016/0042-6822(83)90372-0. [DOI] [PubMed] [Google Scholar]
- Rabinovitz Y. M., Pinus H. A., Kotelnikova A. V. A study of dependence of protein synthesis in mitochondria on the transmembrane potential. Mol Cell Biochem. 1977 Feb 4;14(1-3):109–113. doi: 10.1007/BF01734173. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Naghashfar Z., Cowie A., Carr A., Grisoni M., Kamen R., Cuzin F. Expression of the large T protein of polyoma virus promotes the establishment in culture of "normal" rodent fibroblast cell lines. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4354–4358. doi: 10.1073/pnas.80.14.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C., Kass G. E. Oxidative stress in mitochondria: its relationship to cellular Ca2+ homeostasis, cell death, proliferation, and differentiation. Chem Biol Interact. 1991;77(1):1–23. doi: 10.1016/0009-2797(91)90002-o. [DOI] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Bakker A. C., Vanhaesebroeck B., Beyaert R., Jacob W. A., Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992 Mar 15;267(8):5317–5323. [PubMed] [Google Scholar]
- Schulze-Osthoff K., Beyaert R., Vandevoorde V., Haegeman G., Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 1993 Aug;12(8):3095–3104. doi: 10.1002/j.1460-2075.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Glynn J. M., Guilbert L. J., Cotter T. G., Bissonnette R. P., Green D. R. Role for c-myc in activation-induced apoptotic cell death in T cell hybridomas. Science. 1992 Jul 10;257(5067):212–214. doi: 10.1126/science.1378649. [DOI] [PubMed] [Google Scholar]
- Skowronek P., Haferkamp O., Rödel G. A fluorescence-microscopic and flow-cytometric study of HeLa cells with an experimentally induced respiratory deficiency. Biochem Biophys Res Commun. 1992 Sep 16;187(2):991–998. doi: 10.1016/0006-291x(92)91295-2. [DOI] [PubMed] [Google Scholar]
- Swat W., Ignatowicz L., Kisielow P. Detection of apoptosis of immature CD4+8+ thymocytes by flow cytometry. J Immunol Methods. 1991 Mar 1;137(1):79–87. doi: 10.1016/0022-1759(91)90396-w. [DOI] [PubMed] [Google Scholar]
- Vayssiere J. L., Larcher J. C., Berthelot F., Benlot C., Gros F., Croizat B. Effects on mitochondrial metabolism of CCA, one inducer of neuroblastoma differentiation. Biochem Biophys Res Commun. 1986 Nov 14;140(3):789–796. doi: 10.1016/0006-291x(86)90703-5. [DOI] [PubMed] [Google Scholar]
- Vayssière J. L., Cordeau-Lossouarn L., Larcher J. C., Basseville M., Gros F., Croizat B. Participation of the mitochondrial genome in the differentiation of neuroblastoma cells. In Vitro Cell Dev Biol. 1992 Nov-Dec;28A(11-12):763–772. doi: 10.1007/BF02631065. [DOI] [PubMed] [Google Scholar]
- Vukmanović S., Zamoyska R. Anti-CD3-induced cell death in T cell hybridomas: mitochondrial failure and DNA fragmentation are distinct events. Eur J Immunol. 1991 Feb;21(2):419–424. doi: 10.1002/eji.1830210225. [DOI] [PubMed] [Google Scholar]
- Zheng D. Q., Vayssière J. L., Petit P. X., LeCoeur H., Spatz A., Mignotte B., Feunteun J. Apoptosis is antagonized by large T antigens in the pathway to immortalization by polyomaviruses. Oncogene. 1994 Nov;9(11):3345–3351. [PubMed] [Google Scholar]
- Zoeteweij J. P., van de Water B., de Bont H. J., Mulder G. J., Nagelkerke J. F. Involvement of intracellular Ca2+ and K+ in dissipation of the mitochondrial membrane potential and cell death induced by extracellular ATP in hepatocytes. Biochem J. 1992 Nov 15;288(Pt 1):207–213. doi: 10.1042/bj2880207. [DOI] [PMC free article] [PubMed] [Google Scholar]